ABSTRACT
Macroautophagy/autophagy is a complex self-degradative mechanism responsible for clearance of non functional organelles and proteins. A range of factors influences the autophagic process, and disruptions in autophagy-related mechanisms lead to disease states, and further exacerbation of disease. Despite in-depth research into autophagy and its role in pathophysiological processes, the resources available to use it for therapeutic purposes are currently lacking. Herein we report the Autophagy Small Molecule Database (AutophagySMDB; http://www.autophagysmdb.org/) of small molecules and their cognate protein targets that modulate autophagy. Presently, AutophagySMDB enlists ~10,000 small molecules which regulate 71 target proteins. All entries are comprised of information such as EC50 (half maximal effective concentration), IC50 (half maximal inhibitory concentration), Kd (dissociation constant) and Ki (inhibition constant), IUPAC name, canonical SMILE, structure, molecular weight, QSAR (quantitative structure activity relationship) properties such as hydrogen donor and acceptor count, aromatic rings and XlogP. AutophagySMDB is an exhaustive, cross-platform, manually curated database, where either the cognate targets for small molecule or small molecules for a target can be searched. This database is provided with different search options including text search, advanced search and structure search. Various computational tools such as tree tool, cataloging tools, and clustering tools have also been implemented for advanced analysis. Data and the tools provided in this database helps to identify common or unique scaffolds for designing novel drugs or to improve the existing ones for autophagy small molecule therapeutics. The approach to multitarget drug discovery by identifying common scaffolds has been illustrated with experimental validation.
Abbreviations: AMPK: AMP-activated protein kinase; ATG: autophagy related; AutophagySMDB: autophagy small molecule database; BCL2: BCL2, apoptosis regulator; BECN1: beclin 1; CAPN: calpain; MTOR: mechanistic target of rapamycin kinase; PPARG: peroxisome proliferator activated receptor gamma; SMILES: simplified molecular input line entry system; SQSTM1: sequestosome 1; STAT3: signal transducer and activator of transcription
Introduction
Autophagy is a catabolic trafficking pathway deployed by cells for the destruction of misfolded protein aggregates and dysfunctional organelles, via regulated lysosomal degradation. In eukaryotes, autophagy occurs constitutively to maintain cellular homeostasis; however, it can be induced under several stimuli such as starvation, oxidative stress, and hormonal imbalance. Both its inadequate and overdriven functions thwart cell survival [Citation1,Citation2]. Due to the identification of a full set of essential autophagy genes and proteins in yeast, and their human orthologues, a greater molecular understanding of this fundamental process has been developed [Citation3]. Research into autophagy has revealed its cardinal role in various pathophysiological processes such as cancer, cardiovascular diseases (including ischemic injury and myocardial infarction), and a number of neurodegenerative disorders (including Alzheimer disease [AD], Parkinson disease [PD], and Huntington disease [HD]) and also in infection [Citation4–Citation13]. This dynamic cellular homeostasis process is known to be controlled via a range of signaling proteins and transcription factors [Citation14,Citation15]. For example, MTOR (mechanistic target of rapamycin kinase), phosphoinositide 3-kinase (PI3K), AMP-activated protein kinase (AMPK, also named as protein kinase AMP-activated, or PRKA) and MAPKs (mitogen-activated protein kinases) are signaling proteins that regulate this process; whereas TFEB (transcription factor EB), E2F (E2F transcription factor), HIF1A/HIF-1 alpha (hypoxia inducible factor 1 subunit alpha), and NFKB/NF-ᴋB (nuclear factor kappa B) are examples of transcription factors. Moreover, druggable nuclear receptors such as VDR (vitamin D receptor), RAR (retinoic acid receptor), PPARG/PPAR-γ (peroxisome proliferator activated receptor gamma) and PPARA/PPAR-α (peroxisome proliferator activated receptor alpha), NR3C1/glucocorticoid receptor (nuclear receptor subfamily 3 group C member 1), AR (androgen receptor), and NR1D1/Rev-erb-α (nuclear receptor subfamily 1 group D member 1) regulate autophagy along with the ATG (autophagy related) proteins [Citation16].
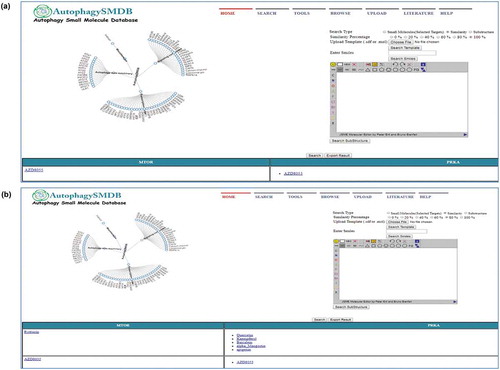
Figure 1. Screenshot of the AutophagySMDB homepage. AutophagySMDB homepage displaying various functionalities such as search, tools, browse, upload and help.

A growing body of evidence depicting its pathophysiological role, demands a meticulous understanding of this network of proteins and associated small molecules modulating autophagy [Citation17–Citation19]. Unfortunately, there is not any platform available on which all the information regarding these is accessible. Presently there are 3 autophagy databases: the Autophagy database, the Human Autophagy Database, and the Autophagy Regulatory Network. The Autophagy database comprehensively provides information on the ATG proteins across species [Citation20]. The Human Autophagy Database is a database for integration and annotation of human genes described to regulate autophagy [Citation21]. The Autophagy Regulatory Network provides information on both curated and predicted interactions of autophagy components, as well as lists several transcription factors and miRNAs that modulate autophagy [Citation22]. However, none of these databases provide information on the small molecules that may regulate autophagy.
Recent research has highlighted the amenability of these protein targets to be regulated by an enormous number of small molecule modulators which would be critical for pharmacological intervention [Citation23]. Of late, a number of resources have become available that give information on drugs or small molecules and their targets, such as drug bank, KEGG DRUG Database, and Orphan nuclear receptor ligand database (ONRLDB) [Citation24–Citation26]. PubChem and ChEMBL databases are known to harbor information on biologically active small molecules for various cognate targets which are having drug like properties. These databases compile information from high throughput screening analysis and are not adequately curated however, AutophagySMDB (Autophagy Small Molecule Database) specifically compiles curated information on all the known autophagy targets and related small molecules [Citation27,Citation28]. This platform can essentially prove to be an asset for the chemists and researchers in the field of drug discovery for autophagy related diseases. As the therapeutic value of autophagy inducers or inhibitors to human diseases is getting increasingly recognized, there is an urgent need of a database for effectual exploitation of existing information [Citation29].
AutophagySMDB offers a platform for gathering and assessing significant information on proteins reported to regulate autophagy and their associated small molecule modulators from direct and indirect evidence, regulating autophagy. All small molecule entries include 2-dimensional (2D) and 3-dimensional (3D) structure files along with a comprehensive array of additional information including the molecular formula, International Union of Pure and Applied Chemistry (IUPAC) name, canonical SMILES (Simplified Molecular Input Line Entry System), function, PubMedID (PMID), U.S. Food and Drug Administration (FDA) approval status, and various experimental values. This database is designed to permit the selection of small molecules based on various properties and to discover common or unique scaffolds for designing new drugs or to improve existing ones.
Results
Database description
AutophagySMDB is a comprehensive database of all the known autophagy targets and their corresponding small molecule modulators. Autophagy can be modulated via several signaling components acting together with transcription factors and autophagy core machinery proteins. The layout of the AutophagySMDB home page depicts functions such as search, tools, browse, upload, literature and help (). The database covers experimentally validated small molecules which modulate these signaling components, transcription factors, or autophagy core machinery proteins. High-throughput screening data were excluded to avoid the issues related with experimental invalidity. This database comprises of 71 target proteins and their ~10,000 small molecule modulators. The targets have been divided into 4 broad categories: signaling components (24 targets, ~2800 small molecules), transcription factors (31 targets, ~7000 small molecules), autophagy core machinery proteins (16 targets, ~30 small molecules) and miscellaneous (~35 molecules). This database also provides various user-friendly search options and several online tools for the analysis of small molecules. Consequently, our database provides a platform for specific and polypharmacological drug discovery for multiple diseases that are modulated by autophagy. Instructions for using AutophagySMDB have been described in a video on the help page of the website.
Data retrieval from AutophagySMDB
The AutophagySMDB interface is comprised of several search options such as text search for both target and small molecule, advanced search, and structure search (). An explicit target search will provide results with a PDB image, and an expandable list of all cognate small molecules with their corresponding physiochemical and experimental properties. A small molecule search will display structure, image, IUPAC name, canonical SMILES, references and manually curated experimental information such as EC50, IC50, Ki and Kd, as well as physiochemical properties such as hydrogen donor and acceptor count, number of rotatable bonds, aromatic rings, molecular weight (MW) and program for prediction of octanol/water partition coefficients of organic compounds (XlogP) (). A specific small molecule search will provide information on targets and off-targets; this function will be useful for finding crossover multi-target small molecules. Link has also been implemented in the small molecule page to allow the user to access associated target pages or vice versa. Furthermore, by using the advanced search, users can be more specific in choosing small molecules based on the previously mentioned physiochemical or experimental properties. Specific, or combinations of properties can be selected via filters using an operator (e.g., equal to, not equal to, greater than or less than) and a value. This search can be further specified by selecting a specific target and/or function such as an activator, or inhibitor.
Figure 2. Screenshots of the text, advanced and structure search from AutophagySMDB. (a and b) The dialog box for target text search (a), small molecule text search (b). (c and d) The dialog box for advanced search (c) and structure search (d). (e) Results of text search for a small molecule pyrazolopyrimidine_derivative_4, displaying QSAR properties of the small molecule, 2D, 3D structure download options along with IUPAC name, canonical SMILE, and experimental values like EC50, IC50 etc.
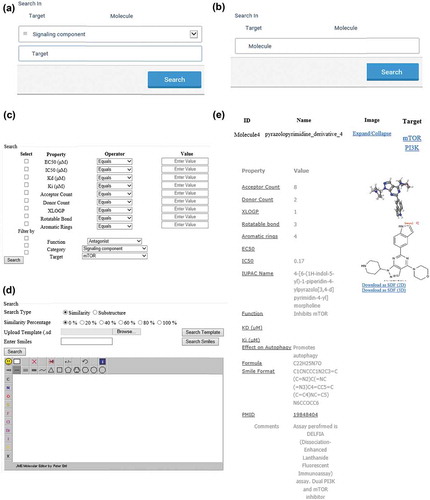
A structure search can be done in 3 ways: (i) by drawing the 2D structure, (ii) by entering SMILES or (iii) uploading the .sdf file or .mol file, for the small molecule. This search can be performed via similarity or substructure. By entering the SMILES format or uploading the SDF file users can retrieve similar compounds. To retrieve compounds by structure search, users can draw the complete or partial structure, using a 2D structure drawing interface.
Tools
AutophagySMDB is provided with various tools such as clustering, catalog, tree view and advanced catalog.
Clustering analysis
Clustering is a method that categorizes small molecules based on their structural backbone (scaffold) and it is a fundamental method for chemists and researchers in the process of drug discovery. We have implemented ChemMine tool (clone) for this purpose. Users can perform binning clustering, hierarchical clustering and multidimensional scaling analysis (MDS).
Catalog tool
Catalog tool implemented in this database can be used to filter small molecules based on target, function, MW and physiochemical properties. Structures of all the small molecules were embedded in Catalog to allow the visualization and to compare structures of small molecules.
Tree tool
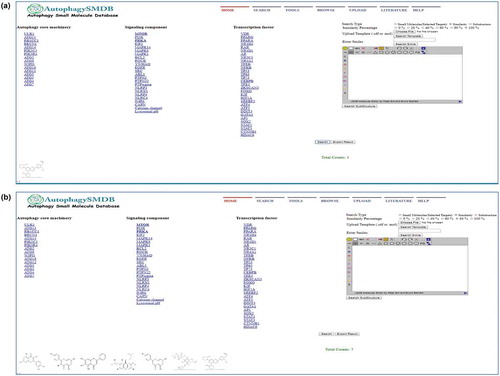
By using tree tool users can retrieve small molecules modulators of one or more selected targets in a single window. The search result will also include information on multiple targets being regulated by small molecules. In addition to it, similarity and substructure features have also been incorporated. By using similarity analysis, users can compare and retrieve percentage similar small molecules between any 2 targets by using the different similarity index viz 100%, 80%, 60% and 40%. A similar analysis can be done with the substructure feature by drawing a substructure of a molecule on the provided Jmol java applet. Server script saves the query as a temporary file and compares it in selected targets to find structurally similar molecules. Additionally, the user can also perform the similarity analysis by uploading either SDF file or SMILES of the molecule. Search output will be in the form of names of the small molecules with their associated targets. Analysis can be retrieved as a PDF file by export result option. For illustrating how useful the tree tool would be for the users in drug discovery we have done a sample exercise described in .
Advanced catalog
The advanced catalog has also been employed wherein a user can select 2 targets and apply options such as union, common, A-B, and B-A between those targets. Target selected first will be assigned as A and the target selected second will be assigned as B. Union option will give the complete list of all the small molecules in both the targets and common will give the result for existing common small molecules between both the targets. A-B and B-A options help to retrieve unique small molecules to each target. Similarity and substructure analysis can also be performed similarly to tree tool. After analysis output in advanced catalog depicts the structures of the small molecules unlike tree tool. Analysis can be retrieved as a PDF file by export result option. illustrate the functionality of advanced catalog.
These tools together make database an interactive knowledgeable resource for multitarget drug discovery.
Convergence and dissonance of autophagy and disease
Most of the autophagy-modulating proteins listed in this database are also reported to influence various pathophysiological conditions such as cancer, infection, neurodegenerative and inflammatory diseases [Citation30–Citation32] () [Citation30,Citation31,Citation33–Citation55].
Table 1. List of target proteins known to modulate autophagy with their role in human diseases.
In the case of cancer, autophagy plays a bimodal role. Tumorigenesis signaling pathways are well related with the regulation of autophagy [Citation56]. Several tumor suppressor genes inhibit MTOR signaling, and thereby stimulate autophagy: TP53/p53 (tumor protein p53) positively modulates autophagy. The proto-oncogene BCL2/Bcl-2 (BCL2, apoptosis regulator) inhibits autophagy by binding to BECN1 (beclin 1), and thereby promotes tumor growth [Citation56]. Genetic deletion of the autophagic gene BECN1 enhances susceptibility to breast, prostate, and ovarian cancers in humans [Citation57]. At the same time basal autophagy has been shown to be upregulated in hypoxic tumors and also in RAS-transformed cancer cells [Citation58]. Besides its role in the clearance of misfolded proteins, autophagy plays a critical role in the clearance of aggregated proteins which are generally associated with several neurodegenerative diseases [Citation59]. As far as host immune system is concerned, autophagy modulates several processes like antigen uptake, killing of pathogens, T cell homeostasis and also inflammation [Citation12]. Polymorphisms in autophagy-related genes contribute to tissue specific inflammatory diseases like inflammatory bowel disease and systemic lupus erythematosus [Citation60]. Autophagy is also a well-documented defense mechanism against several disease causing pathogens including Mycobacterium tuberculosis, Salmonella and Listeria monocytogenes etc [Citation61–Citation63]. In all, this database may prove to be an ideal resource which can be utilized for targeting these diseases via small molecule modulators through regulating autophagy.
Possible usage of autophagysmdb for specific or polypharmacological drug discovery
Polypharmacological drug discovery opens a novel platform for rational drug designing. Polypharmacological phenomena includes: (a) single drug acting on multiple targets of a unique disease pathway, or (b) single drug acting on multiple targets pertaining to multiple disease pathways (c) In addition, polypharmacology for complex diseases is likely to employ multiple drugs acting on distinct targets that are part of networks regulating various physiological responses [Citation64]. The use of a single drug that targets multiple factors involved in a distinct pathological condition may increase the efficacy of the treatment and limits negative aspects of a conventional single-target drug or a combination of multiple drugs. Autophagy is a dynamic cellular homeostasis event controlled by a range of signaling components and transcription factors and its deregulation is implicated in various pathologic processes such as neurodegenerative diseases, infectious diseases, cardiovascular diseases, cancer, and aging. Thus, multitarget modulation of autophagy is of great interest. AutophagySMDB consists of comprehensive information on cellular proteins and their small molecule modulators regulating the autophagic process in mammalian cells. By using this resource and associated tools one can generate unique and common scaffolds information of small molecules regulating key autophagy target proteins.
We have illustrated a polypharmacological (multitarget) approach that helps in designing small molecules that modulates autophagy by using information in AutophagySMDB. In our study we exemplified the polypharmacological phenomena of designing single drug acting on multiple targets in a distinct disease state. Common scaffolds among 2 targets have been identified by the ChemMine tool analysis ().
Figure 5. Common scaffolds among autophagy targets. (a) Structures of common scaffolds in MTOR inhibitors and AMPK activators, (b) Structures of common scaffolds in calcium channel blockers and CAPN inhibitors.
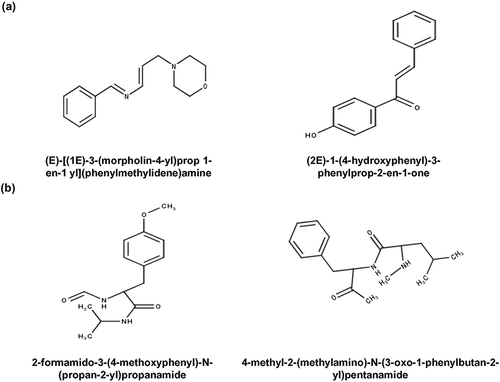
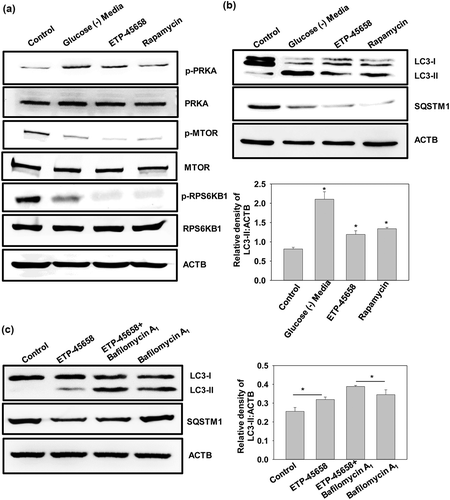
Two master key regulators, MTOR and AMPK, modulate autophagy in opposite directions i.e. MTOR inhibits autophagy and AMPK activates autophagy. Given the importance of MTOR and AMPK in modulating autophagy, these kinases act as an attractive target for polypharmacological drug designing wherein we can modulate activities of both the targets by using a single small molecule to induce autophagy. Our idea is to design one single potent drug that simultaneously inhibits MTOR and activates AMPK thereby promoting autophagy. For that we compared the inhibitors of MTOR and activators of AMPK and identified common scaffolds among them. To identify the common scaffolds among small molecule modulators of MTOR and AMPK we performed binning clustering (Tanimoto coefficient 0.4) analysis using Chemmine tool. Upon comparison we observed 28 similar structures among these targets. We found 2 most abundant scaffolds among the 28 similar structures; (E)-[(1E)-3-(morpholin-4-yl)prop 1-en-1yl](phenylmethylidene)amine and (2E)-1-(4-hydroxyphenyl)-3-phenylprop-2-en-1-one by using maximum common substructure (MCS) analysis provided in ChemMine tool (). One of the common scaffolds, i.e. (E)-[(1E)-3-(morpholin-4-yl)prop 1-en-1yl](phenylmethylidene)amine is found in the molecules; 3_6_dihydro_2H_pyran_1, N_7_methylimidazolopyrimidine_1,tetrahydroquinazoline_derivative_1, dihydrofuropyrimidine_derivative_1, 4_morpholino_6_aryl_1H_pyrazolo_3_4_d_pyrimidines_1 and ETP-45,658, which are known to be the direct binders of MTOR. So, we were intrigued to check the cross modulation of these molecules in directly activating the upstream target AMPK in the modulation of autophagy. ETP-45,658 was commercially available as an MTOR inhibitor and we observed that it significantly induced the phosphorylation of AMPK similarly as observed in glucose starvation () [Citation65]. ETP-45,658 being an MTOR inhibitor also reduced the phosphorylation levels of MTOR and its substrate RPS6KB1/p70S6K (ribosomal protein S6 kinase B1) (). LC3-I to LC3-II conversion and SQSTM1/p62 (sequestosome 1) protein levels were checked upon treatment with ETP-45,658 to monitor autophagy (). Further, to check whether the increase in autophagy was due to increase in autophagic flux, LC3-II accumulation and SQSTM1 levels were also monitored upon treatment with ETP-45,658 in presence of bafilomycin (). These experiments were done in accordance with the autophagy interpretation guidelines [Citation66]. Western blot analysis corroborated with the common scaffold structural interpretation i.e. modulating the 2 different targets MTOR inhibition and AMPK activation simultaneously. Experimental validation of common scaffold analysis confirmed the use of this scaffold structural information for the polypharmacological approach of designing a more potent drug which may overall induce autophagy by simultaneously activating AMPK and inhibiting MTOR.
Figure 6. ETP-45,658 induces AMPKphosphorylation in HEK-293 cells. (a) Western blot analysis of signaling components AMPK, p-AMPK, MTOR, p-MTOR, RPS6KB1 and p- RPS6KB1. Glucose-free media and rapamycin were used as positive control for AMPK phosphorylation and MTOR dephosphorylation respectively (b and c) Western blot analysis of LC3-II and SQSTM1 upon treatment with ETP-45,658 (10 µM) for 4 h in presence and absence of bafilomycin A1 (400 nM). LC3-I to LC3-II conversion is illustrated by LC3-II:ACTB ratios in bar graphs as in insets. Data is representative and mean ± SD from 3 independent experiments. *p < 0.05 as compared to control.
Another common scaffold, (2E)-1-(4-hydroxyphenyl)-3-phenylprop-2-en-1-one is found in 2 small molecules, baicalein and rottlerin; baicalein is well known to activate AMPK and inhibit MTOR complex components and thereby activate autophagy [Citation67]. However, rottlerin is well known to activate autophagy by inhibiting MTOR signaling [Citation68]. Some reports have also shown the effect of rottlerin on AMPK activation as well [Citation69]. These reports strengthen our analysis related to common scaffold identification. This analysis of finding scaffolds would be a stepping stone for multitarget drug discovery. These scaffolds are not complete molecules but they can be used as a starting point for fragment based drug designing.
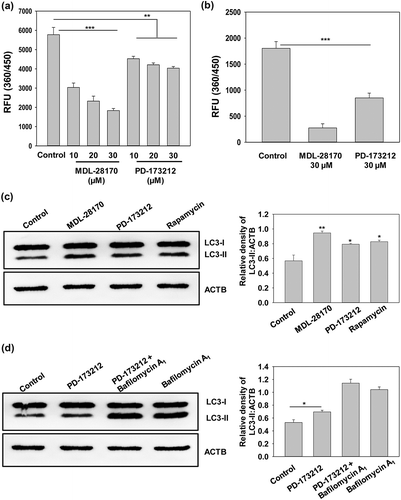
Autophagy acts as a protective mechanism during the initial stages of cancer and neurodegeneration. It has been reported that both calcium channels and CAPN (calpain) are hyperactivated and detrimental during these pathological conditions. We used small molecule information of AutophagySMDB to identify the common scaffolds in calcium channel blockers and CAPN inhibitors by using ChemMine tool. Upon comparison we observed 26 similar structures among these targets. We found 2 most abundant scaffolds among the 26 similar structures; 2-formamido-3-(4-methoxyphenyl)-N-(propan-2-yl)propanamide and 4-methyl-2-(methylamino)-N-(3-oxo-1-phenylbutan-2-yl)pentanamide (). Both of these common scaffolds i.e. 2-formamido-3-(4-methoxyphenyl)-N-(propan-2-yl)propanamide and 4-methyl-2-(methylamino)-N-(3-oxo-1-phenylbutan-2-yl)pentanamide are found in the molecules N_methyl_N_aralkyl_peptidylamines_derivatives_1, N_N_dialkyl_dipeptidylamines_derivatives_1, PD_167341, PD_173212 and CAT_811, which are well known to be the direct binders of calcium channels and CAPN, respectively. We investigated the cross modulation of calcium channel blockers in directly inhibiting the CAPN activity. PD_173212 was available commercially as a potent N-type calcium channel blocker. So, we checked for the direct inhibition of CAPN activity in the SH5YSY cell lysate and of the purified human CAPN upon treatment with PD_173212 in comparison to solvent control and a known CAPN inhibitor MDL-28,170. We observed that it was able to inhibit the CAPN activity significantly in comparison to solvent control (). Further, autophagy was monitored by conversion of LC3-I to LC3-II in SHSY5Y cells upon treatment with the PD_173212 and it was observed that PD_173212 was able to inhibit the CAPN activity significantly along with a slight increase in autophagy (). In order to improve its modulation of autophagy, we need to invoke fragment based drug discovery which employs scaffolds with special 3D binding modes; their fusion and designing to obtain a potent drug molecule. The common scaffold in PD_173212 can be fused to other suitable scaffolds to develop a novel potent drug or it can further be fragmentized to have a new scaffold altogether which can significantly induce autophagy by CAPN inhibition.
Figure 7. Effect of PD-173,212 on CAPN activity. (a) SHSY5Y cell lysate was incubated with the indicated concentrations of PD-173,212 for 30 min and CAPN activity was assessed and represented as relative fluorescence units (360/450). A known inhibitor of CAPN, MDL-28,170, was used as a positive control. (b) Purified human CAPN (1 µg) was incubated at 37°C for 30 min with MDL-28,170 (30 µM), PD-173,212 (30 µM) and solvent vehicle control and CAPN activity was assessed using CAPN substrate (Suc-LLY-AMC) as described in the Materials and Methods section. (c and d) Western blot analysis of LC3-II upon treatment with PD-173,212 (30 µM) for 8 h in presence and absence of bafilomycin A1 (400 nM). LC3-I to LC3-II conversion is illustrated by LC3-II:ACTB ratios in bar graphs as in insets. Data are representative and mean ± SD from 3 independent experiments. *p < 0.05 **p < 0.01 ***p < 0.001 as compared to control.
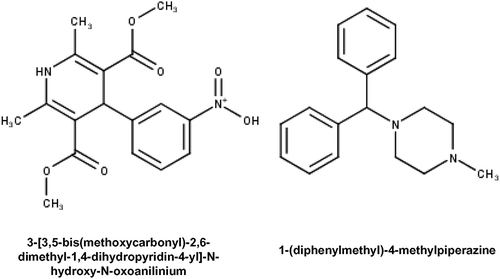
This exercise implies common scaffold structural information can be utilized in identifying the scaffolds which are common to distinct targets for the polypharmacological drug discovery. A similar analysis was done for identifying the unique scaffolds among these targets however, we were able to find 2 scaffolds; 3-[3,5-bis(methoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridin-4-yl]-N-hydroxy-N-oxoanilinium and 1-(diphenylmethyl)-4-methylpiperazine in the calcium channel blockers only (). Identification of the unique scaffold analysis would be beneficial in those cases where we need to design novel drugs which can target a specific autophagic modulator without affecting other modulators.
Further, we have illustrated many small molecules having multiple targets which can be used as an initial point for drug discovery () [Citation68,Citation70–Citation89].
Table 2. List of small molecules having multiple targets.
Discussion
Given the importance of autophagy in many disease conditions and deeper investigation into its mechanism of action has revealed various small molecule modulators that could serve as potential tools for therapeutic purposes [Citation12,Citation56]. The detailed information regarding these small molecules is increasing day by day. Here we present a novel resource on autophagy regulating proteins and their small molecule modulators. AutophagySMDB is a comprehensive database featuring several target proteins from literature and small molecule modulating these proteins which are curated from all the direct and indirect evidence as complete as possible. Selective autophagy has been well reported and various proteins, e.g. SQSTM1, OPTN (optineurin), NBR1 (NBR1, autophagy cargo receptor), CALCOCO2 (calcium binding and coiled-coil domain 2), BNIP3L/NIX (BCL2 interacting protein 3 like), etc. regulating these pathways have been recently identified [Citation90]. Information on small molecules targeting these proteins is mostly available from high throughput screening data therefore these proteins were not included in the database. However, literature related to small molecules targeting these selective autophagy modulators has been provided in the database in the search tab. We anticipate that this database opens new directions in autophagy research and will be valuable to the scientific community. This database allows users to access all the experimental information regarding small molecule interactions with their targets as well as their possible usage in drug development. To compile AutophagySMDB we reviewed over 2500 articles for all relevant information regarding the 71 targets and their small molecule modulators. Metric of relevant articles describing the role of autophagy targets and most cited small molecules has been provided in the database under the search tab. Due to the space constraint not all the reference literature has been included here, however, PubMedIDs of all the relevant articles surveyed for collecting and compiling the information have been provided in the database in the literature page.
To facilitate users in comparing, analyzing, and identifying scaffold similarity we have implemented structure, similarity, clustering, and cataloging tools into this database. Our database will allow users to design unique, common, or similar scaffolds. These functions will also assist in the rational designing of multitarget small molecules, which act on different target proteins that have similar biological functions. We have exemplified this approach to multitarget drug discovery by identifying common scaffold structural information using tools incorporated in the database and also validated it experimentally (–). The complexity of the biological systems limits the usage of small molecules as a therapeutic strategy, as their efficacy may vary among different systems. AutophagySMDB allows chemists and researchers in the field of autophagy to access information for polypharmacological drug designing and this information is not intended as a substitute for medical advice related to disease treatment. We have incorporated the most recent and highly curated information in the database. To cope up with the constantly increasing flow of information the data will be yearly evaluated, and updates will be done regularly for accuracy and to manifest advances in the field.
Materials and methods
Data collection
Data were collected from published literature and the information pertaining to small molecules was retrieved from the articles wherein small molecules were being tested in mammalian cells or in an in vivo setting. For each autophagy modulating target an exhaustive search for all experimental data and structural information was performed, excluding ambiguous data and bioassays. We have manually curated research articles, patents and reviews, as completely as possible. Additionally, we curated every reference cited from review articles. From each article, information regarding the small molecule of interest was gathered including, details of the targets, small molecule structure and function, EC50, IC50, Kd, and Ki, as well as the experimental procedure used to generate the information. The structures of the small molecules were either drawn using Marvin Sketch and saved as SDF files or files were downloaded from PubChem. FDA approval status of the small molecule has been collected from Drug Bank and mentioned in the comment section. Additionally, a list of FDA approved small molecules which modulate autophagy is mentioned under the ‘Search’ menu. Protein Data Bank (PDB) structures available for each target are mentioned under ‘Search’ menu and also included in the comment section of corresponding small molecules.
Database construction
AutophagySMDB web interface has been developed using the web server, Internet Information Server (IIS) 8.0 along with the database server, SQL server 2008 R2.
Clustering tool
The clustering tool ‘ChemMine’ is executed using the source code on the website (https://github.com/TylerBackman/chemminetools) [Citation91]. Users can perform hierarchical clustering, MDS, and binning clustering of small molecules using the ChemMine tool (clone). SDF files, or the SMILES format, of the small molecules provided in the database can be used as the input for clustering analysis.
Tree
In order to generate the tree, the rotatable chart has been used from D3 chart library (https://d3js.org/). All targets are represented as nodes on the tree; by clicking on a specific node users can view all the small molecules associated with that particular target. By clicking on multiple nodes, either from the same category or different categories, users can view all small molecules between the selected targets.
Catalog and advanced catalog
The database provides catalog tools in order to filter and visualize the structures of compounds along with their physiochemical properties. The catalog is implemented in this database using Microsoft Silverlight PivotViewer which functions with the Java applet. Furthermore, our database features an advanced catalog where users can perform extensive cataloging by selecting 2 targets either within or between target categories, and can apply options such as union, common, A-B, and B-A.
Submitting a new target/molecule or program to the database
AutophagySMDB provides a provision for users to submit a new autophagy target or small molecule modulating autophagy under the upload section in the database. Instructions on how to upload a target and molecule have been provided in the help page of the database. Also, there is a provision for suggesting a new program or tool to be integrated in the upload section of the database for its upgradation. Users can suggest for new tools and programs along with their detailed configurations. Suggestions will be evaluated and if found compatible with the database configuration they will be added to the database to make it more valuable.
Cell lines
HEK-293 and SHSY5Y cell lines (ATCC) were cultured in Dulbecco’s modified Eagle media (DMEM) and DMEM/F12 media respectively supplemented with 10% fetal bovine serum and maintained under 5% CO2 at 37°C in a humidified incubator.
Western blotting
Cell lysates were prepared by incubating the treated cells with cell lysis buffer on ice for 30 min and then centrifuged at 16,626 x g at 4°C. The supernatant was collected and the protein concentration was measured with the help of Bradford reagent (Sigma, B6916). For immunoblotting, the whole cell extract was resolved by SDS-PAGE and transferred to polyvinylidene difluoride membrane. Membranes were blocked with 5% bovine serum albumin (Merck Millipore, 12,659) in phosphate-buffered saline (pH 7.2) for 2 h and incubated overnight with primary antibodies. Primary antibodies were anti-LC3 (Sigma, L7543), anti-MTOR, p-MTOR, p-RPS6KB1, RPS6KB1 (Cell Signaling Technology, MTOR substrates antibody sampler kit- 9862), anti-SQSTM1 (BD Biosciences, 610,832) and anti-PRKAA1/2 (Santa Cruz Biotechnology, 25,792), p-PRKAA1/2 (Santa Cruz Biotechnology, 33,524), ACTB/β-actin (Santa Cruz Biotechnology, 47,778). Further, the membranes were incubated with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, 2313, 2314). The blots were visualized with Luminata Forte Western HRP substrate (Millipore, WBLUF0500).
CAPN activity assay
SHSY5Y cells were resuspended in lysis buffer and incubated on ice for 30 min and then centrifuged at 16,626 × g at 4°C. Protein content was measured using the Bradford reagent. CAPN activity was measured using Suc-LLY-AMC as the substrate provided in the CAPN activity assay kit (Merck Millipore, QIA120) as per the manufacturer’s instructions with minor modifications. CAPN activity was also measured with the purified human CAPN provided with the kit. After incubation, fluorescence was read in a Synergy H1 Hybrid Reader (BioTek Instruments, Inc., VT, USA) with excitation at 360 nm and emission at 450 nm and the enzyme activities were expressed as relative fluorescence units.
Disclaimer
The structural and experimental data presented here is collected from published literature. The appropriate references have been provided for all entries in AutophagySMDB. A lot of effort has gone in to make sure the data is scientifically correct. However, users are requested to refer to the original publications cited in the database for each entry. Small molecules are chemical compounds which can be promiscuous in nature. Given the complexity of the biological systems, the efficacy of these compounds can vary in different systems and they may have potential off-target effects. AutophagySMDB helps researchers to access the content for drug discovery purposes. AutophagySMDB is intended for educational and research purposes and not as a substitute for the medical advice or treatment.
Acknowledgments
We thank IMTECH, a constituent laboratory of the CSIR, for facilities and financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463–477. PubMed PMID: 15068787.
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861–2873. PubMed PMID: 18006683.
- Nakatogawa H, Suzuki K, Kamada Y, et al. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10(7):458–467. PubMed PMID: 19491929.
- Brest P, Corcelle EA, Cesaro A, et al. Autophagy and Crohn’s disease: at the crossroads of infection, inflammation, immunity, and cancer. Curr Mol Med. 2010 Jul;10(5):486–502. PubMed PMID: 20540703; PubMed Central PMCID: PMC3655526.
- Gatica D, Chiong M, Lavandero S, et al. Molecular mechanisms of autophagy in the cardiovascular system. Circ Res. 2015 Jan 30;116(3):456–467. PubMed PMID: 25634969; PubMed Central PMCID: PMC4313620.
- Degenhardt K, Mathew R, Beaudoin B, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10(1):51–64. PubMed PMID: 16843265; PubMed Central PMCID: PMCPMC2857533.
- Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100(6):914–922. PubMed PMID: 17332429.
- Komatsu M, Wang QJ, Holstein GR, et al. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A. 2007;104(36):14489–14494. PubMed PMID: 17726112; PubMed Central PMCID: PMCPMC1964831.
- Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–889. PubMed PMID: 16625204.
- Boland B, Kumar A, Lee S, et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci. 2008;28(27):6926–6937. PubMed PMID: 18596167; PubMed Central PMCID: PMCPMC2676733.
- Bjørkøy G, Lamark T, Brech A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171(4):603–614. PubMed PMID: 16286508; PubMed Central PMCID: PMCPMC2171557.
- Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013 Oct;13(10):722–737. PubMed PMID: 24064518; PubMed Central PMCID: PMC5340150.
- Lynch-Day MA, Mao K, Wang K, et al. The role of autophagy in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012 Apr;2(4):a009357. PubMed PMID: 22474616; PubMed Central PMCID: PMC3312403.
- Feng Y, Yao Z, Klionsky DJ. How to control self-digestion: transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol. 2015 Jun;25(6):354–363. PubMed PMID: 25759175; PubMed Central PMCID: PMC4441840.
- Fullgrabe J, Klionsky DJ, Joseph B. The return of the nucleus: transcriptional and epigenetic control of autophagy. Nat Rev Mol Cell Biol. 2014 Jan;15(1):65–74. PubMed PMID: 24326622.
- Chandra V, Bhagyaraj E, Parkesh R, et al. Transcription factors and cognate signalling cascades in the regulation of autophagy. Biol Rev Camb Philos Soc. 2016;91(2):429–451. PubMed PMID: 25651938.
- Baek SH, Kim KI. Epigenetic control of autophagy: nuclear events gain more attention. Mol Cell. 2017 Mar 02;65(5):781–785. PubMed PMID: 28257699.
- Klionsky DJ, Baehrecke EH, Brumell JH, et al. A comprehensive glossary of autophagy-related molecules and processes (2nd edition). Autophagy. 2011 Nov;7(11):1273–1294. PubMed PMID: 21997368; PubMed Central PMCID: PMC3359482.
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004 Nov 5;306(5698):990–995. PubMed PMID: 15528435; PubMed Central PMCID: PMC1705980.
- Homma K, Suzuki K, Sugawara H. The Autophagy Database: an all-inclusive information resource on autophagy that provides nourishment for research. Nucleic Acids Res. 2011;39(Database issue):D986–D990. PubMed PMID: 20972215; PubMed Central PMCID: PMCPMC3013813.
- Moussay E, Kaoma T, Baginska J, et al. The acquisition of resistance to TNFα in breast cancer cells is associated with constitutive activation of autophagy as revealed by a transcriptome analysis using a custom microarray. Autophagy. 2011;7(7):760–770. PubMed PMID: 21490427.
- Türei D, Földvári-Nagy L, Fazekas D, et al. Autophagy Regulatory Network - a systems-level bioinformatics resource for studying the mechanism and regulation of autophagy. Autophagy. 2015;11(1):155–165. PubMed PMID: 25635527; PubMed Central PMCID: PMCPMC4502651.
- Fleming A, Noda T, Yoshimori T, et al. Chemical modulators of autophagy as biological probes and potential therapeutics. Nat Chem Biol. 2011 Jan;7(1):9–17. PubMed PMID: 21164513.
- Nanduri R, Bhutani I, Somavarapu AK, et al. ONRLDB–manually curated database of experimentally validated ligands for orphan nuclear receptors: insights into new drug discovery. Database (Oxford). 2015;2015:bav112. PubMed PMID: 26637529; PubMed Central PMCID: PMCPMC4669993.
- Wishart DS, Knox C, Guo AC, et al. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901–6. PubMed PMID: 18048412; PubMed Central PMCID: PMC2238889.
- Kanehisa M, Goto S, Sato Y, et al. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012 Jan;40(Database issue):D109–14. PubMed PMID: 22080510; PubMed Central PMCID: PMC3245020.
- Gaulton A, Bellis LJ, Bento AP, et al. ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012 Jan;40(Database issue):D1100–D1107. PubMed PMID: 21948594; PubMed Central PMCID: PMC3245175.
- Wang Y, Bolton E, Dracheva S, et al. An overview of the PubChem BioAssay resource. Nucleic Acids Res. 2010 Jan;38(Database issue):D255–D266. PubMed PMID: 19933261; PubMed Central PMCID: PMC2808922.
- Gros F, Muller S. Pharmacological regulators of autophagy and their link with modulators of lupus disease. Br J Pharmacol. 2014 Oct;171(19):4337–4359. PubMed PMID: 24902607; PubMed Central PMCID: PMC4209143.
- Nanduri R, Mahajan S, Bhagyaraj E, et al. The active form of Vitamin D transcriptionally represses Smad7 signaling and activates extracellular signal-regulated kinase (ERK) to inhibit the differentiation of a inflammatory T helper cell subset and suppress experimental autoimmune encephalomyelitis. J Biol Chem. 2015 May 8;290(19):12222–12236. PubMed PMID: 25809484; PubMed Central PMCID: PMC4424354.
- Mahajan S, Dkhar HK, Chandra V, et al. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARgamma and TR4 for survival. J Iimmunol. 2012 Jun 1;188(11):5593–5603. PubMed PMID: 22544925.
- Mahajan S, Saini A, Chandra V, et al. Nuclear receptor Nr4a2 promotes alternative polarization of macrophages and confers protection in sepsis. J Biol Chem. 2015 Jul 24;290(30):18304–18314. PubMed PMID: 25953901; PubMed Central PMCID: PMC4513091.
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012 Apr 13;149(2):274–293. PubMed PMID: 22500797; PubMed Central PMCID: PMC3331679.
- Foster JG, Blunt MD, Carter E, et al. Inhibition of PI3K signaling spurs new therapeutic opportunities in inflammatory/autoimmune diseases and hematological malignancies. Pharmacol Rev. 2012 Oct;64(4):1027–1054. PubMed PMID: 23023033.
- Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009 Jul;89(3):1025–1078. PubMed PMID: 19584320.
- Viollet B, Horman S, Leclerc J, et al. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010 Aug;45(4):276–295. PubMed PMID: 20522000; PubMed Central PMCID: PMC3132561.
- Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010 Apr;1802(4):396–405. PubMed PMID: 20079433.
- Konopleva M JMB KGH, Marzo I, Debose L, et al. Acute lymphoblastic leukemia is a Bcl-2 dependent disease: proteomic profiling and pre-clinical efficacy of a selective Bcl-2 antagonist ABT-199. Blood. 2013;122(21):3919.
- Sassone J, Maraschi A, Sassone F, et al. Defining the role of the Bcl-2 family proteins in Huntington’s disease. Cell Death Dis. 2013 Aug 15;4:e772. PubMed PMID: 23949221; PubMed Central PMCID: PMC3763461.
- Schofield AV, Bernard O. Rho-associated coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem Mol Biol. 2013 Jul-Aug;48(4):301–316. PubMed PMID: 23601011.
- Foote M, Zhou Y. 14-3-3 proteins in neurological disorders. Int J Biochem Mol Biol. 2012;3(2):152–164. PubMed PMID: 22773956; PubMed Central PMCID: PMC3388734.
- Henckaerts L, Cleynen I, Brinar M, et al. Genetic variation in the autophagy gene ULK1 and risk of Crohn’s disease. Inflamm Bowel Dis. 2011 Jun;17(6):1392–1397. PubMed PMID: 21560199.
- Jaber N, Dou Z, Chen JS, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A. 2012 Feb 7;109(6):2003–2008. PubMed PMID: 22308354; PubMed Central PMCID: PMC3277541.
- Salamon H, Bruiners N, Lakehal K, et al. Cutting edge: vitamin D regulates lipid metabolism in Mycobacterium tuberculosis infection. J Iimmunol. 2014 Jul 1;193(1):30–34. PubMed PMID: 24899504; PubMed Central PMCID: PMC4073889.
- Gonzalez-Parra E, Rojas-Rivera J, Tunon J, et al. Vitamin D receptor activation and cardiovascular disease. Nephrol Dialysis Trans. 2012 Dec;27 Suppl 4:iv17–21. PubMed PMID: 23258805.
- Huang JV, Greyson CR, Schwartz GG. PPAR-gamma as a therapeutic target in cardiovascular disease: evidence and uncertainty. J Lipid Res. 2012 Sep;53(9):1738–1754. PubMed PMID: 22685322; PubMed Central PMCID: PMC3413217.
- Altucci L, Leibowitz MD, Ogilvie KM, et al. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007 Oct;6(10):793–810. PubMed PMID: 17906642.
- Chandra V, Bhagyaraj E, Nanduri R, et al. NR1D1 ameliorates Mycobacterium tuberculosis clearance through regulation of autophagy. Autophagy. 2015 Nov 2;11(11):1987–1997. PubMed PMID: 26390081; PubMed Central PMCID: PMC4824569.
- Duez H, Staels B. Rev-erb-alpha: an integrator of circadian rhythms and metabolism. J Appl Physiol. 2009 Dec;107(6):1972–1980. PubMed PMID: 19696364; PubMed Central PMCID: PMC2966474.
- Goumidi L, Grechez A, Dumont J, et al. Impact of REV-ERB alpha gene polymorphisms on obesity phenotypes in adult and adolescent samples. Int J Obesity. 2013 May;37(5):666–672. PubMed PMID: 22828941.
- Matsumoto T, Sakari M, Okada M, et al. The androgen receptor in health and disease. Annu Rev Physiol. 2013;75:201–224. PubMed PMID: 23157556.
- Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci. 2013 Sep;34(9):518–530. PubMed PMID: 23953592; PubMed Central PMCID: PMC3951203.
- Lee SY, Lee SH, Yang EJ, et al. Metformin ameliorates inflammatory bowel disease by suppression of the STAT3 signaling pathway and regulation of the between Th17/Treg balance. PloS one. 2015;10(9):e0135858. PubMed PMID: 26360050; PubMed Central PMCID: PMC4567351.
- Flanagan SE, Haapaniemi E, Russell MA, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet. 2014 Aug;46(8):812–814. PubMed PMID: 25038750; PubMed Central PMCID: PMC4129488.
- Wang H, Lafdil F, Kong X, et al. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int J Biol Sci. 2011 Apr 27;7(5):536–550. PubMed PMID: 21552420; PubMed Central PMCID: PMC3088876.
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008 Jan 11;132(1):27–42. PubMed PMID: 18191218; PubMed Central PMCID: PMC2696814.
- Laddha SV, Ganesan S, Chan CS, et al. Mutational landscape of the essential autophagy gene BECN1 in human cancers. Mol Cancer Res. 2014;12(4):485–490. PubMed PMID: 24478461; PubMed Central PMCID: PMCPMC3989371.
- White E. The role for autophagy in cancer. J Clin Invest. 2015 Jan;125(1):42–46. PubMed PMID: 25654549; PubMed Central PMCID: PMC4382247.
- Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013 Aug;19(8):983–997. PubMed PMID: 23921753.
- Yang Z, Goronzy JJ, Weyand CM. Autophagy in autoimmune disease. J Mol Med. 2015 Jul;93(7):707–717. PubMed PMID: 26054920; PubMed Central PMCID: PMC4486076.
- Benjamin JL, Sumpter R Jr., Levine B, et al. Intestinal epithelial autophagy is essential for host defense against invasive bacteria. Cell Host Microbe. 2013 Jun 12;13(6):723–734. PubMed PMID: 23768496; PubMed Central PMCID: PMC3755484.
- Castillo EF, Dekonenko A, Arko-Mensah J, et al. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proc Natl Acad Sci U S A. 2012 Nov 13;109(46):E3168–76. PubMed PMID: 23093667; PubMed Central PMCID: PMC3503152.
- Gupta M, Shin DM, Ramakrishna L, et al. IRF8 directs stress-induced autophagy in macrophages and promotes clearance of Listeria monocytogenes. Nat Commun. 2015 Mar 16;6:6379. PubMed PMID: 25775030; PubMed Central PMCID: PMC4363081.
- Reddy AS, Zhang S. Polypharmacology: drug discovery for the future. Expert Rev Clin Pharmacol. 2013 Jan;6(1):41–47. PubMed PMID: 23272792; PubMed Central PMCID: PMC3809828.
- Chang C, Su H, Zhang D, et al. AMPK-dependent phosphorylation of GAPDH triggers Sirt1 activation and is necessary for autophagy upon glucose starvation. Mol Cell. 2015 Dec 17;60(6):930–940. PubMed PMID: 26626483.
- Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12(1):1–222. PubMed PMID: 26799652; PubMed Central PMCID: PMC4835977.
- Aryal P, Kim K, Park PH, et al. Baicalein induces autophagic cell death through AMPK/ULK1 activation and downregulation of mTORC1 complex components in human cancer cells. FEBS J. 2014 Oct;281(20):4644–4658. PubMed PMID: 25132405.
- Balgi AD, Fonseca BD, Donohue E, et al. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PloS one. 2009 Sep 22;4(9):e7124. PubMed PMID: 19771169; PubMed Central PMCID: PMC2742736.
- Kumar D, Shankar S, Srivastava RK. Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: molecular mechanisms. Mol Cancer. 2013 Dec 23;12(1):171. PubMed PMID: 24359639; PubMed Central PMCID: PMC3914415.
- Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006 Nov 16;444(7117):337–342. PubMed PMID: 17086191; PubMed Central PMCID: PMC4990206.
- Kotha A, Sekharam M, Cilenti L, et al. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther. 2006 Mar;5(3):621–629. PubMed PMID: 16546976.
- Chang YP, Ka SM, Hsu WH, et al. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J Cell Physiol. 2015 Jul;230(7):1567–1579. PubMed PMID: 25535911.
- Yu DD, Lin W, Chen T, et al. Development of time resolved fluorescence resonance energy transfer-based assay for FXR antagonist discovery. Bioorg Med Chem. 2013 Jul 15;21(14):4266–4278. PubMed PMID: 23688559; PubMed Central PMCID: PMC3691817.
- Kim DE, Kim Y, Cho DH, et al. Raloxifene induces autophagy-dependent cell death in breast cancer cells via the activation of AMP-activated protein kinase. Mol Cells. 2015;38(2):138–144. PubMed PMID: 25537862; PubMed Central PMCID: PMC4332026.
- Bhalla S, Evens AM, Prachand S, et al. Paradoxical regulation of hypoxia inducible factor-1alpha (HIF-1alpha) by histone deacetylase inhibitor in diffuse large B-cell lymphoma. PloS one. 2013;8(11):e81333. PubMed PMID: 24312289; PubMed Central PMCID: PMC3842257.
- Zhang Y, Zhao X, Chang Y, et al. Calcium channel blockers ameliorate iron overload-associated hepatic fibrosis by altering iron transport and stellate cell apoptosis. Toxicol Appl Pharmacol. 2016 Jun 15;301:50–60. PubMed PMID: 27095094.
- Yu HC, Lin CS, Tai WT, et al. Nilotinib induces autophagy in hepatocellular carcinoma through AMPK activation. J Biol Chem. 2013 Jun 21;288(25):18249–18259. PubMed PMID: 23677989; PubMed Central PMCID: PMC3689967.
- Jabbour E, El Ahdab S, Cortes J, et al. Nilotinib: a novel Bcr-Abl tyrosine kinase inhibitor for the treatment of leukemias. Expert Opin Investig Drugs. 2008 Jul;17(7):1127–1136. PubMed PMID: 18549348.
- Ren X, Duan L, He Q, et al. Identification of niclosamide as a new small-molecule inhibitor of the STAT3 signaling pathway. ACS Med Chem Lett. 2010 Dec 9;1(9):454–459. PubMed PMID: 24900231; PubMed Central PMCID: PMC4007964.
- Carrillo AK, Guiguemde WA, Guy RK. Evaluation of histone deacetylase inhibitors (HDACi) as therapeutic leads for human African trypanosomiasis (HAT). Bioorg Med Chem. 2015 Aug 15;23(16):5151–5155. PubMed PMID: 25637120.
- Wang B, Wang XB, Chen LY, et al. Belinostat-induced apoptosis and growth inhibition in pancreatic cancer cells involve activation of TAK1-AMPK signaling axis. Biochem Biophys Res Commun. 2013 Jul 19;437(1):1–6. PubMed PMID: 23743198.
- Zhang CM, Gao L, Zheng YJ, et al. Berbamine protects the heart from ischemia/reperfusion injury by maintaining cytosolic Ca(2+) homeostasis and preventing calpain activation. Circ J. 2012;76(8):1993–2002. PubMed PMID: 22664727.
- Chadalapaka G, Jutooru I, Burghardt R, et al. Drugs that target specificity proteins downregulate epidermal growth factor receptor in bladder cancer cells. Mol Cancer Res. 2010 May;8(5):739–750. PubMed PMID: 20407012; PubMed Central PMCID: PMC2872686.
- Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Iimmunol. 2003 Oct 1;171(7):3863–3871. PubMed PMID: 14500688.
- Shin DS, Kim HN, Shin KD, et al. Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res. 2009 Jan 1;69(1):193–202. PubMed PMID: 19118003.
- Park IJ, Yang WK, Nam SH, et al. Cryptotanshinone induces G1 cell cycle arrest and autophagic cell death by activating the AMP-activated protein kinase signal pathway in HepG2 hepatoma. Apoptosis. 2014 Apr;19(4):615–628. PubMed PMID: 24173372.
- Hollis A, Sperl B, Graber M, et al. The natural product betulinic acid inhibits C/EBP family transcription factors. Chembiochem Eur J Chem Biol. 2012 Jan 23;13(2):302–307. PubMed PMID: 22213238.
- Cheng Y, Ren X, Hait WN, et al. Therapeutic targeting of autophagy in disease: biology and pharmacology. Pharmacol Rev. 2013;65(4):1162–1197. PubMed PMID: 23943849; PubMed Central PMCID: PMC3799234.
- Hu M, Huang H, Zhao R, et al. AZD8055 induces cell death associated with autophagy and activation of AMPK in hepatocellular carcinoma. Oncol Rep. 2014 Feb;31(2):649–656. PubMed PMID: 24297300.
- Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol. 2014 Jun;16(6):495–501. PubMed PMID: 24875736.
- Backman TW, Cao Y, Girke T. ChemMine tools: an online service for analyzing and clustering small molecules. Nucleic Acids Res. 2011 Jul;39(Web Server issue):W486–91. PubMed PMID: 21576229; PubMed Central PMCID: PMC3125754.