ABSTRACT
In Alzheimer disease patients, MAPT/tau pathology and granulovacuolar degeneration bodies (GVBs) co-occur in the same brain regions and in the same cells. The interdependence of these neuropathological hallmarks and the identity of GVBs have long been elusive. Recently, we showed that MAPT pathology causes GVB formation in neurons in vivo and in vitro. Using these novel GVB models, we identified GVBs as lysosomal structures at the convergence of the endo- and autolysosomal pathways. Here, the possible functional consequences of neuronal GVB formation are discussed.
Alzheimer disease (AD) belongs to a class of neurodegenerative disorders that are collectively called tauopathies, as they are characterized by the intracellular aggregation of the microtubule-binding protein MAPT/tau. The extent of MAPT pathology closely correlates with neurodegeneration and thereby clinical symptoms. Yet, how MAPT pathology leads to neurodegeneration is unknown.
In this respect, the presence of granulovacuolar degeneration bodies (GVBs) in neurons with MAPT pathology could provide important clues. GVBs were first described more than a century ago and are defined as membrane-delineated vacuoles that contain a dense core. Postmortem studies have suggested that GVBs are aberrant autophagic organelles, but further experimental evidence to support this is lacking. In the human brain, the amount of neurons with MAPT pathology and GVBs strongly correlate. Zooming in to the cellular level, GVBs are mainly detected in neurons with early-stage MAPT pathology. This suggests that GVB formation is an early event in the disease cascade. Despite this potential involvement in the pathogenesis of tauopathies, GVBs have been relatively underinvestigated. Thus far, further insight into GVB biology has been hampered by the lack of suitable experimental methods to induce GVBs.
In our recent publication [Citation1], we present novel experimental models for GVB formation in mouse brain and cultured primary mouse neurons. GVBs form after the introduction of intracellular MAPT pathology using exposure to human brain-derived or recombinant MAPT aggregates. These experimentally-induced GVBs are positive for established human GVB markers (e.g., CSNK1D/CK1δ, CSNK1E/CK1ε, p-EIF2AK3/PERK, p-EIF2A/eIF2α, p-ERN1/IRE1α and CHMP2B). Mechanistically, we showed that the intracellular aggregation of MAPT, rather than the exposure to extracellular MAPT, leads to the formation of GVBs. Hence, GVBs develop in response to intraneuronal MAPT pathology (). This is an important conclusion, because up to now it was unclear whether these 2 pathological lesions were causally related.
Figure 1. GVBs: a decision point in neuronal fate upon proteostatic stress?
(A) Characterization of GVBs based on the results from the novel models presented in our study. Both endo- and autolysosomal pathways contribute to the content of GVBs. Membrane and proteolytic activity markers characteristic of lysosomal structures are present. (B) Schematic model showing that intraneuronal MAPT pathology precedes and causes GVB formation. (C) Hypothetical model of the role of GVBs in tauopathies. GVBs may either be protective and counteract MAPT pathology (“protective”) or represent failure to cope with MAPT pathology and initiate neurodegeneration (“degenerative”). See text for further detail; black lines, MAPT pathology; yellow circles with dark orange core, GVBs; dashed neuron, neurodegeneration.
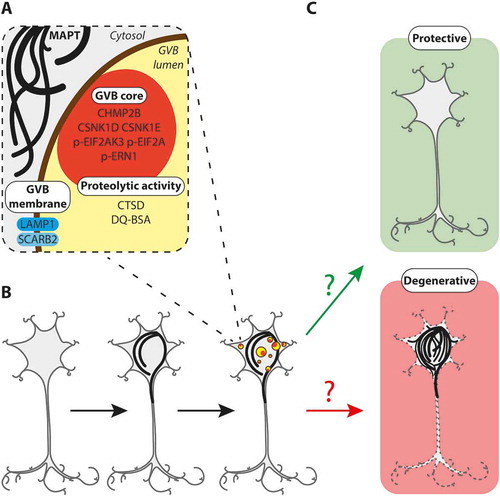
To investigate the identity of GVBs, we analyzed the presence of prototypical markers of organelles in the endo- and autolysosomal pathways, that ultimately deliver extra- and intracellular cargo to lysosomes for degradation. In vitro MAPT-induced GVBs are negative for the early endosome marker EEA1, but positive for the late endosome marker CHMP2B that also recognizes GVBs in the human brain. Furthermore, GVBs lack immunoreactivity for the early macroautophagy/autophagy marker LC3 that localizes to phagophores and autophagosomes. In line with the absence of LC3 immunoreactivity, electron microscopy analysis of the GVBs reveals a single limiting membrane. The GVB membrane is positive for the lysosomal transmembrane proteins LAMP1 and SCARB2/LIMP2. Furthermore, the lysosomal protease CTSD and the endocytosed proteolysis reporter DQ-BSA are found in the majority of GVBs, thus providing the first evidence that GVBs exhibit degradative capacity and are connected to the endolysosomal flux. In addition to endosomal content, GVBs contain autophagic cargo, as shown by their immunoreactivity for the intracellular proteins CSNK1D, CSNK1E, p-EIF2AK3, p-EIF2A and p-ERN1. In conclusion, GVBs are lysosomal organelles that can be distinguished from physiological lysosomes by the accumulation of endosomal and autophagic cargo, despite being proteolytically competent ().
Interestingly, we showed that overexpressed GFP-tagged CSNK1D, but not GFP alone, accumulates in GVBs. The reason for the selective targeting of CSNK1D to GVBs remains to be discovered. Removal of the MAPT kinase CSNK1D could be related to a mechanism to prevent the development of further MAPT pathology. Alternatively, CSNK1D could be part of the functional machinery involved at some step in the process leading to GVB formation. This is supported by studies that functionally implicate the CSNK1D yeast homolog Hrr25 in selective autophagy via phosphorylation of different autophagy receptors, but it is unknown whether this function is conserved in mammalian CSNK1D.
In the human brain, both MAPT pathology and GVBs predominantly occur in neurons. In line with this, we demonstrated that hippocampal, cortical and striatal neurons all form GVBs in culture. In contrast, neither primary mouse glia cells nor HEK293 cells develop GVBs when challenged with intracellular MAPT pathology. Therefore, the neuron-selective occurrence of GVBs is caused by a cell type-specific response to MAPT pathology and not by different susceptibility of neurons and glia to MAPT pathology per se. Interestingly, GVBs are also observed in brains of patients with neurodegenerative diseases characterized by intracellular protein inclusions composed of proteins other than MAPT, namely SNCA/α-synuclein and dipeptide repeat proteins. Therefore, it is conceivable that GVB formation is a common neuronal response to intracellular protein aggregation. In contrast to glia cells that retain proliferative capacity, post-mitotic neurons heavily rely on the lysosomal system to handle the severe proteostatic disturbance imposed by these pathologies. GVB formation appears to reflect a situation of stress in the lysosomal system. Possibly, GVBs are specifically induced as part of a protective lysosomal stress response aimed to restore proteostasis by increasing the neuron’s degradative capacity or by removing specific proteins from the cytosol. The dense proteinaceous core of GVBs could indicate failure of this lysosomal stress response to cope with the persisting proteostatic insult.
The observation that GVBs in the human brain are most abundant in neurons with early-stage MAPT pathology could suggest that GVB-containing neurons are more resilient to MAPT pathology. Alternatively, the formation of GVBs may indicate a transition toward full-blown MAPT pathology and neurodegeneration. In either case, the appearance of GVBs may reflect the ultimate fate of the neuron under proteostatic stress (). Hence, further insight into the functional consequences of GVB formation will contribute to the understanding of the pathogenesis of tauopathies and other neurodegenerative proteinopathies. This urges in-depth investigation of this long overlooked neuropathological hallmark, for which the experimental models that we have established open new avenues.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Reference
- Wiersma VI, van Ziel AM, Vazquez-Sanchez S, et al. Granulovacuolar degeneration bodies are neuron‑selective lysosomal structures induced by intracellular tau pathology. Acta Neuropathol [Internet]. 2019. doi:10.1007/s00401-019-02046-4.
