ABSTRACT
The European autophagy consortium Driving next-generation autophagy researchers towards translation (DRIVE) held its kick-off meeting in Groningen on the 14th and 15th of June 2018. This Marie Skłodowska-Curie Early Training Network was approved under the European Union’s Horizon 2020 Research and Innovation Program and is funded for 4 years. Within DRIVE, 14 European research teams from academia and industry will train 15 PhD students through applied, cross-disciplinary and collaborative macroautophagy/autophagy research. The goal of DRIVE is to stimulate applied approaches in autophagy research and provide training towards translation, while advancing our knowledge on autophagy in specific physiological and pathological states. The strong focus on translation will prepare the PhD students to be at the forefront to exploit autophagy for the development of therapies directly benefitting patients. Thereby, DRIVE will contribute to filling the educational gap that currently exists between academia and industry, and will prepare its PhD students for alternative and highly flexible professional paths.
Autophagy, an emerging field in biomedical life sciences
Autophagy was first characterized in the 1950s but the study of its mechanisms and functions remained mostly descriptive for decades as specific markers to monitor autophagy were missing [Citation1]. In the beginning of the 1990s, genetic screens in yeast led to the identification of a number of genes essential for autophagy, which have been named AuTophaGy-related (ATG) [Citation1]. The discovery of the ATG genes revolutionized the field, and detailed follow-up investigations in yeast deciphered their function and the regulation of autophagy [Citation2]. To date, over 40 ATG genes primarily involved in autophagy have been identified; 16 of them compose the core autophagy machinery required for the formation of autophagosomes in all eukaryotes. The specific molecular roles of the ATG genes and how they act at a mechanistic level, however, remains largely unknown. Nevertheless, several of these proteins have been validated as key autophagy markers and their high degree of conservation incited autophagy research in numerous other model systems, including mouse and human tissues [Citation3]. This new investigative wave has led to a series of seminal discoveries that revealed numerous physiological and pathological implications of autophagy [Citation4,Citation5]. These discoveries have fueled even further autophagy research, making it one of the fastest emerging fields in biomedical life sciences [Citation1,Citation6].
Autophagy acts at a basal level in most cells and, to maintain cellular and organismal homeostasis, can be enhanced in response to cellular and environmental stresses [Citation7]. Cellular constituents can be targeted by either nonselective bulk autophagy or selective types of autophagy, which rely on receptors that allow specific degradation of the unwanted cellular components [Citation8]. Selective types of autophagy include the specific turnover of mitochondria (mitophagy) or the endoplasmic reticulum (reticulophagy). Such flexibility in cargo selection allows organisms to use autophagy for a multitude of physiological functions. Autophagy has been implicated in stem cell maintenance, embryonic development, and the normal formation and functioning of a wide range of organs [Citation9]. It also plays a central role in life span extension (i.e., ageing prevention), metabolism, and multiple aspects of immunity, including the elimination of intracellular pathogens [Citation10]. Defects in autophagy have been implicated in a wide range of neurodegenerative, cardiovascular, chronic inflammatory, muscular and autoimmune diseases, and some malignancies, as well as a recently recognized and rapidly expanding group of Mendelian neurodevelopmental multisystem disorders, congenital disorders of autophagy [Citation11,Citation12]. Not surprisingly in the context of these developments, autophagy has thus emerged as an attractive therapeutic target to prevent the onset and/or the progression of various human illnesses, including specific types of tumors, neurodevelopmental, and neurodegenerative disorders (such as Alzheimer and Parkinson diseases), muscular dystrophies, and specific infections [Citation4]. Thus, the pharmacological manipulation of autophagy holds great promise to change the way we currently treat some of these pathologies [Citation4,Citation13]. Because autophagy can be stimulated by either protein-poor diets (i.e., amino acid starvation) [Citation14] or exercise [Citation15], less invasive combinatorial therapies can also be anticipated.
DRIVE, projecting autophagy research in the future
The manipulation of autophagy has an enormous therapeutic potential to change the way we treat cancers, neurodevelopmental and neurodegenerative disorders, and inflammatory and infectious diseases [Citation4,Citation13]. Despite a rapid increase in our understanding of the mechanisms underlying autophagy and its regulation, translation of this fundamental knowledge into clinical-grade products with immediate benefit for individual and societal health is still lagging behind. This translation is currently hampered by the lack of knowledge about the precise contribution of autophagy in the different physiological and pathological conditions, but also the lack of models, biomarkers, and assays to monitor autophagy in vivo. This gap does not make it possible to effectively test therapies and drugs before advancing towards a clinical trial phase. For example, there are currently just 2 autophagy-modulating drugs in phase I or phase II clinical trials [Citation4], but neither of these (chloroquine/hydroxychloroquine as an inhibitor and rapamycin as an activator) specifically manipulate autophagy. Because these compounds are not specific, they also affect other cellular processes leading to various side effects, such as immunosuppression in the case of rapamycin. This emphasizes the urgent need for models, biomarkers and assays to develop a new generation of specific autophagy modulators, and the need to understand autophagy better at a molecular level.
The Marie Skłodowska-Curie Early Training Network DRIVE (; https://drive-autophagy.eu/) aims at filling this gap by training 15 PhD students through applied, cross-disciplinary and collaborative autophagy research. Through the realization of specific research projects, these young scientists will become experts in the field of autophagy, with an acute awareness for the possible future exploitation of autophagy as a therapeutic target. The DRIVE consortium has enlisted top European autophagy experts (), who have significantly contributed to our fundamental and clinical understanding of autophagy, and leverages their complementary competencies to further stimulate applied autophagy research, through a comprehensive approach ranging from the study of the basic underlying mechanisms to its physiological and pathological relevance.
DRIVE’s goals are directed towards conceptual advances, but also towards the validation of known and new biomarkers, and the adaptation of established assays but also the development of novel ones. For this reason, DRIVE includes 5 industry partners among its members.
The PhD training program of DRIVE
With the help of its autophagy experts, its industrial and other associated partners (see below) ( and ), the goal of DRIVE is to train, over a period of 4 years, a new generation of researchers with the necessary knowledge, skills and networks to facilitate the future translation of autophagy. Although the principal educational aim is to train through research in the laboratory of one of the DRIVE participants, a complementary educational program has been designed to optimally achieve the goals of DRIVE. The PhD students will have at least 2 collaborative research visits at one of the academic, industrial and/or associated partners. These visits will expose the PhD students to different professional settings, but will also allow them to carry out part of their project at a laboratory with relevant complementary expertise. Moreover, DRIVE partners and the PhD students will congregate at the annual DRIVE meetings, where the PhD students will have the opportunity to present and discuss the advances of their projects, and receive dedicated feedback from other specialists. Finally, a series of training courses, arranged at these annual meetings, will equip the PhD students with attributes that are important for scientists interested in translational autophagy research. In addition to knowledge obtained through lectures on key aspects of autophagy, PhD students will thus acquire additional competencies to exploit their results for the development of products and techniques of commercial value, including business and entrepreneurship, patenting, clinical trials, drug discovery, and diagnostic and pharmaceutical commercialization. They will also learn to disseminate results and knowledge through modern channels of communication (topical movies, social media communication, specific reviews and other outreach activities), to network (exchanges with national and international autophagy societies and networks, and patient associations), and to obtain insights into clinical research. As a result, DRIVE PhD students will be very-well prepared for a variety of professional career paths and will be ideal catalysts for partnerships between the public and private sectors.
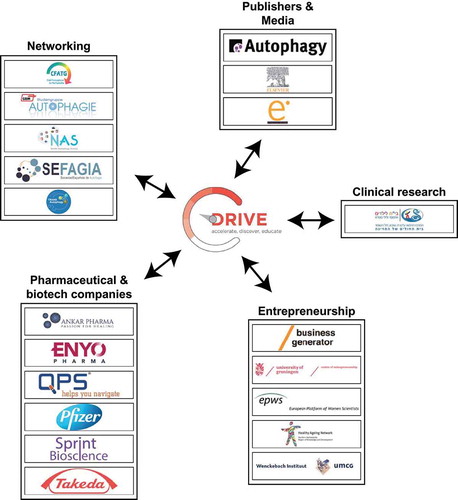
The DRIVE’s associated partners
DRIVE has a series of strategic associated partners that will be pivotal to reach its training goals (). Six international pharmaceutical and biotech companies, ANKAR Pharma (http://www.ankarpharma.com/), ENYO Pharma (http://www.enyopharma.com/), Pfizer (https://www.pfizer.com/), QPS Netherlands (http://www.qps.com/), Sprint Bioscience (http://www.sprintbioscience.com/en/) and Takeda Oncology (http://takedaoncology.com/), will host the PhD students for internships in the private sector and provide lectures on their research on autophagy. The journal Autophagy (https://www.tandfonline.com/toc/kaup20/current), Elsevier (https://www.eu.elsevierhealth.com/) and e.mersion (http://davidbolinsky.com) will provide insights into the media and publishing industry through specific lectures and offers of practical educational tasks. Public and scientific outreach will be achieved via cooperation with the European Transautophagy COST action (http://cost-transautophagy.eu/) and national autophagy networks, including the Club Francophone de l’Autophagie (CFATG, http://cfatg.org/en), the German Autophagy Association (http://autophagie-gbm.de/), the Nordic Autophagy Society (NAS, https://nordicautophagy.org/) and the Sociedad Española de Autofagia (SEFAGIA, http://autofagia.org/). Courses on different aspects of entrepreneurship will be held by the Healthy Aging Network Northern Netherlands (https://www.hannn.eu/), Wenckebach Institute of the University Medical Centre of Groningen (https://www.umcg.nl/EN/corporate/Departments/wenckebach/about/Paginas/default.aspx), the University of Groningen Centre of Entrepreneurship (https://www.rug.nl/society-business/centre-for-entrepreneurship/), the Stichting Business Generator Groningen (https://www.businessgeneratorgroningen.nl/) and the European Platform of Women Scientists (https://epws.org/). Prof. Dr. Heinz Jungbluth, one of the members of the DRIVE consortium, and Prof. Dr. Bruria Ben-Zeev, from The Edmond & Lilly Safra Children’s Hospital at the Sheba Medical Centre (Ramat Gan, Israel, https://eng.sheba-hospital.org.il/children-hospital.aspx) will introduce the PhD students to clinical research, with a particular focus on autophagy.
Conclusions
DRIVE has principally been designed for the training of 15 young scientists, but it also represents a unique platform to initiate new and strengthen existing collaborations between laboratories as well as academic and private partners. The key events for this type of exchange will be the annual DRIVE meetings, but the internships of the PhD students will also be an important vector for the transfer of expertise and techniques, and for the realization of specific aspects of common projects.
Taken together, DRIVE, like the European Transautophagy COST action and the NIH-founded Autophagy, Inflammation and Metabolism (AIM) Center in Albuquerque, New Mexico [Citation16], is a new international initiative that will further promote the fundamental, applied and translational research in the field of autophagy, with the ultimate long-term objective of providing biomedical solutions to unmet medical needs.
Acknowledgments
We thank Catarina Rodrigues for the critical reading of the manuscript. DRIVE is supported by a Marie Skłodowska-Curie ETN grant under the European Union’s Horizon 2020 Research and Innovation Programme (Grant Agreement No 765912). P.B. is also supported by the Ministerio de Ciencia, Innovación y Universidades (BFU2015-65623) and by Redes de la Comunidad de Madrid 2017/BMD-3813. Work in P.C. laboratory is supported by institutional fundings from INSERM, CNRS and University Paris Descartes and by grants from Agence Nationale pour la Recherche (ANR) and Fondation pour la Recherche Médicale (FRM). Z.E. research is supported by the Israel Science Foundation ISF, the Legacy Heritage Fund and the Weizmann Institute Minerva center. E.-L.E. is also supported by The Academy of Finland project grant (286787), by Magnus Ehrnrooth Foundation, and by COST Action 15138 Transautophagy. V.K. is also supported by the CRUK programme grant C2739/A22897. C.K. is also supported by funding from the Vienna Science and Technology Fund (WWTF, VRG10-001) from the FWF Austrian Science Fund (P28113-B28), from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme (grant agreement No 769065), and from the EMBO Young Investigator Program. H.J. is supported by a grant from Action Medical Research (Grant reference GN 2446), United Kingdom. O.P. is also supported by the German Federal Ministry of Education and Research (BMBF) (01EK1608B and 13GW0128B). T.P.-C. is also supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG); SFB/TRR 209 (project B02); FOR 2625 (project 1). A.S. is also supported by the Research Council of Norway (project # 221831 and through its Centres of Excellence funding scheme project # 262652) and the Norwegian Cancer Society (project #190251). F.R. is also supported by ZonMW VICI (016.130.606), ZonMW TOP (91217002), ALW Open Programme (ALWOP.310) and Marie Skłodowska-Curie Cofund (713660) grants.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Mizushima N. A brief history of autophagy from cell biology to physiology and disease. Nat Cell Biol. 2018;20:521–527.
- Nakatogawa H, Suzuki K, Kamada Y, et al. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467.
- Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22:124–131.
- Levine B, Packer M, Codogno P. Development of autophagy inducers in clinical medicine. J Clin Invest. 2015;125:14–24.
- Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–364.
- Levine B, Klionsky DJ. Autophagy wins the 2016 nobel prize in physiology or medicine: breakthroughs in baker’s yeast fuel advances in biomedical research. Proc Natl Acad Sci USA. 2016;114:201–205.
- Mizushima N, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075.
- Kraft C, Peter M, Hofmann K. Selective autophagy: ubiquitin-mediated recognition and beyond. Nat Cell Biol. 2010;12:836–841.
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42.
- Virgin HW, Levine B. Autophagy genes in immunity. Nat Immunol. 2009;10:461–470.
- Menzies FM, Fleming A, Rubinsztein DC. Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci. 2015;16:345–357.
- Van Beek N, Klionsky DJ, Reggiori F. Genetic aberrations in macroautophagy genes leading to diseases. Biochim Biophys Acta. 2018;1865:803–816.
- Morel E, Mehrpour M, Botti J, et al. Autophagy: A druggable process. Annu Rev Pharmacol Toxicol. 2017;57:375–398.
- Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discover. 2012;11:709–730.
- He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515.
- Deretic V, Prossnitz E, Burge M, et al. Autophagy, Inflammation, and Metabolism (AIM) center of biomedical research excellence: supporting the next generation of autophagy researchers and fostering international collaborations. Autophagy. 2018 15548627.2018.1465784.