ABSTRACT
ADIPOQ/adiponectin, an adipocytokine secreted by adipocytes in the breast tumor microenvironment, negatively regulates cancer cell growth hence increased levels of ADIPOQ/adiponectin are associated with decreased breast cancer growth. However, its mechanisms of action remain largely elusive. We report that ADIPOQ/adiponectin induces a robust accumulation of autophagosomes, increases MAP1LC3B-II/LC3B-II and decreases SQSTM1/p62 in breast cancer cells. ADIPOQ/adiponectin-treated cells and xenografts exhibit increased expression of autophagy-related proteins. LysoTracker Red-staining and tandem-mCherry-GFP-LC3B assay show that fusion of autophagosomes and lysosomes is augmented upon ADIPOQ/adiponectin treatment. ADIPOQ/adiponectin significantly inhibits breast cancer growth and induces apoptosis both in vitro and in vivo, and these events are preceded by macroautophagy/autophagy, which is integral for ADIPOQ/adiponectin-mediated cell death. Accordingly, blunting autophagosome formation, blocking autophagosome-lysosome fusion or genetic-knockout of BECN1/Beclin1 and ATG7 effectively impedes ADIPOQ/adiponectin induced growth-inhibition and apoptosis-induction. Mechanistic studies show that ADIPOQ/adiponectin reduces intracellular ATP levels and increases PRKAA1 phosphorylation leading to ULK1 activation. AMPK-inhibition abrogates ADIPOQ/adiponectin-induced ULK1-activation, LC3B-turnover and SQSTM1/p62-degradation while AMPK-activation potentiates ADIPOQ/adiponectin's effects. Further, ADIPOQ/adiponectin-mediated AMPK-activation and autophagy-induction are regulated by upstream master-kinase STK11/LKB1, which is a key node in antitumor function of ADIPOQ/adiponectin as STK11/LKB1-knockout abrogates ADIPOQ/adiponectin-mediated inhibition of breast tumorigenesis and molecular analyses of tumors corroborate in vitro mechanistic findings. ADIPOQ/adiponectin increases the efficacy of chemotherapeutic agents. Notably, high expression of ADIPOQ receptor ADIPOR2, ADIPOQ/adiponectin and BECN1 significantly correlates with increased overall survival in chemotherapy-treated breast cancer patients. Collectively, these data uncover that ADIPOQ/adiponectin induces autophagic cell death in breast cancer and provide in vitro and in vivo evidence for the integral role of STK11/LKB1-AMPK-ULK1 axis in ADIPOQ/adiponectin-mediated cytotoxic autophagy.
Introduction
Adipocytes are major constituents of mammary stroma, long considered as mere fat-storing units, but now established as active players within the breast tumor microenvironment, that influence growth and malignant progression of breast cancer.Citation1-Citation5 Adipocytes secrete a large number of bioactive molecules called adipocytokines that function in an autocrine, paracrine and endocrine manner to exert their biological effects.Citation2,Citation3,Citation6 While most adipocytokines are positively associated with breast cancer growth and their increased levels are considered a risk-factor due to their pro-oncogenic role,Citation7 ADIPOQ/adiponectin/ACRP30/apM1/GBP28 is the only adipocytokine known for its anticancer role.Citation8-Citation10 In fact, ADIPOQ/adiponectinCitation11-Citation14 is the most abundant adipose tissue secreted protein and is recognized as ‘guardian angel adipocytokine’ owing to its protective role against the pathogenesis of many disease-states.Citation15,Citation16 ADIPOQ/adiponectin usually circulates in oligomeric complexes as trimers, hexamers and multimers. A globular form of ADIPOQ/adiponectin is generated via proteolytic cleavage of the monomers. Functionally, ADIPOQ/adiponectin multimers mediate peripheral functions whereas monomers get transported to brain and mediate several CNS functions.Citation17-Citation20 ADIPOQ/adiponectin can exist as full-length or smaller globular fragment; however, almost all ADIPOQ/adiponectin appears to exist as full-length ADIPOQ/adiponectin in plasma, and only a small amount of globular ADIPOQ/adiponectin has been detected in human plasma.Citation21 Experimental settings use the globular fragment of ADIPOQ/adiponectin that mimics the full-length ADIPOQ/adiponectin.Citation22-Citation24 Various epidemiological studies have shown that low levels of ADIPOQ/adiponectin are associated with increased risk of multiple cancers including breast cancer. A more aggressive phenotype of breast cancer with larger tumor size, higher tumor-grade, increased angiogenesis and metastasis is observed in women with low ADIPOQ/adiponectin levels.Citation19,Citation25-Citation28 A study evaluating the expression of ADIPOQ/adiponectin shows high levels of ADIPOQ/adiponectin in the adjacent tissue of breast cancer but baseline expression levels in normal breast tissue are not analyzed.Citation29 Evaluation of ADIPOQ/adiponectin in ductal carcinoma in situ and invasive ductal carcinoma samples shows that high ADIPOQ/adiponectin expression may be associated with invasive progressionCitation30,Citation31 but the authors do not evaluate ADIPOQ/adiponectin expression in peritumoral normal tissue. While the anticancer role of ADIPOQ/adiponectin in breast cancer is well supported with epidemiological studies and preclinical findings, studies evaluating the expression of ADIPOQ/adiponectin in breast cancer samples are limited. ADIPOQ/adiponectin treatment has shown promise in insulin-sensitizing, anti-inflammatory, antiatherogenic as well as anticancer activities.Citation32-Citation38 ADIPOQ/adiponectin induces cell cycle arrest and apoptosis and increases expression of proapoptotic genes BAD (BCL2 associated agonist of cell death), TP53 (tumor protein p53) and PTEN (phosphatase and tensin homolog), and decreases anti-apoptotic genes BCL2 and BIRC5/Survivin (baculoviral IAP repeat containing 5) and reduces the expression of CCND1 (cyclin D1) and CCNE2 (cyclin E2) in breast cancer cells.Citation39-Citation41 Proangiogenic and metastatic features such as invasion and migration are also inhibited with ADIPOQ/adiponectin treatment.Citation42,Citation43 Various studies from others and our group have shown that ADIPOQ/adiponectin treatment inhibits growth, invasion, and migration and induces apoptosis of cancer cells.Citation37,Citation44-Citation47
In recent years, macroautophagy (referred to as autophagy hereafter), a catabolic process in which the cells utilize a vacuolar, lysosomal degradation pathway and recycle their cytoplasmic constituents to primarily prevent accumulation of damaged proteins or provide energy in stress conditions, has emerged as an important regulator of cell death and survival.Citation48,Citation49 Four forms of autophagy have been proposed based on their functional impact—cytoprotective autophagy that may bestow therapeutic resistance and increase apoptosis when blocked; cytotoxic autophagy that promotes apoptosis and cell death; cytostatic autophagy that may mediate growth inhibition and senescence; and nonprotective autophagy that exerts no influence on therapeutic sensitivity.Citation50-Citation52 At the molecular level, autophagy is regulated by concerted actions of multiple autophagy-related (ATG) proteins that mediate the formation of autophagosomes, sequestration of proteins and organelles, fusion of autophagosomes with lysosomes and disintegration of the cargo. Emerging evidence indicates the involvement of autophagy in breast tumor suppression and suggests that autophagy and apoptosis respond to similar tumor-microenvironmental and external cues.Citation53-Citation55
We report here that ADIPOQ/adiponectin stimulates autophagic flux in breast cancer cells and suppressing autophagy by inhibiting autophagosome-formation using 3-methyladenine, blocking autophagosome-lysosome fusion, with bafilomycin A1 (Baf), or CRISPR/Cas9-mediated knockout of BECN1 (Beclin 1) and ATG7 (autophagy-related 7) effectively blocked ADIPOQ/adiponectin induced growth-inhibition and stimulated apoptosis. Notably, we demonstrate that ADIPOQ/adiponectin induced ULK1 (unc-51 like autophagy activating kinase 1) via AMPK (5′ adenosine monophosphate-activated protein kinase) activation, which in turn is regulated by ADIPOQ/adiponectin-induced STK11/LKB1 (serine/threonine kinase 11/Liver Kinase B1). We present that STK11/LKB1 is integral to ADIPOQ/adiponectin-mediated cytotoxic autophagy and STK11/LKB1-silencing inhibits ADIPOQ/adiponectin-mediated AMPK-activation, autophagy induction and tumor inhibition. We further investigated the effects of ADIPOQ/adiponectin treatment on the therapeutic efficacy of chemotherapeutic drugs and show that cotreatment of ADIPOQ/adiponectin markedly decreased growth and increased apoptosis in breast cancer cells treated with various chemotherapeutic agents. Collectively, these data provide the first in vitro and in vivo evidence to support a novel role of ADIPOQ/adiponectin as an inducer of cytotoxic-autophagy in breast cancer and show an integral role of the STK11/LKB1-AMPK-ULK1 axis.
Results
ADIPOQ/adiponectin inhibits breast cancer growth in vitro and in vivo
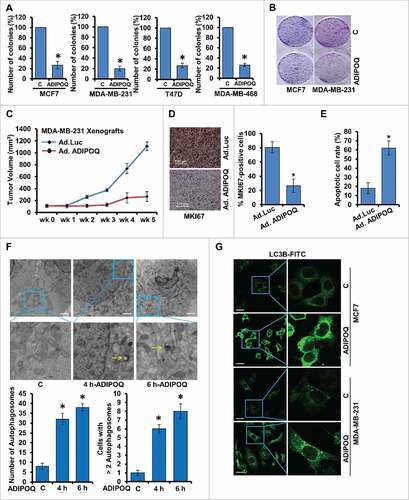
ADIPOQ/adiponectin exerted a significant decrease in cell-number in a temporal manner and more effective inhibition was observed within 48 to 60 h of treatment (Fig. S1A to C). ADIPOQ/adiponectin inhibited soft-agar colony-formation and clonogenicity of breast cancer cells in comparison to control cells (, ). It is interesting to note that ADIPOQ/adiponectin-mediated inhibition of breast cancer growth was associated with increased apoptotic cell death (Fig. S1D). Subsequently, we examined the in vivo physiological relevance of our in vitro findings by evaluating whether ADIPOQ/adiponectin-administration inhibited breast carcinoma growth in athymic nude mice. We observed that ADIPOQ/adiponectin treatment inhibited MDA-MB-231 xenografts in athymic nude mice while the vehicle treated-group showed increased tumor growth (). In line with in vitro studies, breast tumors from ADIPOQ/adiponectin-treated mice showed significantly decreased MKI67/Ki-67 (marker of proliferation) expression () and increased number of TUNEL-positive apoptotic cells in comparison to the control group (). These results suggest that ADIPOQ/adiponectin induces apoptosis and inhibits breast tumor growth.
Figure 1. ADIPOQ/adiponectin inhibits breast cancer growth and induces autophagosome accumulation. (A) Breast cancer cells were treated with 5 µg/ml ADIPOQ/adiponectin and subjected to soft-agar colony-formation assay for 3 wk. Histogram represents average number of colonies counted (in 6 microfields). *, P < 0.001, compared with untreated controls. Vehicle-treated cells, denoted with the letter “C.” (B) Clonogenicity of breast cancer cells treated with 5 µg/ml ADIPOQ/adiponectin as indicated. (C) MDA-MB-231 cell-derived xenografts were developed in nude mice and treated with control-adenoviral (Ad-Luc) and ADIPOQ/adiponectin-adenoviral (Ad-ADIPOQ) (108 pfu). Tumor growth was monitored by measuring the tumor volume for 6 wk. (n = 8 mice per group). Ad-ADIPOQ/adiponectin treatment reduced tumor size as compared with Ad-Luc, *P < 0.001. (D) Tumors from vehicle (V) and ADIPOQ/adiponectin-treated mice were subjected to immunohistochemical analysis using MKI67 antibodies. Scale bar: 100 µm. Bar diagrams show quantification of immunohistochemical analysis. *P < 0.001, compared with control. (E) TUNEL-positive cells in tumor sections were counted. Each bar represents the mean apoptotic cell rate (n = 6–8). *, P < 0.01, compared with untreated controls. (F) MCF7 cells were treated with 5 µg/ml ADIPOQ/adiponectin as indicated and visualized under an electron microscope. Scale bar: 2 µm. Top pictures are shown with approximately 7,400x magnifications. Double-membrane autophagosomes were counted in randomly selected ∼100 cells. The number of autophagosomes was counted from randomly selected fields. Cells with >2 autophagosomes were counted. (G) MCF7 and MDA-MB-231 cells were treated with 5 µg/ml ADIPOQ/adiponectin and subjected to immunocytochemistry using LC3B antibody. Scale bar: 20 µm. Representative immunofluorescence images are shown.
ADIPOQ/adiponectin treatment induces autophagy in breast cancer cells
We next examined the ability of ADIPOQ/adiponectin to modulate autophagy in breast cancer cells. Transmission electron microscopy (TEM) was used to observe ultrastructural changes in breast cancer cells treated with ADIPOQ/adiponectin. The TEM studies revealed that ADIPOQ/adiponectin-treated breast cancer cells acquired significantly higher number (4-fold increase) of autophagic vacuoles in comparison to control cells. Accordingly, number of breast cancer cells exhibiting autophagosomes (more than 2) was increased upon ADIPOQ/adiponectin-treatment (). Redistribution of MAP1LC3B/LC3B (microtubule-associated protein 1 light chain 3 α) from the cytosol to autophagosomes denotes the formation of autophagosomes and can be detected with confocal-microscopy. A marked increase in LC3B puncta formation was observed in ADIPOQ/adiponectin-treated MDA-MB-231 and MCF7 cells while control cells exhibited a diffuse green fluorescence in the cytosol (, Fig. S2). Conversion of LC3B-I to LC3B-II, an important indicator of autophagy activity, involves cleavage of LC3B by ATG4 to generate the cytoplasmic-form LC3B-I (18 kDa), which conjugates with the lipid phosphatidylethanolamine and converts to phagophore-associated LC3B-II (16 kDa).Citation56 We examined the effect of ADIPOQ/adiponectin treatment on LC3B conversion, and found a time-dependent accumulation of LC3B-II in MCF7 and MDA-MB-231 cells ().
Figure 2. ADIPOQ/adiponectin induces autophagy in breast cancer cells. (A) Breast cancer cells were treated with 5 µg/ml ADIPOQ/adiponectin, and total cell lysates were immunoblotted for LC3B and SQSTM1/p62 expression. ACTB was used as a loading control. Bar diagram shows quantification of western blot signals from multiple independent experiments. (B) Immunoblot analysis of BECN1, ATG5, ATG7 and ATG12 in breast cancer cells treated with 5 µg/ml ADIPOQ/adiponectin as indicated. ACTB was used as a loading control. (C) Total protein lysates from tumors from control-adenoviral (Ad-Luc) and ADIPOQ/adiponectin -adenoviral (Ad-ADIPOQ)-treated mice were examined for the expression of BECN1, ATG7, LC3B and SQSTM1/p62. ACTB was used as a loading control. (D) Tumors from vehicle (V) and ADIPOQ/adiponectin-treated mice were subjected to immunohistochemical (IHC) analysis using BECN1, ATG5, SQSTM1/p62 and LC3B antibodies. Scale bar: 100 µm. Bar diagrams show quantification of IHC analysis. *P < 0.01, compared with control. (E,F) Breast cancer cells were transfected with an LC3B-encoding plasmid, treated with 5 µg/ml ADIPOQ/adiponectin and stained with LysoTracker Red. Scale bar: 20 µm. Representative immunofluorescence images are shown.
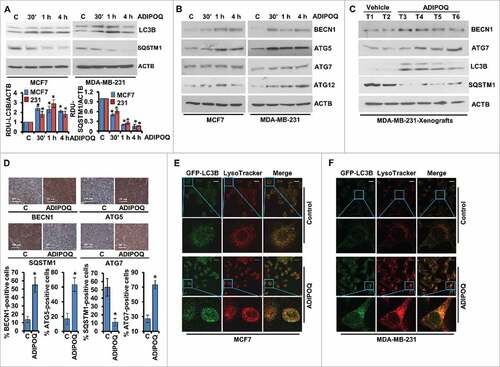
The ubiquitin-binding protein SQSTM1/p62 (sequestosome 1) functions as a link between LC3B and ubiquitinated substrates and is degraded in autolysosomes. The degradation of SQSTM1/p62 is accelerated when autophagic flux is activated resulting in decreased SQSTM1/p62 levels whereas inhibition of autophagy results in accumulation of SQSTM1/p62 in the cell.Citation57-Citation59 Immunoblot analysis of ADIPOQ/adiponectin-treated breast cancer cells showed a significant temporal decrease in SQSTM1/p62 levels (). In addition to increased LC3B-conversion and decreased SQSTM1/p62-levels, other autophagy-related (ATG) proteins, for instance, BECN1, ATG5, ATG7 and ATG12 were also modulated upon ADIPOQ/adiponectin treatment. MCF7 and MDA-MB-231 cells treated with ADIPOQ/adiponectin showed increased expression of BECN1, LAMP1 (lysosomal associated membrane protein 1), ATG5, ATG7 and ATG12 (, Fig. S3). These in vitro observations were also supported by our in vivo findings as immunoblotting of MDA-MB-231 xenograft-tumors from ADIPOQ/adiponectin-treated mice showed increased expression of BECN1, ATG7, LC3B-II and decreased SQSTM1/p62 protein levels (). Immunohistochemical analysis also showed increased expression of BECN1, ATG5, ATG7 and decreased SQSTM1/p62 level in breast tumors treated with ADIPOQ/adiponectin (). We further investigated the effect of ADIPOQ/adiponectin on lysosomes using LysoTracker Red (an acidic pH marker for lysosomes)-staining and confocal microscopy and observed that ADIPOQ/adiponectin increased lysosomal signals in breast cancer cells (Fig. S4). To provide evidence that ADIPOQ/adiponectin induces autophagic flux in breast cancer cells, we transfected MCF7 cells with a plasmid encoding membrane-localized red fluorescent protein (mRFP)-EGFP-LC3B (tandem fluorescent-tagged LC3B [tfLC3B]) which results in both green and red fluorescence.Citation60 EGFP fluorescence is quenched in acidic compartments whereas mRFP is more stable in low-pH environment. Accordingly, tfLC3B can be used to ascertain autophagosomes (GFP-positive and RFP-positive; merged as yellow) and autolysosomes (GFP-negative and RFP-positive; merged as red) and both yellow and red punctate fluorescence will increase in autophagy-activation. Analysis of the distribution of the GFP-RFP-LC3B fusion protein in ADIPOQ/adiponectin-treated breast cancer cells exhibited increased red and yellow fluorescence similar to rapamycin (an activator of autophagy), which also caused an increase in both red and yellow punctate fluorescence (Fig. S5), indicating that autophagic flux had increased. The final stage of autophagy is the fusion of autophagosomes with lysosomes, which was examined by staining GFP-LC3B-transfected MCF7 and MDA-MB-231 cells with LysoTracker Red. Significant overlap between LC3B and lysosomal signals was observed upon ADIPOQ/adiponectin treatment indicating that ADIPOQ/adiponectin induced autophagosome-lysosome fusion (, ). Taken together, these results provide strong evidence that ADIPOQ/adiponectin increases autophagy in breast cancer cells.
Impairment of autophagy inhibits ADIPOQ/adiponectin-mediated reduction in cell survival and apoptosis induction in breast cancer cells
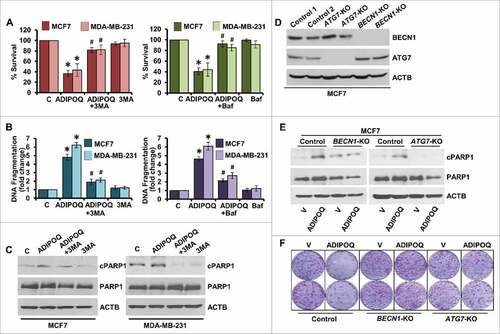
Next, the link between induction of autophagy and apoptosis by ADIPOQ/adiponectin in breast cancer cells was investigated. Autophagy was blocked in ADIPOQ/adiponectin -treated MDA-MB-231 and MCF7 cells using 3-methyladenine (3-MA), a phosphatidylinositol 3-kinase (PtdIns3K/Vps34) inhibitor that hinders autophagosome formationCitation61 or Baf, a specific vacuolar type H+-ATPase inhibitor which blocks autophagosome-lysosome fusion.Citation62 We found that ADIPOQ/adiponectin-mediated inhibition of growth of breast cancer cells was abrogated (56% to 60% vs. 15% to 20% growth-inhibition) upon combined treatment with 3-MA or Baf (). Also, DNA-fragmentation observed upon ADIPOQ/adiponectin -treatment was significantly reduced (6- to 7-fold vs. 2- to 2.5-fold) in cells cotreated with 3-MA and Baf (). Immunoblot analysis of MCF7 and MDA-MB-231 cells treated with combination of ADIPOQ/adiponectin and 3-MA showed decreased cleavage of PARP1 in comparison to ADIPOQ/adiponectin-treated cells (). Autophagy is mediated by the coordinated actions of many ATG proteins.Citation49 BECN1 and ATG7 were knocked out (KO) in MCF7 cells using CRISPR/Cas9 technology (). We found that ADIPOQ/adiponectin treatment did not induce PARP1 cleavage in BECN1-KO and ATG7-KO cells (). Also, ADIPOQ/adiponectin-mediated inhibition of growth of breast cancer cells was significantly blocked upon BECN1 and ATG7 knockout as BECN1-KO and ATG7-KO MCF7 cells showed normal anchorage-dependent-growth even in the presence of ADIPOQ/adiponectin (). Collectively, these data showed that sensitivity to ADIPOQ/adiponectin-mediated inhibition of growth and apoptosis-induction was significantly impaired by inhibition of autophagy indicating that ADIPOQ/adiponectin induces cytotoxic-autophagy in breast cancer cells, which is integral for its function.
Figure 3. Inhibition of autophagy inhibits ADIPOQ/adiponectin-mediated reduction in cell-survival and apoptosis-induction in breast cancer cells. (A) MCF7 and MDA-MB-231 cells were treated with 5 µg/ml ADIPOQ/adiponectin alone or in combination with 200 nM Baf and 2 mM 3-MA as indicated and subjected to XTT assay. *P < 0.001, compared with control; # P < 0.005, compared with ADIPOQ/adiponectin-treated cells. (B) Breast cancer cells were treated as in (A) and subjected to DNA fragmentation assay. *P < 0.01, compared with control; # P < 0.01, compared with ADIPOQ/adiponectin -treated cells. (C) MCF7 and MDA-MB-231 cells were treated with 5 µg/ml ADIPOQ/adiponectin and 2 mM 3-MA and total cell lysates were immunoblotted for cleaved PARP1 (cPARP1), PARP1 and ACTB as indicated. (D) BECN1 and ATG7 were knocked out in MCF7 cells using CRISPR/Cas9 and total cell lysates were immunoblotted for BECN1 and ATG7. ACTB was used as loading control. (E) Control, BECN1-KO and ATG7-KO MCF7 cells were treated with 5 µg/ml ADIPOQ/adiponectin and total cell lysates were immunoblotted for cleaved-PARP1 and total-PARP1 expression levels. (F) Clonogenicity of control, BECN1-KO and ATG7-KO MCF7 cells treated with 5 µg/ml ADIPOQ/adiponectin as indicated.
ADIPOQ/adiponectin induces energy-depletion and AMPK-activation in breast cancer cells and inhibition of AMPK impedes ADIPOQ/adiponectin-mediated modulation of autophagy markers
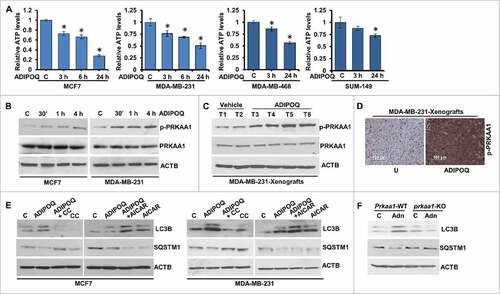
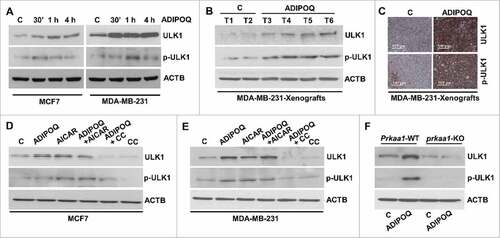
ADIPOQ/adiponectin treatment increases the AMP:ATP ratio altering the cellular energy levels.Citation63 To investigate whether ADIPOQ/adiponectin affects cellular energy levels in breast cancer cells, we measured the intracellular production of ATP in MCF7, MDA-MB-231, MDA-MB-468 and SUM-149 breast cancer cells upon ADIPOQ/adiponectin treatment. Indeed, breast cancer cells exhibited a significant decrease in intracellular ATP levels in the presence of ADIPOQ/adiponectin (). Accordingly, Thr172-phosphorylation of the PRKAA1 subunit of the cellular energy sensor kinase AMPK was also remarkably increased in ADIPOQ/adiponectin-treated MCF7 and MDA-MB-231 breast cancer cells () indicating an increased AMP:ATP ratio in the presence of ADIPOQ/adiponectin. Next, we examined PRKAA1 phosphorylation in MDA-MB-231 xenograft-tumors from ADIPOQ/adiponectin-treated and vehicle-treated mice and found that PRKAA1 phosphorylation was increased in tumors from ADIPOQ/adiponectin-treated mice in comparison to vehicle-treated controls while total-PRKAA1 levels remained unaffected (). Immunohistochemical analysis also showed increased phosphorylation of PRKAA1 in breast tumors treated with ADIPOQ/adiponectin () supporting our in vitro observations. We then investigated the functional relevance of AMPK-activation in ADIPOQ/adiponectin-induced autophagy. Inhibition of AMPK-activation with compound C led to decreased LC3B-II and increased SQSTM1/p62 levels in ADIPOQ/adiponectin-treated MCF7 and MDA-MB-231 cells whereas the AMPK activator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) potentiated the effects of ADIPOQ/adiponectin on LC3B-II and SQSTM1/p62 levels (). Furthermore, immunoblot analysis of ADIPOQ/adiponectin-treated prkaa1 knockout mouse embryonic fibroblasts (MEFs) exhibited decreased LC3B-II and high SQSTM1/p62 levels while Prkaa1 WT MEFs showed increased LC3B-II and decreased SQSTM1/p62 levels (). These results supported the notion that AMPK activation plays an important role in ADIPOQ/adiponectin-induced autophagy.
Figure 4. ADIPOQ/adiponectin induces energy depletion and AMPK-activation in breast cancer cells and inhibition of AMPK hinders ADIPOQ/adiponectin-mediated modulation of autophagy markers. (A) Intracellular ATP production was measured in MCF7, MDA-MB-231, MDA-MB-468 and SUM149 cells treated with ADIPOQ/adiponectin (5 µg/ml) for the indicated times. Relative ATP levels are expressed as fold change with respect to control. *P < 0.01, compared with control. (B) Total cell lysates from MCF7 and MDA-MB-231 cells treated with 5 µg/ml ADIPOQ/adiponectin for the indicated times were immunoblotted for phospho-PRKAA1 (p-PRKAA1), and total PRKAA1. ACTB was used as a loading control. (C) Total protein lysates from tumors from control-adenoviral (Ad-Luc) and ADIPOQ/adiponectin-adenoviral (Ad-ADIPOQ)-treated mice were examined for the expression of p-PRKAA1 and PRKAA1. ACTB was used as a loading control. (D) Tumors from vehicle (V) and ADIPOQ/adiponectin-treated mice were subjected to immunohistochemical analysis using p-PRKAA1 antibodies. Scale bar: 100 µm. (E) MCF7 and MDA-MB-231 cells were treated with ADIPOQ/adiponectin (5 µg/ml), compound C (CC) and AICAR alone and in combination as indicated, and total cell lysates were immunoblotted for LC3B and SQSTM1/p62 expression. ACTB was used as a loading control. (F) Prkaa1 wild-type (Prkaa1-WT) and prkaa1 knockout (prkaa1-KO) MEFs were treated with 5 µg/ml ADIPOQ/adiponectin and total cell lysates were immunoblotted for LC3B and SQSTM1/p62 expression. ACTB was used as a loading control.
ADIPOQ/adiponectin increases ULK1 expression and phosphorylation via AMPK in breast cancer cells
The serine/threonine kinase ULK1 is a key autophagy-initiating kinase that integrates cellular nutrient and energy cues and regulates autophagy-induction.Citation64 We examined the effect of ADIPOQ/adiponectin on ULK1; and immunoblot analysis showed increased expression and phosphorylation of ULK1 in ADIPOQ/adiponectin-treated MCF7 and MDA-MB-231 cells (). MDA-MB-231 xenografts also displayed increased expression and phosphorylation of ULK1 as evident in immunoblot and immunohistochemical analyses (, ). ULK1 itself is now known to be regulated in part by the energy-sensing kinase AMPK, which associates with the ULK1 complex and phosphorylates ULK1.Citation65,Citation66 Therefore, we examined the involvement of AMPK in ADIPOQ/adiponectin-induced ULK1 activation and observed that AMPK inhibition with compound C caused a decrease in ULK1 and phospho-ULK1 levels whereas AMPK-stimulation with AICAR resulted in increased expression of ULK1 (, ). The importance of AMPK in ADIPOQ/adiponectin-mediated induction of ULK1 was further supported by analyses of prkaa1 knockout MEFs. ADIPOQ/adiponectin treatment induced ULK1 expression and phosphorylation in Prkaa1 wild-type MEFs but not in prkaa1 null MEFs (). Together, these results show that AMPK activation is imperative for ADIPOQ/adiponectin-mediated activation of ULK1.
Figure 5. ADIPOQ/adiponectin increases ULK1 expression via AMPK in breast cancer cells. (A) MCF7 and MDA-MB-231 cells were treated with 5 µg/ml ADIPOQ/adiponectin for various time intervals as indicated and total cell lysates were immunoblotted for ULK1 and phospho-ULK1 (p-ULK1) expression. ACTB is used as a loading control. (B) Total protein lysates from tumors from control-adenoviral (Ad-Luc) (denoted as C) and ADIPOQ/adiponectin-adenoviral (Ad-ADIPOQ)-treated mice were examined for the expression of ULK1 and p-ULK1. ACTB was used as a loading control. (C) Tumors from vehicle (V) and ADIPOQ/adiponectin-treated mice were subjected to immunohistochemical analysis using ULK1 and p-ULK1 antibodies. Scale bar: 100 µm. (D, E) MCF7 and MDA-MB-231 cells were treated with ADIPOQ/adiponectin (5 µg/ml), compound C and AICAR alone and in combination as indicated, and total cell lysates were immunoblotted for ULK1 and p-ULK1 expression. ACTB was used as a loading control. (F) Prkaa1 wild-type (Prkaa1-WT) and prkaa1 knockout (prkaa1-KO) MEFs were treated with 5 µg/ml ADIPOQ/adiponectin and total cell lysates were immunoblotted for ULK1 and p-ULK1 expression. ACTB was used as control.
STK11/LKB1 plays an integral role in ADIPOQ/adiponectin-mediated cytotoxic-autophagy and tumor growth-inhibition
STK11/LKB1 (serine/threonine kinase 11), an upstream kinase and tumor suppressor that phosphorylates PRKAA1 and activates AMPK in response to various cellular and environmental signals,Citation67,Citation68 has been recently shown to be involved in promoting autophagic activity.Citation69,Citation70 We showed that ADIPOQ/adiponectin treatment increased the expression of STK11/LKB1 in MCF7 and MDA-MB-231 cells (Fig. S6A). MDA-MB-231 xenografts from athymic nude mice treated with ADIPOQ/adiponectin or vehicle-control for 5 wk were examined for STK11/LKB1 expression and tumors from ADIPOQ/adiponectin-treated-mice showed increased expression of STK11/LKB1 in comparison to tumors from vehicle-treated mice (Fig. S6B). The upstream kinase STK11/LKB1 is phosphorylated by MAPK1/ERK2 (mitogen-activated protein kinase 1), which abrogates the ability of STK11/LKB1 to bind and activate PRKAA1.Citation71,Citation72 We observed that ADIPOQ/adiponectin treatment not only inhibited the phosphorylation of MAPK1/ERK2, a negative regulator of STK11/LKB1, but also inhibited negative phosphorylation of STK11/LKB1 in MCF7 and MDA-MB-231 cells (Fig. S6C). We examined the impact of elimination of MAPK1/ERK2-activity on ADIPOQ/adiponectin-induced autophagy markers. As expected, cotreatment with MAPK1/ERK2-inhibitor, U0126, potentiated ADIPOQ/adiponectin-mediated LC3B-II induction and SQSTM1/p62 reduction in breast cancer cells (Fig. S6D).
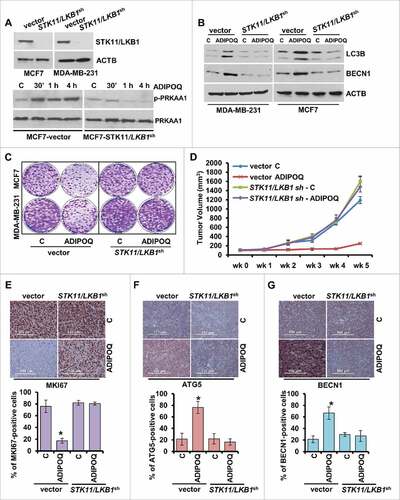
We questioned the significance of STK11/LKB1 in ADIPOQ/adiponectin-mediated cytotoxic autophagy and breast cancer growth-inhibition. Stable pools of breast cancer cells with STK11/LKB1-depletion were developed using STK11/LKB1 shRNA lentiviruses and puromycin selection, and the knockdown (KD) of STK11/LKB1 protein was confirmed in western blots (). ADIPOQ/adiponectin treatment increased PRKAA1 phosphorylation in MCF7 cells infected with vector (pLKO.1) whereas MCF7 cells infected with STK11/LKB1 shRNAexhibited no PRKAA1 phosphorylation (). Next, we compared the autophagy levels by evaluating the well-established autophagy markers LC3B-II and BECN1 in cells infected with STK11/LKB1 vector and STK11/LKB1-null cells. We found that ADIPOQ/adiponectin treatment increased levels of LC3B-II and BECN1 in the MCF7 cells infected with vector and MDA-MB-231 cells infected with vector in comparison to MCF7 cells infected with STK11/LKB1 shRNAandMDA-MB-231 cells infected with STK11/LKB1 shRNA that showed no change (). ADIPOQ/adiponectin also inhibited clonogenic growth of MCF7 cells infected with vector and MDA-MB-231 cells infected with vector, whereas no alteration in growth of MCF7 cells infected with STK11/LKB1 shRNAandMDA-MB-231 cells infected with STK11/LKB1 shRNA cells was observed (). Subsequently, the in vivo physiological relevance of our in vitro results was investigated by examining whether STK11/LKB1 plays an integral role in ADIPOQ/adiponectin mediated autophagy-induction and tumor-growth inhibition. Xenografts of MDA-MB-231 cells infected with STK11/LKB1 shRNAandMDA-MB-231 cells infected with vector were developed in athymic-nude mice followed by ADIPOQ/adiponectin treatment of 5 wk. Tumor growth was significantly inhibited in the ADIPOQ/adiponectin-treated MDA-MB-231-vector-infected control group whereas ADIPOQ/adiponectin treatment did not inhibit tumor growth in the group with MDA-MB-231 cells infected with STK11/LKB1 shRNA (). Immunohistochemical staining of the marker of proliferation MKI67/Ki-67, ATG5 and BECN1 showed that the ADIPOQ/adiponectin-treated MDA-MB-231-vector-infected control group displayed reduced expression of MKI67/Ki-67 and increased expression of ATG5 and BECN1. Conversely, ADIPOQ/adiponectin treatment did not affect expression of MKI67/Ki-67, ATG5 or BECN1 in the group with MDA-MB-231 cells infected with STK11/LKB1 shRNA ( to ).
Figure 6. STK11/LKB1 plays an important role in ADIPOQ/adiponectin-mediated cytotoxic-autophagy and inhibition of tumor growth. (A) Total protein lysates of MCF7 and MDA-MB-231 cells infected with STK11/LKB1 shRNA (STK11/LKB1sh ) and vector-pLKO.1 control (vector) were immunoblotted for the expression of STK11/LKB1. MCF7 cells containing STK11/LKB1 shRNA and MCF7 cells containing vector were treated with 5 µg/ml ADIPOQ/adiponectin and total protein lysates were examined for p-PRKAA1 and PRKAA1 expression in an immunoblot assay. (B) MCF7 cells containing STK11/LKB1 shRNA, MCF7 cells containing vector, MDA-MB-231 cells containing STK11/LKB1 shRNA, and MDA-MB-231 cells containing vector were treated with 5 µg/ml ADIPOQ/adiponectin and total protein lysates were examined for LC3B and BECN1 expression in an immunoblot assay. (C) MCF7 cells with STK11/LKB1 shRNA, MCF7 cells with vector, MDA-MB-231 cells with STK11/LKB1 shRNA, and MDA-MB-231 cells with vector were treated with 5 µg/ml ADIPOQ/adiponectin and subjected to a clonogenicity assay. (D) Tumors derived from MDA-MB-231 cells with STK11/LKB1 shRNA and MDA-MB-231 cells with vector-pLKO.1 control (vector) were developed in nude mice and treated with control-adenoviral-Ad-Luc (C) and ADIPOQ/adiponectin-adenoviral (Ad-ADIPOQ) (108 pfu). Tumor growth was monitored by measuring the tumor volume for 5 weeks (n = 8–10); (P < 0.001), vector + ADIPOQ/adiponectin compared with STK11/LKB1 shRNA+ ADIPOQ/adiponectin. (E-G) Tumors from vehicle (V) and ADIPOQ/adiponectin-treated mice were subjected to immunohistochemical analysis using MKI67, ATG5 and BECN1 antibodies. Scale bar: 100 µm. Bar diagrams show quantification of IHC-analysis. *P < 0.01, compared with control.
ADIPOQ/adiponectin treatment enhances the effect of reduced dose of multiple chemotherapeutic agents
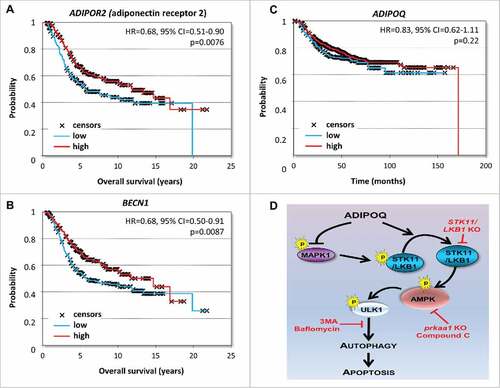
The cytoprotective function of autophagy may reduce therapeutic advantage; therefore, its inhibition is proposed to sensitize tumor cells to treatment modalities. Indeed, it has been shown that combined treatment with autophagy inhibitors increase the efficacy of chemotherapy.Citation73,Citation74 Another form of autophagy is cytoxic-autophagy that is usually not associated with conventional therapies but can mediate tumor growth-inhibition and apoptosisCitation49 and hence may enhance the effect of conventional chemotherapeutic regimens. Conventional chemotherapy causes some unwanted toxicity and side effects; therefore, a foremost challenge is to develop regimens with lower doses of chemotherapeutic agents without compromising their effectiveness. Since ADIPOQ/adiponectin induces cytotoxic-autophagy, we reasoned that combination of ADIPOQ/adiponectin treatment with lower doses of doxorubicin, paclitaxel and carboplatin might be effective in breast cancer. We found that half-doses of doxorubicin, paclitaxel and carboplatin alone did not inhibit growth and clonogenicity of breast cancer cells but a combined treatment with ADIPOQ/adiponectin resulted in effective growth-inhibition (, ). The combination of ADIPOQ/adiponectin with reduced dose of chemotherapeutic agents also resulted in increased TUNEL staining and PARP1 cleavage (, ). Next, we examined the correlation between expression of ADIPOR2 (adiponectin receptor 2) and BECN1 with overall survival in chemotherapy-treated patients. The survival analysis was restricted to chemotherapy treated patients (n = 416 in the Metabric cohort and n = 791 in the Affymetrix cohort). In the Metabric data set, higher expression of ADIPOR2 and BECN1 correlated with better survival (HR = 0.68, 95% CI = 0.51–0.90, P = 0.0076 and HR = 0.68, 95% CI = 0.50–0.91, P = 0.0087, respectively). In the Affymetrix cohort, ADIPOQ/adiponectin levels were correlated with survival (HR = 0.83, 95% CI = 0.62–1.11, P = 0.22) but the results were not statistically significant. Survival plots are displayed in , , . In the chemotherapy-treated patients in the Affymetrix cohort, higher expression of ADIPOR2 and BECN1 correlated with better relapse-free survival (HR = 0.63, 95% CI = 0.47–0.84, P = 0.0013 and HR = 0.73, 95% CI = 0.73–1.00, P = 0.049, respectively). The survival analyses using Metabric and Affymetric cohort showed that lower expression of ADIPOR1 (Metabric: HR = 1.6, 95% CI = 1.2–2.1, P = 0.0029; Affymetric: HR = 1.4, 95% CI = 1.1–1.9, P = 0.013) correlated with better relapse-free survival. Survival plots are presented in Fig. S7A to D. Survival analysis using overall survival data in the Affymetrix cohort delivered similar results for ADIPOR2 (HR = 0.78, 95% CI = 0.58–0.92, P = 0.0083) and BECN1 (HR = 0.74, 95% CI = 0.6–0.92, P = 0.0062) (Plots not shown).
Figure 7. ADIPOQ/adiponectin sensitizes breast cancer cells to a variety of chemotherapy drugs. (A, B) MCF7 and MDA-MB-231 cells were treated with 5 µg/ml ADIPOQ/adiponectin, carboplatin (Car.), paclitaxel (Pac.) or doxorubicin (Dox.) either alone or in combination and subjected to clonogenicity. *P < 0.01, compared with control; **P < 0.001, compared with control; # P < 0.05, compared with cells treated with carboplatin, paclitaxel and doxorubicin alone. (C) MCF7 and MDA-MB-231 cells were treated as in (A) and subjected to TUNEL assay. *P < 0.05, compared with control; **P < 0.01, compared with carboplatin alone; # P < 0.01, compared with paclitaxel alone; ## P < 0.01, compared with doxorubicin alone. (D) MCF7 cells were treated with 5 µg/ml ADIPOQ/adiponectin, carboplatin (Car.), paclitaxel (Pac.) and or doxorubicin (Dox.) either alone or in combination and total cell lysates were subjected to immunoblot analysis using cleaved-PARP1, total-PARP1 antibodies. ACTB was included as a loading control.
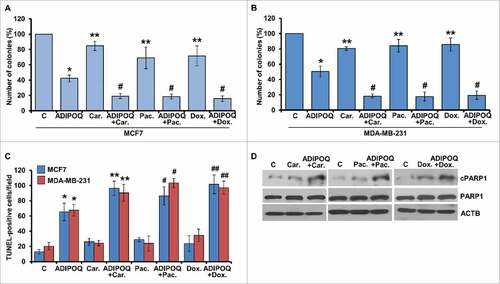
Figure 8. Higher expression of ADIPOQ/adiponectin-receptor and BECN1 correlates with increased overall survival. (A, B) Chemotherapy-treated patients (n = 416 in the Metabric cohort and n = 791 in the Affymetrix cohort) were included in a survival analysis. In the Metabric data set, higher expression of ADIPOR2 (adiponectin receptor 2) and BECN1 correlated with better survival (HR = 0.68, 95% CI = 0.51–0.90, P = 0.0076 and HR = 0.68, 95% CI = 0.50–0.91, P = 0.0087, respectively). (C) ADIPOQ/adiponectin levels were correlated with survival in the Affymetrix cohort but the results were not statistically significant (HR = 0.83, 95% CI = 0.62–1.11, P = 0.22). (D) Schematic representation of ADIPOQ/adiponectin-mediated activation of cytotoxic autophagy via the STK11/LKB1 axis. ADIPOQ/adiponectin treatment increases expression and cytoplasmic localization of STK11/LKB1 and reduces negative phosphorylation of STK11/LKB1 via reduction of phospho-MAPK1/ERK2. ADIPOQ-induced STK11/LKB1 leads to AMPK-activation, which in turn increases ULK1 expression and phosphorylation leading to cytotoxic autophagy and tumor growth inhibition.
Collectively, the in vitro and in vivo findings presented here reveal a novel role of ADIPOQ/adiponectin as an inducer of autophagic-cell-death in breast cancer cells and provide evidence for the integral role of the STK11/LKB1-AMPK-ULK1 axis in ADIPOQ/adiponectin-mediated cytotoxic-autophagy (). These studies also indicate that ADIPOQ/adiponectin treatment may be used to lower the chemotherapy dose in breast cancer.
Discussion
Autophagy is the basic vacuolar, lysosomal degradation process known to help preventing the accumulation of damaged proteins and organelles, recycling cytoplasmic constituents and maintaining cellular homeostasis. Recent studies have proposed a much more complex role of autophagy that differentially affects cells based on different intracellular and extracellular cues. Accordingly, the autophagic process can be cytoprotective, cytotoxic, cytostatic or nonprotective. Blocking cytoprotective autophagy can lead to increased sensitivity to therapy whereas obstructing cytotoxic autophagy can result in reduced drug sensitivity. Inducing cytotoxic autophagy or cytostatic autophagy promotes cell death via apoptotic induction or growth inhibition respectively. Conversely, nonprotective autophagy does not influence therapeutic effects when modulated.Citation50-Citation52 Therefore, it is deemed necessary to decipher the functional classification of autophagy before designing any intervention modalities.
In the present study, we hypothesized that ADIPOQ/adiponectin-mediated breast cancer inhibition might involve autophagy and investigated the underlying molecular mechanisms. Previous reports have shown that breast cancer cells undergo cytotoxic autophagy involving apoptosis in response to various combination treatments such as vitamin D and radiation, eribulin and AURKA (aurora kinase A) inhibitor, and the bioactive compound C from Celastrus paniculatus.Citation75-Citation78 Breast cancer cells can undergo autophagic cell death via the canonical pathway involving BECN1 or the noncanonical pathway independent of BECN1 and autophagy can serve as an integral prerequisite to promote apoptosis in a drug-dependent manner (reviewed in 51). We provide clear evidence that ADIPOQ/adiponectin induces the accumulation of autophagosomes and autophagosome-lysosome fusion and breast cancer cells undergo ‘autophagic cell death’ upon ADIPOQ/adiponectin treatment as inhibiting autophagy with 3-MA or Baf or knockout of BECN1 and ATG7 rescues ADIPOQ/adiponectin-treated breast cancer cells. ADIPOQ/adiponectin mediated autophagic-induction results in apoptotic cell death. Further, our results demonstrate that ADIPOQ/adiponectin reduces cellular ATP production altering the AMP:ATP ratio leading to a significant increase in PRKAA1 phosphorylation that further induces the phosphorylation of ULK1 and accordingly, AMPK inhibition inhibits while AMPK stimulation further stimulates ULK1 expression and phosphorylation levels in ADIPOQ/adiponectin-treated cells. We also found that the upstream master kinase, tumor suppressor STK11/LKB1 plays an integral role in mediating ADIPOQ/adiponectin-mediated autophagy-induction and tumor-suppression. We used only single dose of ADIPOQ/adiponectin in our studies and we acknowledge that lower and/or higher doses of ADIPOQ/adiponectin may result in different mechanistic effects. Our in vivo studies corroborate the in vitro findings. Importantly, ADIPOQ/adiponectin treatment sensitizes breast cancer cells to chemotherapy and indeed higher expression of ADIPOR2 and BECN1 correlate with longer overall survival and relapse-free survival in chemotherapy-treated patients in Metabric and Affymetrix cohorts.
STK11/LKB1 is a tumor suppressor and an upstream kinase that modulates diverse cellular functions including the cell cycle, apoptosis, cell polarity and metabolism via activating AMPK and several AMPK-related kinases.Citation79 STK11/LKB1 has been recently implicated in autophagy, and reduced expression of STK11/LKB1 and BECN1 associates with poor survival in nonsmall cell lung cancer.Citation80 We show that STK11/LKB1 is important for ADIPOQ/adiponectin-mediated autophagy as ADIPOQ/adiponectin treatment fails to induce the cleavage of LC3B or BECN1-induction in STK11/LKB1-silenced breast cancer cells. Autophagy is a tightly regulated process mediated by concerted actions of ATG proteins. Among the autophagy-related proteins, ULK1, the most upstream component of core-autophagy-machinery forms a complex with ATG13 and RB1CC1/Atg17 (RB1 inducible coiled-coil 1) and plays a key role in autophagy initiation.Citation81 Core components of autophagy machinery are conserved from yeast to mammals.Citation82-Citation84 AMPK associates with and directly phosphorylates ULK1/Atg1 and this phosphorylation-dependent modification of ULK1 is integral for autophagy induction.Citation66 We found that ADIPOQ/adiponectin treatment resulted in AMPK activation, which, in a ‘feed-forward’ manner, led to ULK1 phosphorylation in breast cancer cells. Although previous studies have examined the connection between ADIPOQ/adiponectin and ULK1/Atg1 in LPS-induced cardiac anomalies, inflammation, diabetes, insulin sensitivity in high-fat-diet-fed obese mice, and acetaminophen-induced liver injury,Citation85-Citation87 these are the first studies showing that ADIPOQ/adiponectin treatment leads to ULK1 activation in cancer cells mechanistically connecting ADIPOQ/adiponectin with autophagy-induction-components in breast cancer cells.
Owing to the cytoprotective role of autophagy, many clinical trials are currently investigating the efficacy of autophagy inhibition using FDA-approved chloroquine or hydroxychloroquine in combination with chemotherapy in different cancers including breast cancer.Citation88-Citation92 Contrary to these studies showing that inhibiting autophagy enhances the efficacy of chemotherapy, we observed that stimulating cytotoxic-autophagy with ADIPOQ/adiponectin treatment sensitizes breast cancer cells to doxorubicin, paclitaxel and carboplatin. In addition, ADIPOQ/adiponectin is effective in combination with reduced dosages of chemotherapy drugs. High ADIPOQ/adiponectin levels have been linked with better response to therapy, overall survival and progression-free survival while low ADIPOQ/adiponectin levels associate with more aggressive progression of breast cancer manifested as larger tumor size, higher tumor-grade, increased angiogenesis and metastasis.Citation19,Citation25-Citation28 Systemic review and meta-analysis show that a low calorie diet and weight-loss result in increased ADIPOQ/adiponectin levels,Citation93 in addition, several strategies have been put forth to potentiate the level of ADIPOQ/adiponectin using ADIPOR agonists, AMPK activators and PPARG/PPARγ (peroxisome proliferator activated receptor gamma) agonists including troglitazone, rosiglitazone, pioglitazone and ciglitazone.Citation15,Citation94 In light of our observations, combination of ADIPOQ/adiponectin enhancing strategies and chemotherapy might be useful in circumventing chemotherapy-associated comorbidities while enhancing the efficacy of chemotherapy regimens.
In conclusion, we present that ADIPOQ/adiponectin is a potent inducer of cytotoxic autophagy that leads to breast cancer inhibition and the mechanistic underpinnings involve modulation of the STK11/LKB1 and AMPK-ULK1 axis. These studies also indicate the broader effects of ADIPOQ/adiponectin not only limited to efficacy as a single agent but also useful for reducing the toxicity associated with standard chemotherapy in combination regimens.
Materials and methods
Cell culture and reagents
Breast cancer cell lines MDA-MB-231, MCF7, T47D, and MDA-MB-468 were obtained from the American Type Culture Collection (HTB-26, HTB-22, HTB-133, HTB-132) thawed from early-passage stocks kept in liquid nitrogen vapor as required, and cultured according to the supplier's instructions. Cell lines were authenticated by analyzing known genetic markers or responses (e.g., expression of estrogen receptor and TP53 and estrogen responsiveness) and all cells were cultured for less than 3 mo before reinitiating fresh cultures and were routinely inspected by microscopy for a stable phenotype. prkaa1 null and Prkaa1 WT immortalized MEFs were kindly provided by Dr. Keith R. Laderoute (SRI International, Menlo Park, CA, USA).Citation95 Human recombinant full-length ADIPOQ/adiponectin was procured from BioVendor (BioVendor Research and Diagnostic Products, RD172029100). Our preliminary dose-dependent and time-dependent studies showed that 5µg/ml ADIPOQ/adiponectin is an effective dose in breast cancer cells therefore we treated breast cancer cells with 5µg/ml ADIPOQ/adiponectin. Antibodies for MAP1LC3B/LC3B (3868), SQSTM1/p62 (5114), STK11/LKB1 (3050), phospho-STK11/LKB1 (Ser428) (3482), PRKAA1 (2532), phospho-PRKAA1 (Thr172) (2535), BECN1 (3495), ULK1 (4773), phospho-ULK1 (Ser555) (5869), ATG5 (12994), ATG7 (8558), ATG12 (4180), cleaved PARP1 (5625), and PARP1 (9532) were purchased from Cell Signaling Technology. ACTB/β-actin (A5441) antibody was purchased from Sigma-Aldrich. LysoTracker Red DND-99 (L7528) was purchased from Invitrogen. 3-Methyladenine (M9281) was purchased from Sigma-Aldrich. AICAR (A9978) was purchased from Sigma. Compound C (171260) was purchased from Calbiochem. Alexa Fluor 488 (A-11008) and Alexa Fluor 555 (A-21428) were purchased from ThermoFisher Scientific.
ATG7 and BECN1 knockout with CRISPR/Cas9
We used the following oligos: ATG7 (NM_006395.2): set1-Forward 1: CAC CGA ATC AAG TAT GAT GAG AAC A and Reverse 1: AAA CTG TTC TCA TCA TAC TTG ATT C; set2- Forward 2: AAA CTG TTC TCA TCA TAC TTG ATT C and Reverse 2: AAA CGG ACG ACT CAC AGT GCA CTG C. BECN1 1(NM_003766.3): set1-Forward 1: AAA CGG ACG ACT CAC AGT GCA CTG C and Reverse 1: AAA CGC ATG GTG CTG TTG TTG GAC C; set2- Forward 2: CAC CGG CCA ACA GCT TCA CTC TGA T and Reverse 2: AAA CAT CAG AGT GAA GCT GTT GGC C. Digested and purified LentiCRISPRv2 plasmid [lentiCRISPRv2 was a gift from Feng Zhang (Addgene, 52961)] was incubated with phosphorylated, annealed oligos for ATG7 and BECN1 in a ligation reaction, transformed into Stbl3 bacteria (ThermoFisher Scientific, C7373–03). Plasmid DNA was purified and sequenced for verification of correct insertion. lentiCRISPR with inserted sequences were cotransfected into HEK293T cells with packinging plasmids pVSVg [pVSVg was a gift from Tannishtha Reya (Addgene, 14888)] and psPAX2 [psPAX2 was a gift from Didier Trono (Addgene, 12260)]. MCF7 cells were transfected twice, and selected for a wk. MCF7 cells were examined for the ATG7 and BECN1 knockout using immunoblot analyses.
STK11/LKB1 stable knockdown using lentiviral short hairpin RNA
Five premade lentiviral STK11/LKB1 short hairpin RNA (shRNA) constructs and a negative control construct created in the same vector system (pLKO.1) were purchased from Open Biosystems (RHS4533). Paired STK11/LKB1 stable knockdown cells were generated following our previously established protocol.Citation45 Transient lentivirus stocks were prepared following the manufacturer's protocol. One d before transfection, 1.5x 106 293T cells were plated in 100-mm dishes. Cells were cotransfectedwith shRNA constructs (3 µg) together with 3 µgpCMV-dR8.2 dvpr [pCMV-dR8.2 dvpr was a gift from Bob Weinberg (Addgene, 8455)] and 0.3 µg pCMV-VSV-G [pCMV-VSV-G was a gift from Bob Weinberg (Addgene, 8454)] helper constructs using Lipofectamine 2000 (ThermoFisher Scientific, 11668027) or Fugene (Promega, E2311).Two days later, viral stocks were harvested from the culture medium and filtered to remove nonadherent 293T cells.To select for the MCF7 and MDA-MB-231 cells that were stably expressing shRNAconstructs, cells were plated at subconfluent densities andinfected with a cocktail of 1 mL of virus-containing medium,3 mL of regular medium, and 8 µg/mL polybrene (Sigma-Aldrich, TR-1003). Selectionwith 0.5 to 2 µg/mL of puromycin (Sigma-Aldrich, P9620) was started 48 h after lentivirus infection. After 4 wk of selection for MCF7 and MDA-MB-231 cells, monolayers of stably infected pooled clones were harvested for use andcryopreserved.
Breast tumorigenesis assay
Subcutaneous xenografts with MDA-MB-231 cells and MDA-MB-231 cells infected with the pLKO.1 vector, as well as MDA-MB-231 cells infected with STK11/LKB1 shRNA were generated in the lower flank of the athymic nude mice as described previously.Citation96 Tumor-bearing mice were grouped in 2 experimental groups (8 mice/group) and treated with intraperitoneal injections of 1) recombinant adenovirus (108 plaque-forming units [pfu]) expressing ADIPOQ/adiponectin or 2) adenovirus expressing luciferase (Ad-Luc; control) (kind gift from Dr. Yu Wang, University of Hong Kong).Citation97 Tumors were collected after 6 wk of treatment; measured, weighed, and subjected to further analysis by immunohistochemistry (IHC), RT-PCR and western blotting. For IHC, at least 4 random, nonoverlapping representative images from each tumor section from 8 tumors of each group were captured using ImagePro software for quantification. All animal studies were in accordance with the guidelines of Johns Hopkins University IACUC.
Transmission electron microscopy
Breast cancer cells were treated with ADIPOQ/adiponectin or vehicle-control for 4 or 6 h. Treated cells were fixed with electron microscopy fixing buffer (2.5% glutaraldehyde, 3 mM MgCl2, in 0.1 M sodium cacodylate buffer, pH 7.2) at 4°C overnight. Cells were rinsed with 3 mM MgCl2, 3% sucrose (Sigma, S0389), 0.1 M sodium cacodylate, thrice, and postfixed in reduced 1% osmium tetroxide with 0.8% potassium ferrocyanide, 0.1 M sodium cacodylate for 2 h on ice in the dark. Following 3 rinses with 0.1 M Maleate buffer (pH 6.2), specimens were stained with 2% uranyl acetate (0.22 µm filtered, 1 h, dark) in 0.1 M maleate buffer and dehydrated through a graded series of ethanol (30 to 100%). Specimens were embedded in EPON, sectioned, stained and examined with an H7600 transmission electron microscope (Hitachi, Tokyo, Japan).
Immunofluorescence and confocal imaging
Breast cancer cells were subjected to immunofluorescence analysis.Citation98 Fixed and immunofluorescently stained cells were imaged using a Zeiss LSM510 Meta (Zeiss, Dublin, California, USA) laser scanning confocal system configured to a Zeiss Axioplan 2 upright microscope (Zeiss, Dublin, California, USA). All experiments were performed multiple times using independent biological replicates. For measurement of intralysosomal function using LysoTracker Red, breast cancer cells were cultured on coverglass slide chamber and treated with vehicle or ADIPOQ/adiponectin. Treated cells were incubated with 50 nM LysoTracker Red DND-99, and the fluorescence intensity was observed under the confocal microscope. Multiple view-fields were examined and representative cells from 3 view-fields were photographed. For the mRFP-EGFP-LC3B assay, breast cancer cells were seeded in 6-well plates with microscope cover glasses, transfected with mRFP-EGFP-LC3B (Addgene, 21074; deposited by Tamotsu Yoshimori) using Fugene (Promega, E2311) for 24 h, and treated with vehicle-control or ADIPOQ/adiponectin. Following treatment, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS; FisherScientific, 70–013–032) and examined with a confocal microscope. Cells with GFP-LC3B+ puncta (green) or mRFP-LC3B+ (red) or GFP+ mRFP+ (yellow) puncta were examined and at least 50 to 100 cells/sample were counted in triplicates.
Cell viability assay
Cell viability assay was performed by estimating the reduction of XTT (2, 3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxyanilide), using a commercially available kit (Sigma, 11465015001) following the manufacturer's instructions. Breast cancer cells were plated in 96-well plates at an initial density of 4 × 103 cells/well for 24 h followed by ADIPOQ/adiponectin treatment as indicated. XTT labeling reagent was added to each culture well to attain a final concentration of 0.3 mg/ml. After a 4 h exposure at 37°C, absorbance was measured between 450 and 690 nm using a 96-well plate reader (SPECTRAmax PLUS, Molecular Devices, Sunnyvale, CA, USA). Pilot experiments verified that the cell densities used in experiments performed were within the linear range of the XTT assay. A standard curve was prepared using cell densities from 1 × 103 to 1 × 106, and the results were calculated with respect to the number of cells.
Clonogenicity assay
Clonogenicity assayCitation99 was conducted in the presence of ADIPOQ/adiponectin for 10 d. Single-cell suspension of breast cancer cells were plated in 12-well plates at a density of 250 cells per well overnight. Cells were treated with ADIPOQ/adiponectin (BioVendor Research and Diagnostic Products, RD172029100) and the medium was replaced with fresh medium containing the treatments every 3 d. Post-treatment period, the medium was removed and colonies were stained with crystal violet (0.1% in 20% methanol) (Sigma-Aldrich, C3886) and colony numbers were assessed visually. Colonies containing >50 normal-appearing cells were counted and pictures were taken using a digital camera.
Anchorage-independent growth assay
Anchorage-independent growthCitation96 of breast cancer cells was assayed by colony formation in soft agar in the presence of ADIPOQ/adiponectin. Briefly, equal volumes of Noble agar (1.2%; Sigma-Aldrich, A5431) and complete medium were mixed to make 0.6% agar growth medium solution in 6-well tissue culture plates. Cells (2 × 103 cells/well) were suspended in media with or without ADIPOQ/adiponectin treatment followed by mixing with an equal volume of agarose (0.6%; Sigma-Aldrich, A9045). Cell suspension-agarose mix (2 ml) was then added to each well. Plates were incubated at 37°C with 5% CO2 in a humidified incubator for 3 wk, and media with or without ADIPOQ/adiponectin treatment were added every 3 d. Colonies were stained with 0.005% crystal violet in PBS for 1 h at room temperature and observed using Olympus IX50 inverted microscope (Olympus, Center Valley, PA). Colonies were counted in 5 randomly selected fields at 10x magnification. Results are expressed as an average number of colonies counted per microfield.
TUNEL assay
TUNEL (Abcam, ab66108) staining was conducted according to the supplier's instructions. Four to 5 randomly selected and nonoverlapping fields were imaged to score TUNEL-positive cells.
RNA isolation and RT-PCR
For RNA isolation and RT-PCR,Citation100,Citation101 total cellular RNA was extracted using the TRIzol Reagent (ThermoFisher Scientific, 15596026). RT-PCR was performed using specific sense and antisense PCR primers. BECN1: Forward 5′- ACC TCA GCC GAA GAC TGA AG-3′, Reverse 5′- TGT CAG AAC TAC AAA CGC TGT T-3′; LAMP1: Forward 5′- TTG GTT AAT GGC TCC GTT TTC A-3′, Reverse 5′- CAG ATG ACG ACA ACT TCC TTG T-3′; ATG5: Forward 5′ TTG ACG TTG GTA ACT GAC AAA GT-3′, Reverse 5′- GCT CTT CCT TGG AAC A-3′.
Immunoblotting
Whole cell lysate was prepared by scraping breast cancer cells in modified RIPA buffer.Citation102,Citation103 Equal amounts of lysate protein were resolved on sodium-dodecyl sulfate polyacrylamide gels, and transferred to nitrocellulose membrane followed by western blot analysis. Immunodetection was performed using an enhanced chemiluminescence ECL system (GE Healthcare Life Sciences, RPN2106) according to the manufacturer's instructions. The blots are representative of multiple independent experiments and bar diagrams are included showing quantification of western blot signals.
ATP assay
Breast cancer cells were treated with vehicle or ADIPOQ/adiponectin for 3, 6 or 24 h, lysed in cell lysis solution (Perkin Elmer, 6016739). The substrate solution was added to the breast cancer cell lysates and ATP levels were detected by measuring luminescence. ATP levels were measured using the ATPlite luminescence assay system (Perkin Elmer, 6016739) according to the manufacturer's instructions.
Survival analysis
Correlation to survival was assessed using 2 independent data sets. The first database investigated includes the Metabric patients.Citation104 Here, all together 1,959 samples were measured using Illumina gene chips (Illumina, San Diego, CA). Only overall survival data was available for these patients. A second database was set up using Affymetrix gene chips as described previously.Citation105 Altogether 4,073 patients were investigated by Affymetrix HGU133A and plus2 gene chips. For these patients both overall survival and relapse-free survival were available. Survival analysis was done for ADIPOR1, ADIPOR2, ADIPOQ and BECN1. For the Metabric cohort, the probes ILMN_1688322, ILMN_2224833, and ILMN_1744822 were used for these genes, respectively. For the Affymetrix cohort, the probe sets 217748_at, 201346_at, and 208946_s_at were used, respectively. Survival analysis was performed in the R statistical environment (www.r-project.org) using the survival Bioconductor library as described previously.Citation105 Cox proportional hazard regression was computed with significance, hazard rate and 95% confidence intervals, and Kaplan-Meier plots were generated to display the survival curves.
Statistical analysis
All experiments were performed thrice in triplicates. Statistical analysis was performed using Microsoft Excel software. Significant differences were analyzed using the Student t test and 2-tailed distribution. Results were considered to be statistically significant if P < 0.05. Results were expressed as mean ± SE between triplicate experiments performed thrice.
Abbreviations
| ACTB | = | actin, β |
| ADIPOR1 | = | adiponectin receptor 1 |
| ADIPOR2 | = | adiponectin receptor 2 |
| ADIPOQ | = | adiponectin, C1Q and collagen domain containing |
| AICAR | = | 5-aminoimidazole-4-carboxamide ribonucleotide |
| AMPK | = | AMP-activated protein kinase |
| ATG5 | = | autophagy-related 5 |
| ATG7 | = | autophagy-related 7 |
| ATG12 | = | autophagy-related 12 |
| ATP | = | adenosine triphosphate |
| BECN1 | = | Beclin 1 |
| GFP | = | green fluorescent protein |
| LAMP1 | = | lysosomal associated membrane protein 1 |
| MAPK1/ERK2 | = | mitogen-activated protein kinase 1 |
| MAP1LC3B/LC3B | = | microtubule associated protein 1 light chain 3 β |
| 3-MA | = | 3-methyladenine |
| MEFs | = | mouse embryonic fibroblasts |
| MKI67 | = | marker of proliferation Ki-67 |
| PARP1 | = | poly(ADP-ribose) polymerase1 |
| Pfu | = | plaque-forming units |
| PRKAA1 | = | protein kinaseAMP-activatedα 1 catalytic subunit |
| RB1CC1 | = | RB1 inducible coiled-coil 1 |
| SQSTM1/p62 | = | sequestosome 1 |
| STK11/LKB1 | = | serine/threonine kinase 11 |
| TUNEL | = | terminal deoxynucleotidyl transferase dUTP nick end labeling |
| ULK1 | = | unc-51 like autophagy activating kinase 1 |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_material.zip
Download Zip (4.1 MB)Funding
This work was supported by NCI NIH, 1R21CA185943–01A1 (to NKS); NCI NIH R01CA131294, NCI NIH R21CA155686, Avon Foundation, Breast Cancer Research Foundation (BCRF) 90047965 and The Fetting Fund (to DS).
References
- Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev 2007; 8:395-408; PMID:17716297; https://doi.org/10.1111/j.1467-789X.2007.00396.x
- Sharma D, Davidson NE. Obesity and breast cancer: a multipartite connection. J Mammary Gland Biol Neoplasia 2013; 18:253-5; PMID:24190309; https://doi.org/10.1007/s10911-013-9306-4
- Iyengar NM, Zhou XK, Gucalp A, Morris PG, Howe LR, Giri DD, Morrow M, Wang H, Pollak M, Jones LW, et al. Systemic Correlates of White Adipose Tissue Inflammation in Early-Stage Breast Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research 2016; 22:2283-89; PMID:26712688; https://doi.org/10.1158/1078-0432.CCR-15-2239
- Lee Y, Jung WH, Koo JS. Adipocytes can induce epithelial-mesenchymal transition in breast cancer cells. Breast cancer research and treatment 2015; 153:323-35; PMID:26285644; https://doi.org/10.1007/s10549-015-3550-9
- Santander AM, Lopez-Ocejo O, Casas O, Agostini T, Sanchez L, Lamas-Basulto E, Carrio R, Cleary MP, Gonzalez-Perez RR, Torroella-Kouri M. Paracrine Interactions between Adipocytes and Tumor Cells Recruit and Modify Macrophages to the Mammary Tumor Microenvironment: The Role of Obesity and Inflammation in Breast Adipose Tissue. Cancers 2015; 7:143-78; PMID:25599228; https://doi.org/10.3390/cancers7010143
- Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 2007; 14:189-206; PMID:17639037; https://doi.org/10.1677/ERC-06-0068
- Saxena NK, Sharma D. Multifaceted leptin network: the molecular connection between obesity and breast cancer. J Mammary Gland Biol Neoplasia 2013; 18:309-20; PMID:24214584; https://doi.org/10.1007/s10911-013-9308-2
- Ye JJ, Jia J, Dong SJ, Zhang CL, Yu SQ, Li LX, Mao C, Wang D, Chen J, Yuan G. Circulating adiponectin levels and the risk of breast cancer: a meta-analysis. Eur J Cancer Prev 2014; 23:158-65; PMID:23929213; https://doi.org/10.1097/CEJ.0b013e328364f293
- Kishida K, Funahashi T, Shimomura I. Adiponectin as a routine clinical biomarker. Best practice & research Clinical endocrinology & metabolism 2014; 28:119-30; https://doi.org/10.1016/j.beem.2013.08.006
- Delort L, Jarde T, Dubois V, Vasson MP, Caldefie-Chezet F. New insights into anticarcinogenic properties of adiponectin: a potential therapeutic approach in breast cancer? Vitamins and hormones 2012; 90:397-417; PMID:23017724
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 1996; 271:10697-703; PMID:8631877; https://doi.org/10.1074/jbc.271.18.10697
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 1995; 270:26746-9; PMID:7592907; https://doi.org/10.1074/jbc.270.45.26746
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun 1996; 221:286-9; PMID:8619847; https://doi.org/10.1006/bbrc.1996.0587
- Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem 1996; 120:803-12; PMID:8947845; https://doi.org/10.1093/oxfordjournals.jbchem.a021483
- Surmacz E. Leptin and adiponectin: emerging therapeutic targets in breast cancer. J Mammary Gland Biol Neoplasia 2013; 18:321-32; PMID:24136336; https://doi.org/10.1007/s10911-013-9302-8
- Perrier S, Jarde T. Adiponectin, an anti-carcinogenic hormone? A systematic review on breast, colorectal, liver and prostate cancer. Current medicinal chemistry 2012; 19:5501-12; PMID:22876928; https://doi.org/10.2174/092986712803833137
- Chen X, Wang Y. Adiponectin and breast cancer. Med Oncol 2011; 28:1288-95; PMID:20625941; https://doi.org/10.1007/s12032-010-9617-x
- Shibata R, Ouchi N, Murohara T. Adiponectin and cardiovascular disease. Circ J 2009; 73:608-14; PMID:19261992; https://doi.org/10.1253/circj.CJ-09-0057
- Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr 2007; 86:s858-866; PMID:18265479
- Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol 2012; 165:313-327; PMID:21718299; https://doi.org/10.1111/j.1476-5381.2011.01560.x
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocrine Rev 2005; 26:439-51; PMID:15897298; https://doi.org/10.1210/er.2005-0005
- Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes 2003; 52:1355-63; PMID:12765944; https://doi.org/10.2337/diabetes.52.6.1355
- Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, Heuser JE, Lodish HF. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem 2003; 278:50810-17
- Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A 2002; 99:16309-13; PMID:12456889; https://doi.org/10.1073/pnas.222657499
- Macis D, Guerrieri-Gonzaga A, Gandini S. Circulating adiponectin and breast cancer risk: a systematic review and meta-analysis. International journal of epidemiology 2014; 43:1226-36; PMID:24737805; https://doi.org/10.1093/ije/dyu088
- Shahar S, Salleh RM, Ghazali AR, Koon PB, Mohamud WN. Roles of adiposity, lifetime physical activity and serum adiponectin in occurrence of breast cancer among Malaysian women in Klang Valley. Asian Pacific journal of cancer prevention: APJCP 2010; 11:61-6; PMID:20593932
- Michalakis K, Williams CJ, Mitsiades N, Blakeman J, Balafouta-Tselenis S, Giannopoulos A, Mantzoros CS. Serum adiponectin concentrations and tissue expression of adiponectin receptors are reduced in patients with prostate cancer: a case control study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2007; 16:308-13; https://doi.org/10.1158/1055-9965.EPI-06-0621
- Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, et al. Association of serum adiponectin levels with breast cancer risk. Clinical cancer research: an official journal of the American Association for Cancer Research 2003; 9:5699-704; PMID:14654554
- Basu S, Combe K, Kwiatkowski F, Caldefie-Chezet F, Penault-Llorca F, Bignon YJ, Vasson MP. Cellular Expression of Cyclooxygenase, Aromatase, Adipokines, Inflammation and Cell Proliferation Markers in Breast Cancer Specimen. PloS one 2015; 10:e0138443; PMID:26431176; https://doi.org/10.1371/journal.pone.0138443
- Jeong YJ, Bong JG, Park SH, Choi JH, Oh HK. Expression of leptin, leptin receptor, adiponectin, and adiponectin receptor in ductal carcinoma in situ and invasive breast cancer. Journal of breast cancer 2011; 14:96-103; PMID:21847403; https://doi.org/10.4048/jbc.2011.14.2.96
- Karaduman M, Bilici A, Ozet A, Sengul A, Musabak U, Alomeroglu M. Tissue levels of adiponectin in breast cancer patients. Med Oncol 2007; 24:361-66; PMID:17917082; https://doi.org/10.1007/s12032-007-0021-0
- Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer 2006; 94:1221-25; PMID:16570048; https://doi.org/10.1038/sj.bjc.6603051
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 2001; 7:947-53; PMID:11479628; https://doi.org/10.1038/90992
- Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem 2003; 278:2461-68; PMID:12431986; https://doi.org/10.1074/jbc.M209033200
- Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A 2001; 98:2005-10; PMID:11172066; https://doi.org/10.1073/pnas.98.4.2005
- Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 2003; 112:91-100; PMID:12840063; https://doi.org/10.1172/JCI200317797
- Saxena NK, Sharma D. Metastasis suppression by adiponectin: LKB1 rises up to the challenge. Cell adhesion & migration 2010; 4:358-62; https://doi.org/10.4161/cam.4.3.11541
- Taliaferro-Smith L, Nagalingam A, Knight BB, Oberlick E, Saxena NK, Sharma D. Integral role of PTP1B in adiponectin-mediated inhibition of oncogenic actions of leptin in breast carcinogenesis. Neoplasia 2013; 15:23-38; PMID:23358729; https://doi.org/10.1593/neo.121502
- Shrestha A, Nepal S, Kim MJ, Chang JH, Kim SH, Jeong GS, Jeong CH, Park GH, Jung S, Lim J, et al. Critical Role of AMPK/FoxO3A Axis in Globular Adiponectin-Induced Cell Cycle Arrest and Apoptosis in Cancer Cells. J Cell Physiol 2016; 231:357-369; PMID:26089158; https://doi.org/10.1002/jcp.25080
- Rahal OM, Simmen RC. Paracrine-acting adiponectin promotes mammary epithelial differentiation and synergizes with genistein to enhance transcriptional response to estrogen receptor beta signaling. Endocrinology 2011; 152:3409-21; PMID:21712365; https://doi.org/10.1210/en.2011-1085
- Li G, Cong L, Gasser J, Zhao J, Chen K, Li F. Mechanisms underlying the anti-proliferative actions of adiponectin in human breast cancer cells, MCF7-dependency on the cAMP/protein kinase-A pathway. Nutr Cancer 2011; 63:80-8; PMID:21108124
- Dubois V, Delort L, Billard H, Vasson MP, Caldefie-Chezet F. Breast cancer and obesity: in vitro interferences between adipokines and proangiogenic features and/or antitumor therapies? PloS one 2013; 8:e58541; PMID:23554900; https://doi.org/10.1371/journal.pone.0058541
- Jarde T, Caldefie-Chezet F, Goncalves-Mendes N, Mishellany F, Buechler C, Penault-Llorca F, Vasson MP. Involvement of adiponectin and leptin in breast cancer: clinical and in vitro studies. Endocr Relat Cancer 2009; 16:1197-210; PMID:19661131; https://doi.org/10.1677/ERC-09-0043
- Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y, Pecquery R. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncology reports 2008; 20:971-7; PMID:18813842
- Taliaferro-Smith L, Nagalingam A, Zhong D, Zhou W, Saxena NK, Sharma D. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene 2009; 28:2621-33; PMID:19483724; https://doi.org/10.1038/onc.2009.129
- Sharma D, Wang J, Fu PP, Sharma S, Nagalingam A, Mells J, Handy J, Page AJ, Cohen C, Anania FA, et al. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology 2010; 52:1713-22; PMID:20941777; https://doi.org/10.1002/hep.23892
- Saxena NK, Fu PP, Nagalingam A, Wang J, Handy J, Cohen C, Tighiouart M, Sharma D, Anania FA. Adiponectin modulates C-jun N-terminal kinase and mammalian target of rapamycin and inhibits hepatocellular carcinoma. Gastroenterology 2010; 139:1762-73, 1773 e1761-1765; PMID:20637208; https://doi.org/10.1053/j.gastro.2010.07.001
- Nagelkerke A, Bussink J, Geurts-Moespot A, Sweep FC, Span PN. Therapeutic targeting of autophagy in cancer. Part II: pharmacological modulation of treatment-induced autophagy. Seminars in cancer biology 2015; 31:99-105
- Nagelkerke A, Sweep FC, Geurts-Moespot A, Bussink J, Span PN. Therapeutic targeting of autophagy in cancer. Part I: molecular pathways controlling autophagy. Seminars in cancer biology 2015; 31:89-98
- Gewirtz DA. The four faces of autophagy: implications for cancer therapy. Cancer research 2014; 74:647-51; PMID:24459182; https://doi.org/10.1158/0008-5472.CAN-13-2966
- Sharma K, Le N, Alotaibi M, Gewirtz DA. Cytotoxic autophagy in cancer therapy. International journal of molecular sciences 2014; 15:10034-10051; PMID:24905404; https://doi.org/10.3390/ijms150610034
- Gewirtz DA. When cytoprotective autophagy isn't… and even when it is. Autophagy 2014; 10:391-92; PMID:24419177; https://doi.org/10.4161/auto.27719
- Yu L, Lenardo MJ, Baehrecke EH. Autophagy and caspases: a new cell death program. Cell Cycle 2004; 3:1124-26; PMID:15326383; https://doi.org/10.4161/cc.3.9.1097
- Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 2004; 304:1500-02; PMID:15131264; https://doi.org/10.1126/science.1096645
- Luo S, Rubinsztein DC. Atg5 and Bcl-2 provide novel insights into the interplay between apoptosis and autophagy. Cell death and differentiation 2007; 14:1247-50; PMID:17431417; https://doi.org/10.1038/sj.cdd.4402149
- Green DR, Levine B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell 2014; 157:65-75; PMID:24679527; https://doi.org/10.1016/j.cell.2014.02.049
- Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 2005; 171:603-14; PMID:16286508; https://doi.org/10.1083/jcb.200507002
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Øvervatn A, Bjørkøy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 2007; 282:24131-45; PMID:17580304; https://doi.org/10.1074/jbc.M702824200
- Bjorkoy G, Lamark T, Johansen T. p62/SQSTM1: a missing link between protein aggregates and the autophagy machinery. Autophagy 2006; 2:138-9; PMID:16874037; https://doi.org/10.4161/auto.2.2.2405
- Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 2007; 3:452-60; PMID:17534139; https://doi.org/10.4161/auto.4451
- Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 2010; 285:10850-61; PMID:20123989; https://doi.org/10.1074/jbc.M109.080796
- Rubinsztein DC, Cuervo AM, Ravikumar B, Sarkar S, Korolchuk V, Kaushik S, Klionsky DJ. In search of an “autophagomometer.” Autophagy 2009; 5:585-589; PMID:19411822; https://doi.org/10.4161/auto.5.5.8823
- Liu Q, Gauthier MS, Sun L, Ruderman N, Lodish H. Activation of AMP-activated protein kinase signaling pathway by adiponectin and insulin in mouse adipocytes: requirement of acyl-CoA synthetases FATP1 and Acsl1 and association with an elevation in AMP/ATP ratio. Faseb J 2010; 24:4229-39; PMID:20667975; https://doi.org/10.1096/fj.10-159723
- Wong PM, Puente C, Ganley IG, Jiang X. The ULK1 complex: sensing nutrient signals for autophagy activation. Autophagy 2013; 9:124-37; PMID:23295650; https://doi.org/10.4161/auto.23323
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al. Phosphorylation of ULK1 (hULK1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 2011; 331:456-61; PMID:21205641; https://doi.org/10.1126/science.1196371
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature cell biology 2011; 13:132-41; PMID:21258367; https://doi.org/10.1038/ncb2152
- Vaahtomeri K, Makela TP. Molecular mechanisms of tumor suppression by LKB1. FEBS letters 2011; 585:944-51; PMID:21192934; https://doi.org/10.1016/j.febslet.2010.12.034
- Hardie DG. New roles for the LKB1 ->AMPK pathway. Current opinion in cell biology 2005; 17:167-73; PMID:15780593; https://doi.org/10.1016/j.ceb.2005.01.006
- Lu C, Xie C. Radiation-induced autophagy promotes esophageal squamous cell carcinoma cell survival via the LKB1 pathway. Oncol Rep 2016; 35:3559-65; PMID:27109915
- Sun A, Li C, Chen R, Huang Y, Chen Q, Cui X, Liu H, Thrasher JB, Li B. GSK-3beta controls autophagy by modulating LKB1-AMPK pathway in prostate cancer cells. The Prostate 2016; 76:172-83; PMID:26440826; https://doi.org/10.1002/pros.23106
- Zheng B, Jeong JH, Asara JM, Yuan YY, Granter SR, Chin L, Cantley LC. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Molecular cell 2009; 33:237-47; PMID:19187764; https://doi.org/10.1016/j.molcel.2008.12.026
- Kawashima I, Mitsumori T, Nozaki Y, Yamamoto T, Shobu-Sueki Y, Nakajima K, Kirito K. Negative regulation of the LKB1/AMPK pathway by ERK in human acute myeloid leukemia cells. Experimental hematology 2015; 43:524-533 e521; PMID:25846811; https://doi.org/10.1016/j.exphem.2015.03.005
- Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol 2011; 8:528-39; PMID:21587219; https://doi.org/10.1038/nrclinonc.2011.71
- Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 2012; 11:709-730; PMID:22935804; https://doi.org/10.1038/nrd3802
- Bristol ML, Di X, Beckman MJ, Wilson EN, Henderson SC, Maiti A, Fan Z, Gewirtz DA. Dual functions of autophagy in the response of breast tumor cells to radiation: cytoprotective autophagy with radiation alone and cytotoxic autophagy in radiosensitization by vitamin D 3. Autophagy 2012; 8:739-53; PMID:22498493; https://doi.org/10.4161/auto.19313
- Wilson EN, Bristol ML, Di X, Maltese WA, Koterba K, Beckman MJ, Gewirtz DA. A switch between cytoprotective and cytotoxic autophagy in the radiosensitization of breast tumor cells by chloroquine and vitamin D. Hormones & cancer 2011; 2:272-85; https://doi.org/10.1007/s12672-011-0081-7
- Kozyreva VK, Kiseleva AA, Ice RJ, Jones BC, Loskutov YV, Matalkah F, Smolkin MB, Marinak K, Livengood RH, Salkeni MA, et al. Combination of Eribulin and Aurora A Inhibitor MLN8237 Prevents Metastatic Colonization and Induces Cytotoxic Autophagy in Breast Cancer. Molecular cancer therapeutics 2016; 15:1809-1822; PMID:27235164; https://doi.org/10.1158/1535-7163.MCT-15-0688
- Weng JR, Yen MH, Lin WY. Cytotoxic constituents from Celastrus paniculatus induce apoptosis and autophagy in breast cancer cells. Phytochemistry 2013; 94:211-9; PMID:23810286; https://doi.org/10.1016/j.phytochem.2013.05.022
- Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nature reviews Cancer 2009; 9:563-75; PMID:19629071; https://doi.org/10.1038/nrc2676
- Jiang L, Liang X, Liu M, Wang W, Ma J, Guo Q, Han L, Yang C, Nan K. Reduced expression of liver kinase B1 and Beclin1 is associated with the poor survival of patients with non-small cell lung cancer. Oncology reports 2014; 32:1931-8; PMID:25175672
- Mizushima N. The role of the ULK1/ATG1 complex in autophagy regulation. Curr Opin Cell Biol 2010; 22:132-9; PMID:20056399; https://doi.org/10.1016/j.ceb.2009.12.004
- Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, Mizushima N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol 2008; 181:497-10; PMID:18443221; https://doi.org/10.1083/jcb.200712064
- Ganley IG, Lam du H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. The Journal of biological chemistry 2009; 284:12297-305; PMID:19258318; https://doi.org/10.1074/jbc.M900573200
- Hosokawa N, Sasaki T, Iemura S, Natsume T, Hara T, Mizushima N. ULK101, a novel mammalian autophagy protein interacting with ULK13. Autophagy 2009; 5:973-9; PMID:19597335; https://doi.org/10.4161/auto.5.7.9296
- Ren J, Xu X, Wang Q, Ren SY, Dong M, Zhang Y. Permissive role of AMPK and autophagy in adiponectin deficiency-accentuated myocardial injury and inflammation in endotoxemia. J Mol Cell Cardiol 2016; 93:18-31; PMID:26906634; https://doi.org/10.1016/j.yjmcc.2016.02.002
- Yao F, Zhang M, Chen L. 5′-Monophosphate-activated protein kinase (AMPK) improves autophagic activity in diabetes and diabetic complications. Acta Pharm Sin B 2016; 6:20-5; PMID:26904395; https://doi.org/10.1016/j.apsb.2015.07.009
- Lin Z, Wu F, Lin S, Pan X, Jin L, Lu T, Shi L, Wang Y, Xu A, Li X. Adiponectin protects against acetaminophen-induced mitochondrial dysfunction and acute liver injury by promoting autophagy in mice. J Hepatol 2014; 61:825-31; PMID:24882054; https://doi.org/10.1016/j.jhep.2014.05.033
- Mahalingam D, Mita M, Sarantopoulos J, Wood L, Amaravadi RK, Davis LE, Mita AC, Curiel TJ, Espitia CM, Nawrocki ST, et al. Combined autophagy and HDAC inhibition: a phase I safety, tolerability, pharmacokinetic, and pharmacodynamic analysis of hydroxychloroquine in combination with the HDAC inhibitor vorinostat in patients with advanced solid tumors. Autophagy 2014; 10:1403-14; PMID:24991835; https://doi.org/10.4161/auto.29231
- Rangwala R, Chang YC, Hu J, Algazy KM, Evans TL, Fecher LA, Schuchter LM, Torigian DA, Panosian JT, Troxel AB, et al. Combined MTOR and autophagy inhibition: phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy 2014; 10:1391-402; PMID:24991838; https://doi.org/10.4161/auto.29119
- Vogl DT, Stadtmauer EA, Tan KS, Heitjan DF, Davis LE, Pontiggia L, Rangwala R, Piao S, Chang YC, Scott EC, et al. Combined autophagy and proteasome inhibition: a phase 1 trial of hydroxychloroquine and bortezomib in patients with relapsed/refractory myeloma. Autophagy 2014; 10:1380-90; PMID:24991834; https://doi.org/10.4161/auto.29264
- Guo W, Wang Y, Wang Z, Wang YP, Zheng H. Inhibiting Autophagy Increases Epirubicin's Cytotoxicity in Breast Cancer Cells. Cancer Sci 2016; 107(11):1610-21; https://doi.org/10.1111/cas.13059
- Sun R, Shen S, Zhang YJ, Xu CF, Cao ZT, Wen LP, Wang J. Nanoparticle-facilitated autophagy inhibition promotes the efficacy of chemotherapeutics against breast cancer stem cells. Biomaterials 2016; 103:44-55; PMID:27376558; https://doi.org/10.1016/j.biomaterials.2016.06.038
- Salehi-Abargouei A, Izadi V, Azadbakht L. The effect of low calorie diet on adiponectin concentration: a systematic review and meta-analysis. Horm Metab Res 2015; 47:549-55; PMID:25985324; https://doi.org/10.1055/s-0035-1549878
- Otvos L, Jr., Haspinger E, La Russa F, Maspero F, Graziano P, Kovalszky I, Lovas S, Nama K, Hoffmann R, Knappe D, et al. Design and development of a peptide-based adiponectin receptor agonist for cancer treatment. BMC Biotechnol 2011; 11:90; PMID:21974986; https://doi.org/10.1186/1472-6750-11-90
- Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol 2006; 26:5336-47; PMID:16809770; https://doi.org/10.1128/MCB.00166-06
- Nagalingam A, Arbiser JL, Bonner MY, Saxena NK, Sharma D. Honokiol activates AMP-activated protein kinase in breast cancer cells via an LKB1-dependent pathway and inhibits breast carcinogenesis. Breast cancer research: BCR 2012; 14:R35; PMID:22353783; https://doi.org/10.1186/bcr3128
- Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, Wu D, Cooper GJ, Xu A. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res 2006; 66:11462-70; PMID:17145894; https://doi.org/10.1158/0008-5472.CAN-06-1969
- Nagalingam A, Tighiouart M, Ryden L, Joseph L, Landberg G, Saxena NK, Sharma D. Med1 plays a critical role in the development of tamoxifen resistance. Carcinogenesis 2012; 33:918-30; PMID:22345290; https://doi.org/10.1093/carcin/bgs105
- Knight BB, Oprea-Ilies GM, Nagalingam A, Yang L, Cohen C, Saxena NK, Sharma D. Survivin upregulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin-induced migration of breast carcinoma cells. Endocr Relat Cancer 2011; 18:413-428; PMID:21555376; https://doi.org/10.1530/ERC-11-0075
- Ohri S, Sharma D, Dixit A. Modulation of c-myc and c-fos gene expression in regenerating rat liver by 2-mercaptopropionylglycine. Cell Biol Int 2002; 26:187-92; PMID:11846448; https://doi.org/10.1006/cbir.2001.0825
- Avtanski DB, Nagalingam A, Bonner MY, Arbiser JL, Saxena NK, Sharma D. Honokiol inhibits epithelial-mesenchymal transition in breast cancer cells by targeting signal transducer and activator of transcription 3/Zeb1/E-cadherin axis. Molecular oncology 2014; 8:565-80; PMID:24508063; https://doi.org/10.1016/j.molonc.2014.01.004
- Saxena NK, Taliaferro-Smith L, Knight BB, Merlin D, Anania FA, O'Regan RM, Sharma D. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res 2008; 68:9712-22; PMID:19047149; https://doi.org/10.1158/0008-5472.CAN-08-1952
- Nagalingam A, Kuppusamy P, Singh SV, Sharma D, Saxena NK. Mechanistic elucidation of the antitumor properties of withaferin a in breast cancer. Cancer Res 2014; 74:2617-29; PMID:24732433; https://doi.org/10.1158/0008-5472.CAN-13-2081
- Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012; 486:346-352; PMID:22522925
- Mihaly Z, Kormos M, Lanczky A, Dank M, Budczies J, Szasz MA, Győrffy B. A meta-analysis of gene expression-based biomarkers predicting outcome after tamoxifen treatment in breast cancer. Breast Cancer Res Treat 2013; 140:219-32; PMID:23836010; https://doi.org/10.1007/s10549-013-2622-y
