ABSTRACT
The Golgi apparatus is a central intracellular membrane organelle in the secretory pathway. The formation of the unique stacked architecture of the Golgi ensures accurate protein glycosylation and sorting. However, how the Golgi structure and function respond to extracellular stresses is largely unexplored. In a recent study, we reported that under short-term glucose deprivation, a subpopulation of the Golgi stacking protein GORASP2/GRASP55 is targeted from the Golgi to the interface between autophagosomes and lysosomes to promote autophagosome maturation; this process is regulated by O-GlcNAcylation. Under growth condition, GORASP2 is O-GlcNAcylated and functions as a stacking protein in the Golgi. Upon glucose starvation, GORASP2 is de-O-GlcNAcylated and is partially relocated from the Golgi to the autophagosome-lysosome interface, where it interacts with lipidated LC3 on autophagosomes and LAMP2 on lysosomes, and functions as a membrane tether to facilitate autophagosome-lysosome fusion. Therefore, our study uncovered an unconventional role of the Golgi ‘glue’ protein in autophagy that acts by sensing the glucose level.
The Golgi apparatus is the central hub for protein trafficking and glycosylation in the secretory pathway. This organelle is characterized by stacks of tightly aligned flat cisternal membranes. Extensive research has been carried out to explore the mechanism of Golgi stack formation over the past few decades. GORASP1/GRASP65 (golgi reassembly stacking protein 1) and GORASP2/GRASP55, are at present the only proteins shown to function in Golgi stacking. Both are peripheral membrane proteins on the cytoplasmic face of the Golgi cisternae that first form homo-dimers that then oligomerize in trans through their conserved N-terminal GRASP domain, thereby functioning as the ‘glue’ to stick adjacent cisternae together into stacks and to link Golgi stacks into a ribbon. GORASP2 is located in the medial-trans Golgi, whereas its homolog GORASP1 concentrates in the cis-Golgi; these 2 proteins coordinate with each other to organize Golgi membranes into a stacked structure (). Depletion of GORASP1 and GORASP2 in cells disrupts the Golgi structure and results in accelerated protein trafficking and defective glycosylation. Therefore, structure formation is required for proper functioning of the Golgi. The question is whether and how the Golgi adjusts its structure and function in response to extracellular stimuli or stresses.
Figure 1. Schematic model of GORASP2/GRASP55 function in Golgi stacking and autophagosome-lysosome tethering. In growth conditions, GORASP2 is O-GlcNAcylated and localized in the medial-trans Golgi to glue adjacent cisternae together. Upon glucose starvation, GORASP2 is de-O-GlcNAcylated and a subpopulation of the protein is targeted to the autophagosome and lysosome interface, where it binds LC3-II on autophagosomes and LAMP2 on lysosomes, and thereby facilitates the fusion of these 2 membrane organelles
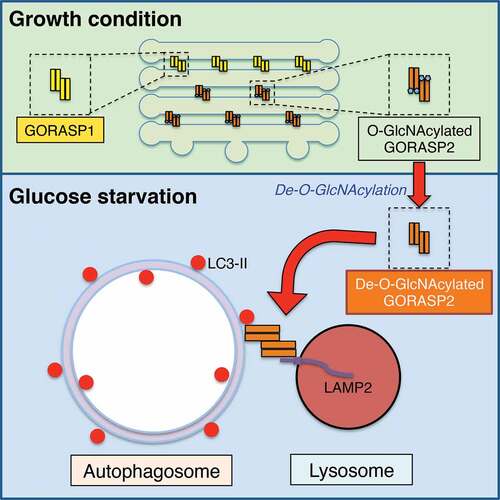
In an effort to study how the Golgi responds to energy deprivation, we screened over a dozen critical Golgi structural proteins for O-GlcNAcylation, a cytosolic and nuclear glycosylation that serves as a nutrient sensor to regulate many processes of cell physiology. Among these proteins, only GORASP2, but not its homolog GORASP1 or any of the other proteins we examined, is O-GlcNAcylated [Citation1]. Under growth condition, GORASP2 is O-GlcNAcylated by the O-GlcNAc transferase OGT; upon glucoses starvation, GORASP2 O-GlcNAcylation is significantly reduced (). This effect is quick; a short 4-h glucose starvation could almost completely abolish GORASP2 O-GlcNAcylation, consistent with a role of GORASP2 in energy sensing.
Because glucose deprivation induces autophagy, we considered the role of GORASP2 in autophagy. Indeed, GORASP2 knockdown dramatically increases the number of autophagosomes, as well as both LC3-II and SQSTM1/p62 protein levels. To understand how such a Golgi structural protein functions in autophagy, we determined the subcellular localization of GORASP2 after glucose starvation. Surprisingly, GORASP2 forms puncta outside of the Golgi area, which colocalize with autophagosomes indicated by the dual-color of GFP and RFP signals of the mRFP-GFP-LC3 reporter (). We excluded this colocalization as corresponding to ‘Golgiphagy’ based on the following 3 reasons: 1) except for GORASP2, no other Golgi proteins were detected by fluorescence microscopy on those autophagosomes; 2) GORASP2 is localized at the surface of autophagosomes, as it is not protected by the autophagosome membranes from protease digestion; 3) GORASP2 localization at the autophagosome surface was directly visualized by immuno-electron microscopy (immuno-EM). Additional experiments demonstrated that GORASP2 puncta also partially colocalize with LAMP2, a lysosomal marker, during glucose starvation.
To address the question of how GORASP2 O-GlcNAcylation regulates its targeting and functioning on autophagosomes, we identified the O-GlcNAcylation sites and generated a mutant that is deficient in O-GlcNAcylation. When expressed in cells, this mutant is better targeted to autophagosomes than wild-type GORASP2, and its expression promotes autophagic activity. These results demonstrate that de-O-GlcNAcylation allows GORASP2 to function in autophagy.
GORASP2 links Golgi cisternal membranes into stacks by forming trans-oligomers. The localization of GORASP2 to the autophagosome-lysosome interface prompted us to consider the possibility that GORASP2 oligomers may also tether autophagosomes to lysosomes. Indeed, GORASP2 co-immunoprecipitates with lipidated LC3 on autophagosomes, and this interaction requires GORASP2 de-O-GlcNAcylation. The interaction between these 2 proteins maps to a specific LC3-interacting region (LIR) motif in the GRASP domain of GORASP2; mutation of this site abolishes the GORASP2-LC3 interaction, as well as GORASP2 targeting to autophagosomes. On the lysosome side, GORASP2 co-immunoprecipitates with LAMP2, although this interaction appears not to be regulated by GORASP2 O-GlcNAcylation.
To directly test the hypothesis that GORASP2 tethers autophagosomes and lysosomes through the interactions with LC3 and LAMP2, we applied the following 3 complementary approaches: First, we determined LC3-LAMP2 colocalization in the absence or presence of GORASP2 in the cell. During glucose starvation, LC3 and LAMP2 largely colocalize when autophagosome-lysosome fusion is blocked by treatment with bafilomycin A1. Depletion of GORASP2 significantly reduces their colocalization, which is rescued by the expression of wild-type GORASP2 but not the LIR mutant that fails to interact with LC3. Second, we determined the dependence of GORASP2 on LC3-LAMP2 interaction by co-immunoprecipitation. Under normal condition, LC3 and LAMP2 exhibit a specific but weak interaction. This interaction is significantly enhanced by the addition of non-O-GlcNAcylated GORASP2 in a dose-dependent manner, but less by O-GlcNAcylated GORASP2 or the LIR mutant. Third, we determined the capability of GORASP2 in promoting the fusion of LC3-positive autophagosomes and LAMP2-positive lysosomes in vitro. Non-O-GlcNAcylated GORASP2, but not O-GlcNAcylated GORASP2 or the LIR mutant, facilitates autophagosome-lysosome fusion.
Collectively, our study has made an unexpected finding that the Golgi stacking protein GORASP2 functions as a membrane tether in autophagosome-lysosome fusion by bridging LC3 and LAMP2; this function is regulated by GORASP2 O-GlcNAcylation (). Therefore, this study uncovered a novel autophagosome and lysosome tethering mechanism that is different from, but may also cooperate with, the previously described RAB7-HOPS-SNARE machinery. GORASP2 acts as a membrane tether for autophagy in a similar way, as it stacks Golgi cisternae, indicating an extensive role of GORASP2 in membrane-related processes. In addition, the lysosome membrane protein LAMP2 has long been shown to be essential for autophagosome maturation, but the mechanism was unknown. Here, the GORASP2-LAMP2 interaction provides an explanation as to how LAMP2 is required for autophagy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zhang X, Wang L, Lak B, et al. GRASP55 senses glucose deprivation through O-GlcNAcylation to promote autophagosome-lysosome fusion. Dev Cell. 2018;45:245–61 e6.
