ABSTRACT
A recent prospective epidemiological study suggested that an increase in the nutritional uptake of the natural polyamine spermidine is associated with reduced overall and cancer-specific mortality. Here, we speculate through which mechanisms spermidine might exert such oncopreventive effects.
Abbreviations: ACLY, ATP citrate lyase; ATG, autophagy-related gene; CoA, coenzyme A; NSCLC, non-small cell lung cancer
Spermidine, a polyamine that is particularly abundant in sperm, is a polycation that is associated with DNA in most, if not all, living organisms. If in excess over DNA, spermidine is volatile and confers the characteristic smell and taste to sperm. Spermidine is contained in all food items containing nuclei (such as vegetable, meat and fish), is scarcely present in milk and hyperprocessed food items, yet is overabundant in food products generated by bacterial or fungal fermentation (such as smelly mature cheese and the soya fermentation product natto) or specific fruits with a sperm-like odor (such as durian fruit)
A recent prospective, observational epidemiological study revealed that individuals that have been eating a diet that is spermidine-rich are characterized by a reduced overall mortality, as well as a decreased mortality by each of the major causes of death, namely (i) cardiovascular, (ii) cancer and (iii) ‘other’ causes [Citation1]. The association between high spermidine uptake and reduced mortality is independent from confounding factors including age, sex, body mass index, consumption of alcohol or aspirin, metabolic syndrome, diabetes, physical activity, and socioeconomic status, as well as conventional dietary scores distinguishing healthy from unhealthy eating. These results confirm our long-lasting suspicion that spermidine has broad health-improving effects [Citation2–Citation9], based on our observation that nutritional supplementation of this polyamine can extend the longevity of multiple model species, including yeast, nematodes, flies and mice [Citation10–Citation12]. Here, we explore the literature linking spermidine to reduced cancer risk.
Beyond its metabolic function as an intermediate in the conversion of putrescine to spermine, and it role as a precursor of acetyl-spermidine [Citation13,Citation14], spermidine functions as a signaling molecule that acts as an endogenous inhibitor of the acetyl transferase EP300, an enzyme that transfers acetyl groups from acetyl coenzyme A (CoA) on lysine residues of cytoplasmic and nuclear proteins ()) [Citation15]. This inhibitory effect results from the sterical competition between spermidine and acetyl CoA for binding to the EP300 catalytic site [Citation12]. Acetyl CoA functions as an endogenous inhibitor of macroautophagy/autophagy, meaning that excess acetyl CoA (and in particular its cytosolic pool) cause the hyperacetylation of multiple proteins involved in the regulation or execution of autophagy, thus stalling the process [Citation16–Citation18]. This may constitute a phylogenetically ancient mechanism through which nutrient excess, which results in an increase in cellular acetyl CoA, inhibits autophagy [Citation19,Citation20]. Conversely, fasting or caloric restriction cause a reduction in acetyl CoA levels, thereby triggering autophagy [Citation21]. This ancient regulatory system (conserved throughout eukaryotic evolution) facilitates the adaptation of organisms to dwindling nutrient resources. Intriguingly, this regulatory system may be modulated by endogenous metabolites such as spermidine, as well as external agents such as aspirin. Aspirin, which – like spermidine – inhibits EP300 by competing with acetyl CoA [Citation22], is well known for its cancer-preventive action [Citation23,Citation24] in particular on the gastrointestinal system (a 30% decrease of cancer in long-term users) [Citation25] but also lung [Citation26], mammary [Citation27] and prostate carcinomas (a 10% decrease) [Citation28].
Figure 1. Mechanism of action of spermidine with respect to its anticancer effects. (a) Molecular mode of action. Spermidine competitively inhibits the enzymatic activity of the acetyl transferase EP300, resulting in the deacetylation of multiple autophagy-related proteins, thus triggering autophagy. (b) Anticancer activity of spermidine. Spermidine may exert cell-autonomous effects on cancer cells, yet may also influence their dialog with immune effectors to facilitate the recognition of tumor-associated antigens and the elimination of cancer cells. DAMP, danger-associated molecular pattern; TME, tumor microenvironment.
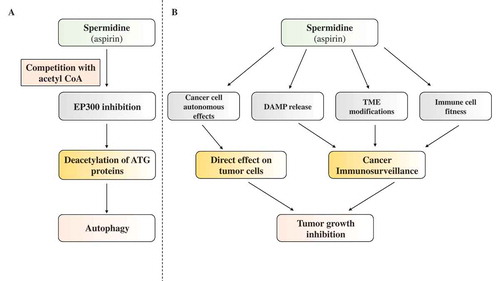
It is hence tempting to speculate, yet remains to be demonstrated, that both spermidine and aspirin share a common mode of action with respect to the prophylaxis of malignant disease. As mentioned above, spermidine is a potent inducer of autophagy, both in vitro (in cultured cells) and in vivo (when spermidine is supplied with the food or drinking water). In yeast, nematodes and flies, life span extension by spermidine is lost upon knockout/knockdown of essential autophagy-related (ATG) genes (such as ATG5, ATG7 or BECN1) [Citation10].
Similarly, in mice, the capacity of spermidine to prevent cardiac aging [Citation29] is fully lost upon cardiomyocyte-specific knockout of Atg7 [Citation11,Citation15]. These findings suggest that the desirable effects of spermidine on health are secondary to the induction of autophagy. In a model of cancer prophylaxis using hydroxycitrate, an inhibitor of the acetyl CoA-generating enzyme ACLY (ATP citrate lyase), which reduces intracellular acetyl CoA levels, we observed that inactivation of ATG5 in KRAS-expressing non-small cell lung cancer (NSCLC) cells abolishes the chemopreventive effect [Citation30,Citation31]. Similarly, both hydroxycitrate and spermidine could enhance the anticancer immune response induced by immunogenic chemotherapy, thus improving the control of established tumors. Again, this effect is lost upon knockdown of essential autophagy genes (ATG5 or ATG7) [Citation31,Citation32], supporting the idea that spermidine might induce autophagy in cancer cells to improve anticancer immunosurveillance. Along the same line of evidences, the activation of autophagy elicited by spermidine administration accounts for the prevention of hepatocellular carcinoma formation [Citation33], reduces the burden of colorectal cancer allografts [Citation34] and promotes the rejuvenation of the adaptive branch of the immune system [Citation35,Citation36].
The history of tumor biology has been marked by a long period during which malignancy was thought to solely result from genetic and epigenetic aberrations in the cancer cells. However, it has become clear over the past decade that beyond such cell-autonomous events, cancer will only emerge and develop into a life-threatening disease if the immune system fails to detect and eliminate malignant cells ()) [Citation37–Citation39]. Autophagy has classically been studied with respect to cancer, while adopting the cell-autonomous point of view, often in vitro and in xenotransplantation experiments (in which human cancer cells are implanted into immunodeficient hosts). Many of these studies came to the conclusion that autophagy can increase the fitness of malignant cells, acting as part of a cellular defense system against stressful or harmful conditions, while some investigators noted the capacity of autophagy to suppress malignant transformation in specific circumstances, for instance by inhibiting pro-carcinogenic inflammatory reactions [Citation40–Citation45]. However, it appears important to note that, in the presence of an immune system, the anticancer effects of autophagy induction within malignant cells prevail because of the effective induction of antitumor immunity [Citation46–Citation52].
It will be important to study the detailed mechanisms through which spermidine and aspirin can stimulate immunosurveillance and to develop strategies for replacing them with more effective EP300 inhibitors or combining them with other immunostimulants.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; the European Commission (ArtForce); European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); the European Research Council (ERC); Fondation Carrefour; Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI). F.M. is grateful to the Austrian Science Fund FWF (Austria) for grants P23490-B20, P29262, P24381, P29203 P27893, I1000, “SFB Lipotox” (F3012), and DKplus Metabolic and Cardiovascular Diseases (W1226), as well as to Bundesministerium für Wissenschaft, Forschung und Wirtschaft and the Karl-Franzens University for grants “Unkonventionelle Forschung”. We acknowledge support from NAWI Graz and the BioTechMed-Graz flagship project “EPIAge.” FC is supported by the Ligue contre le Cancer.
Disclosure statement
Frank Madeo, Didac Carmona-Gutierrez, Oliver Kepp and Guido Kroemer are the scientific co-founders of Samsara Therapeutics. Frank Madeo and Didac Carmona-Gutierrez are scientific co-founders of Longevity Labs.
Additional information
Funding
References
- Kiechl S, Pechlaner R, Willeit P, et al. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr. 2018 Aug 1;108(2):371–380. PubMed PMID: 29955838.
- Minois N. Molecular basis of the ‘anti-aging’ effect of spermidine and other natural polyamines - a mini-review. Gerontology. 2014;60(4):319–326. PubMed PMID: 24481223.
- Schwarz C, Stekovic S, Wirth M, et al. Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging (Albany NY). 2018 Jan 8;10(1):19–33. PubMed PMID: 29315079; PubMed Central PMCID: PMC5807086.
- Gupta VK, Pech U, Bhukel A, et al. Spermidine suppresses age-associated memory impairment by preventing adverse increase of presynaptic active zone size and release. PLoS Biol. 2016 Sep;14(9):e1002563. PubMed PMID: 27684064; PubMed Central PMCID: PMC5042543.
- Yang Y, Chen S, Zhang Y, et al. Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death Dis. 2017 Apr 6;8(4):e2738. PubMed PMID: 28383560; PubMed Central PMCID: PMC5477584.
- Fan J, Yang X, Li J, et al. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget. 2017 Mar 14;8(11):17475–17490. PubMed PMID: 28407698; PubMed Central PMCID: PMC5392263.
- Yang Q, Zheng C, Cao J, et al. Spermidine alleviates experimental autoimmune encephalomyelitis through inducing inhibitory macrophages. Cell Death Differ. 2016 Nov 1;23(11):1850–1861. PubMed PMID: 27447115; PubMed Central PMCID: PMC5071574.
- Michiels CF, Kurdi A, Timmermans JP, et al. Spermidine reduces lipid accumulation and necrotic core formation in atherosclerotic plaques via induction of autophagy. Atherosclerosis. 2016 Aug;251:319–327. PubMed PMID: 27450786.
- Paul S, Kang SC. Natural polyamine inhibits mouse skin inflammation and macrophage activation. Inflamm Res. 2013 Jul;62(7):681–688. PubMed PMID: 23603994.
- Eisenberg T, Knauer H, Schauer A, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009 Nov;11(11):1305–1314. PubMed PMID: 19801973.
- Eisenberg T, Abdellatif M, Schroeder S, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016 Dec;22(12):1428–1438. PubMed PMID: 27841876; PubMed Central PMCID: PMC5806691.
- Pietrocola F, Lachkar S, Enot DP, et al. Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ. 2015 Mar;22(3):509–516. PubMed PMID: 25526088; PubMed Central PMCID: PMC4326581.
- Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009 Sep;61(9):880–894. PubMed PMID: 19603518; PubMed Central PMCID: PMC2753421.
- Murray-Stewart TR, Woster PM, Casero RA. Targeting polyamine metabolism for cancer therapy and prevention. Biochem J. 2016 Oct 1;473(19):2937–2953. PubMed PMID: 27679855; PubMed Central PMCID: PMC5711482.
- Madeo F, Eisenberg T, Pietrocola F, et al. Spermidine in health and disease. Science. 2018 Jan 26;359(6374). PubMed PMID: 29371440. DOI:10.1126/science.aan2788.
- Morselli E, Marino G, Bennetzen MV, et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol. 2011 Feb 21;192(4):615–629. PubMed PMID: 21339330; PubMed Central PMCID: PMC3044119.
- Eisenberg T, Schroeder S, Andryushkova A, et al. Nucleocytosolic depletion of the energy metabolite acetyl-coenzyme a stimulates autophagy and prolongs lifespan. Cell Metab. 2014 Mar 4;19(3):431–444. PubMed PMID: 24606900; PubMed Central PMCID: PMC3988959.
- Marino G, Pietrocola F, Eisenberg T, et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol Cell. 2014 Mar 6;53(5):710–725. PubMed PMID: 24560926.
- Madeo F, Pietrocola F, Eisenberg T, et al. Caloric restriction mimetics: towards a molecular definition. Nat Rev Drug Discov. 2014 Oct;13(10):727–740. PubMed PMID: 25212602.
- Lopez-Otin C, Galluzzi L, Freije JMP, et al. Metabolic control of longevity. Cell. 2016 Aug 11;166(4):802–821. PubMed PMID: 27518560.
- Pietrocola F, Galluzzi L, Bravo-San Pedro JM, et al. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 2015 Jun 2;21(6):805–821. PubMed PMID: 26039447.
- Pietrocola F, Castoldi F, Markaki M, et al. Aspirin recapitulates features of caloric restriction. Cell Rep. 2018 Feb 27;22(9):2395–2407. PubMed PMID: 29490275; PubMed Central PMCID: PMC5848858.
- Rothwell PM, Fowkes FG, Belch JF, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011 Jan 1;377(9759):31–41. PubMed PMID: 21144578.
- Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012 Apr 3;9(5):259–267. PubMed PMID: 22473097.
- Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010 Nov 20;376(9754):1741–1750. PubMed PMID: 20970847.
- Moysich KB, Menezes RJ, Ronsani A, et al. Regular aspirin use and lung cancer risk. BMC Cancer. 2002 Nov 26;2:31. PubMed PMID: 12453317; PubMed Central PMCID: PMC138809.
- Clarke CA, Canchola AJ, Moy LM, et al. Regular and low-dose aspirin, other non-steroidal anti-inflammatory medications and prospective risk of HER2-defined breast cancer: the California Teachers Study. Breast Cancer Res. 2017 May 1;19(1):52. PubMed PMID: 28460643; PubMed Central PMCID: PMC5410689.
- Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014 Oct;15(11):e484–92. PubMed PMID: 25281467; PubMed Central PMCID: PMC4203149.
- Zhang H, Wang J, Li L, et al. Spermine and spermidine reversed age-related cardiac deterioration in rats. Oncotarget. 2017 Sep 12;8(39):64793–64808. PubMed PMID: 29029392; PubMed Central PMCID: PMC5630292.
- Rao S, Tortola L, Perlot T, et al. A dual role for autophagy in a murine model of lung cancer. Nat Commun. 2014;5:3056. PubMed PMID: 24445999.
- Pietrocola F, Pol J, Vacchelli E, et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell. 2016 Jul 11;30(1):147–160. PubMed PMID: 27411589; PubMed Central PMCID: PMC5715805.
- Michaud M, Martins I, Sukkurwala AQ, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011 Dec 16;334(6062):1573–1577. PubMed PMID: 22174255.
- Yue F, Li W, Zou J, et al. Spermidine prolongs lifespan and prevents liver fibrosis and hepatocellular carcinoma by activating MAP1S-mediated autophagy. Cancer Res. 2017 Jun 1;77(11):2938–2951. PubMed PMID: 28386016; PubMed Central PMCID: PMC5489339.
- Miao H, Ou J, Peng Y, et al. Macrophage ABHD5 promotes colorectal cancer growth by suppressing spermidine production by SRM. Nat Commun. 2016 May;18(7):11716. PubMed PMID: 27189574; PubMed Central PMCID: PMC4873969.
- Puleston DJ, Zhang H, Powell TJ, et al. Autophagy is a critical regulator of memory CD8(+) T cell formation. Elife. 2014 Nov 11;3. PubMed PMID: 25385531; PubMed Central PMCID: PMC4225493. DOI:10.7554/eLife.03706.
- Puleston DJ, Simon AK. New roles for autophagy and spermidine in T cells. Microb Cell. 2015 Mar 2;2(3):91–93. PubMed PMID: 28357282; PubMed Central PMCID: PMC5349183.
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011 Mar 25;331(6024):1565–1570. PubMed PMID: 21436444.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646–674. PubMed PMID: 21376230.
- Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006 Oct;6(10):715–727. PubMed PMID: 16977338.
- Degenhardt K, Mathew R, Beaudoin B, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006 Jul;10(1):51–64. PubMed PMID: 16843265; PubMed Central PMCID: PMC2857533.
- Galluzzi L, Pietrocola F, Bravo-San Pedro JM, et al. Autophagy in malignant transformation and cancer progression. Embo J. 2015 Apr 1;34(7):856–880. PubMed PMID: 25712477; PubMed Central PMCID: PMC4388596.
- Rybstein MD, Bravo-San Pedro JM, Kroemer G, et al. The autophagic network and cancer. Nat Cell Biol. 2018 Mar;20(3):243–251. PubMed PMID: 29476153.
- Zhong Z, Sanchez-Lopez E, Karin M. Autophagy, Inflammation, and Immunity: A Troika Governing Cancer and Its Treatment. Cell. 2016 Jul 14;166(2):288–298. PubMed PMID: 27419869; PubMed Central PMCID: PMC4947210.
- Monkkonen T, Debnath J. Inflammatory signaling cascades and autophagy in cancer. Autophagy. 2018;14(2):190–198. PubMed PMID: 28813180; PubMed Central PMCID: PMC5902219.
- Netea-Maier RT, Plantinga TS, van de Veerdonk FL, et al. Modulation of inflammation by autophagy: consequences for human disease. Autophagy. 2016;12(2):245–260. PubMed PMID: 26222012; PubMed Central PMCID: PMC4836004.
- Ma Y, Adjemian S, Mattarollo SR, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013 Apr 18;38(4):729–741. PubMed PMID: 23562161.
- Ma Y, Galluzzi L, Zitvogel L, et al. Autophagy and cellular immune responses. Immunity. 2013 Aug 22;39(2):211–227. PubMed PMID: 23973220.
- Michaud M, Xie X, Bravo-San Pedro JM, et al. An autophagy-dependent anticancer immune response determines the efficacy of melanoma chemotherapy. Oncoimmunology. 2014;3(7):e944047. PubMed PMID: 25610726; PubMed Central PMCID: PMC4292732.
- Ko A, Kanehisa A, Martins I, et al. Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ. 2014 Jan;21(1):92–99. PubMed PMID: 24037090; PubMed Central PMCID: PMC3857616.
- Ladoire S, Enot D, Senovilla L, et al. The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Autophagy. 2016 May 3;12(5):864–875. PubMed PMID: 26979828; PubMed Central PMCID: PMC4854557.
- Ladoire S, Penault-Llorca F, Senovilla L, et al. Combined evaluation of LC3B puncta and HMGB1 expression predicts residual risk of relapse after adjuvant chemotherapy in breast cancer. Autophagy. 2015;11(10):1878–1890. PubMed PMID: 26506894; PubMed Central PMCID: PMC4824597.
- Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013 Oct;13(10):722–737. PubMed PMID: 24064518; PubMed Central PMCID: PMC5340150.
