ABSTRACT
SLC3A2/CD98hc (solute carrier family 3 member 2) and its light chain subunits constitute the heterodimeric transmembrane complexes that mediate amino acid transport and regulate MTOR and macroautophagy/autophagy. Despite the proven tumorigenic role of SLC3A2 in a number of cancers including head and neck squamous cell carcinomas (HNSCC), the link between SLC3A2, autophagy regulation and tumor radioresistance remained elusive. In a recently published study we demonstrated that low levels of SLC3A2 and SLC7A5/LAT1 protein expression significantly correlate with good clinical prognosis in locally advanced HNSCC treated with primary radiochemotherapy. The SLC3A2-deficient HNSCC cells show a higher radiosensitivity and increased autophagy levels. We found that autophagy activation is a tumor survival strategy to overcome nutrient stress by lack of SLC3A2 and to withstand radiation-mediated cell damage. Inhibition of the autophagy activation in SLC3A2 knockout HNSCC cells by knockdown of ATG5 expression or treatment with bafilomycin A1 results in radiosensitivity. Consequently, the expression levels of ATG5 correlates with overall survival in HNSCC patients, and autophagy inhibition in combination with SLC3A2-targeted therapy can be a promising strategy for HNSCC radiosensitization.
Abbreviations
CD98hc: CD98 heavy chain CSC cancer stem cells; EAA: essential amino acids; GSH: glutathione; MTOR: mammalian target of rapamycin; HNSCC: head and neck squamous cell carcinoma; RCTx: primary radiochemotherapy; PORT-C: postoperative radiochemotherapy; ROS: reactive oxygen species; SLC3A2: solute carrier family 3 member 2; TCA cycle: tricarboxylic acid cycle.
Primary radiochemotherapy (RCTx) and postoperative radiochemotherapy (PORT-C) are conventional treatment for patients with locally advanced head and neck squamous cell carcinomas (HNSCC). Based on the results shown in the large population-based clinical trials, patients with locally advanced HNSCC show a heterogeneous response to the current state-of-the-art radiochemotherapy. In the last decades, the human papilloma virus (HPV) status has been identified as a prognostic parameter for patients with HNSCC. To further adapt the radiotherapy on the patients’ individual tumor biology, the development of new biomarkers is needed. From preclinical studies it is known that the eradication or at least inactivation of all cancer stem cells (CSC) is of paramount importance for the achievement of permanent tumor control after curatively intended radiotherapy. Tumor radiocurability depends on the density of CSCs within the irradiated target volume, but also on the intrinsic mechanisms of CSC radioresistance such as activation of the DNA repair pathways and pro-survival mechanisms among which autophagy might play a critical role.
SLC3A2 is a chaperone for light chain subunits such as SLC7A5 and SLC7A11/xCT by regulating their recruitment to the plasma membrane (). By regulation of amino acid transport, SLC3A2 and SLC7A5 play an important role in tumor growth and controlling oxidative stress. Owing to these functions, an overexpression of SLC3A2 and SLC7A11 was associated with the development and progression of various types of cancer. The preclinical evidence that SLC3A2 is engaged in the maintenance of HNSCC stem cells has motivated analysis of its prognostic relevance for this type of malignancy. Previous studies indicated a potential role for SLC3A2 as a prognostic marker for patients with oropharyngeal tumors. Later, the correlation of SLC3A2 mRNA expression levels with locoregional control after radiochemotherapy has been analyzed within the retrospective, multicentric clinical studies of the German Cancer Consortium – Radiation Oncology Group (DKTK-ROG). It was shown that increased SLC3A2 expression is significantly associated with poor loco-regional control after RCTx and PORT-C in locally advanced HNSCC, and may be also prognostic for a subgroup of patients with HPV-negative HNSCC. Taken together, this makes a SLC3A2 a promising prognostic biomarker for patients with locally advanced HNSCC and a target for potential radiosensitization.
Figure 1. The role of SLC3A2 in regulation of autophagy activation and cell radiosensitivity. (A) In HNSCC cells, SLC3A2 and its light chain subunit SLC7A5 play an important role in transport of essential amino acids (EAA) such as histidine, isoleucine, leucine, methionine, valine, tryptophan, phenylalanine and threonine as well as conditionally essential ones such as cysteine and tyrosine. This amino acid transport is important for biosynthesis, energy production in the tricarboxylic acid cycle (TCA cycle), and activation of the MTOR signaling pathway. SLC3A2 is also associated with the cystine (CSSC) transporter SLC7A11 which is critical for synthesis of glutathione, one of the major scavengers of reactive oxygen species (ROS). (B) Blocking of the SLC3A2-dependent amino acid transport inhibits the MTOR pathway, downregulates the TCA cycle and restrains tumor cell proliferation. This results in the activation of autophagy as a pro-survival mechanism.
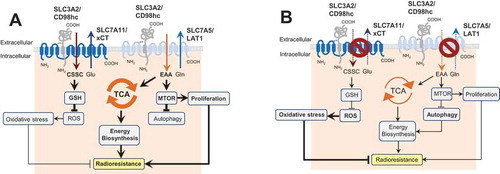
In our recent study [Citation1] we demonstrated that SLC3A2 and SLC7A5 are colocalized, and expression levels of these proteins are significantly coregulated in cancer cell lines and in HNSCC tumor tissues. Similar to our previous study, which showed prognostic value of SLC3A2 mRNA levels for HNSCC, our recent findings demonstrate that low levels of SLC3A2 and SLC7A5 protein expression also serve as markers of good clinical prognosis in locally advanced HNSCC treated with RCTx. This pivotal role of the SLC3A2-SLC7A5 transporter in HNSCC progression and therapy resistance can be explained by the high bioenergetics demands of the rapidly growing HNSCC tumors. This was confirmed in experiments with SLC3A2 knockout cells, which exhibited slow proliferation, low basal oxygen consumption rate and downregulation of Krebs cycle intermediates. SLC3A2 is also associated with cystine transporter SLC7A11, which is critical for synthesis of glutathione (GSH), one of the major scavengers of reactive oxygen species (ROS). Consequently, knockout of SLC3A2 expression is also associated with decreased level of reduced GSH and induction of oxidative stress (). Downregulation of SLC3A2 and SLC7A5 expression via knockdown or knockout results in the inhibition of the MTOR signaling pathway and induction of autophagy, as was confirmed by global gene expression profiling, fluorescence cytometry and analysis of LC3 conversion. Of importance, treatment of HNSCC cells with the PI3K-MTOR inhibitor BEZ235 also results in activation of autophagy as a pro-survival mechanism of cellular stress response. One of the key autophagy regulators, ATG5, is significantly upregulated in radioresistant HNSCC cell lines as compared to their isogenic parental cells, and its low expression levels significantly correlate with better overall survival in HNSCC patients. Consistent with these data, knockdown of ATG5 expression or treatment with bafilomycin A1 inhibits activation of autophagy in response to SLC3A2 downregulation, and results in significant HNSCC radiosensitization. Of note, bafilomycin A1 and chloroquine more potently inhibit the viability of SLC3A2 knockout cells, which are also more sensitive to bafilomycin A1-induced apoptosis than wild-type counterparts. Taken together, our study suggests that SLC3A2-deficient HNSCC cells activate autophagy as a pro-survival mechanism. Combination of the SLC3A2-targeted treatment with autophagy inhibition can be a potential strategy for HNSCC radiosensitization.
The role of autophagy as a modulator of tumor radioresistance is still debatable as it serves as both a therapy sensitizing and pro-survival mechanism depending on tumor type and stage of malignancy. Nevertheless, the vast majority of current clinical studies are focused on autophagy inhibition. Furthermore, early stage clinical studies are paving the way to bring forward autophagy inhibition as a radiosensitizing treatment to patients. This for example includes clinical trials in glioblastoma multiforme combining the conventional treatment (concurrent radiochemotherapy with temozolomide) with autophagy inhibition by chloroquine (NCT02378532, NCT02432417). At the same time a humanized anti-SLC3A2 antibody is being examined in a phase I clinical trial in patients with leukemia (NCT02040506).
Future studies are warranted to validate our findings by a combination of radiotherapy with autophagy inhibition alone or with anti-SLC3A2 treatment in xenograft mouse models and by validation of autophagy regulators such as ATG5 as potential prognosticators of clinical outcome in HNSCC after radiotherapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Reference
- Digomann D, Kurth I, Tyutyunnykova A, et al. The CD98 heavy chain is a marker and regulator of head and neck squamous cell carcinoma radiosensitivity. Clin Cancer Res. 2019;25(10):3152–3163.
