ABSTRACT
The modular complex TRAPP acts as an activator of a subgroup of Ypt/RAB GTPases. The substrate GTPases and TRAPP are conserved from yeast to human cells, required for secretion and macroautophagy/autophagy and implicated in human disease. All TRAPP complexes contain four core subunits essential for cell viability, and until recently there were no human diseases associated with any core TRAPP subunit. Recently, we reported a neurological disorder associated with a pathogenic variant of the core TRAPP subunit TRAPPC4. This variant results in lower levels of full-length TRAPPC4 protein and the TRAPP complex. A conditional mutation of the yeast homolog of TRAPPC4, Trs23, also results in a lower level of the protein and the TRAPP complex. Phenotypic analysis of the yeast mutant cells reveals a minor defect in secretion and a major defect in autophagy. Similarly, primary fibroblasts derived from human patients also exhibit minor and severe defects in secretion and autophagy, respectively. We propose that the autophagy defect caused by the pathogenic-TRAPPC4 variant results in the severe neurological disorder. Moreover, we hypothesize that low levels of the core TRAPP complex are more detrimental to autophagy than to secretion, and that the long-term autophagy defect is especially harmful to neuronal cells.
Abbreviations: ER: endoplasmic reticulum; PM: plasma membrane; TRAPP: transport protein particle; Ypt: yeast protein transport
Identifying a human TRAPPC4 variant
Family-based rare variant analysis using exome sequencing has become a powerful approach for identifying “orphan” genetic variants of undiagnosed diseases. Using this approach for patients with a severe neurological disorder, a recessive pathogenic-TRAPPC4 variant was identified in three unrelated families [Citation1]. Interestingly, all patients have an identical pathogenic variant in an intron-exon junction, resulting in an incompletely penetrant splicing defect. This defect results in a reduction in the wild-type transcript and the full-length TRAPPC4 protein (with no truncated protein evident by immuno-blot analysis).
The TRAPP complex
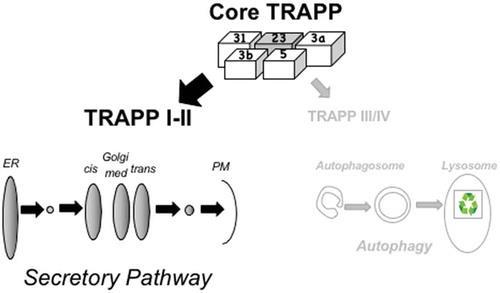
TRAPPC4 is one of the core TRAPP subunits. Deletion of the genes each of these subunits, including TRAPPC4, is lethal. All four subunits are conserved from yeast to human cells and are essential for yeast cell viability as well (, top). Core TRAPP is shared by a number of TRAPP complexes that contain additional complex-specific subunits. TRAPP complexes act as activators of a subgroup of Ypt/RAB GTPases also conserved from yeast to human cells: Ypt1/RAB1 and Ypt31/RAB11. These Ypt/RABs are essential for the secretory pathway, and Ypt1/RAB1 is also essential for the onset of autophagy. Both these GTPases and their TRAPP activators are implicated in multiple human diseases ranging from cancer to neurodegenerative disorders. However, until now, no human disease was associated with subunits of core TRAPP. One reason might be because complete depletion of any of these subunits is lethal. It is likely that the incompletely penetrant phenotype of the TRAPPC4 splicing variants identified here rendered viable progeny, but the reduction in the level of the full-length protein caused severe neurological problems.
Figure 1. A model explaining the reason for a severe autophagy defect in Trs23/TRAPPC4 mutant cells. Top: molecular architecture of the yeast core TRAPP (TRAPP I), which contains four subunits (3a and 3b, two copies of Bet3; 5, Bet5; 31, Trs31; 23, Trs23). TRAPP II–IV complexes contain core TRAPP and additional complex-specific subunits. Mutant Trs23/TRAPPC4 result in a low level of this core TRAPP subunit and the core TRAPP complex. Because the secretory pathway (left) is important for cell viability under normal growth conditions, we propose that most of the available core TRAPP is shuttled to this process by assembling TRAPP complexes required for secretion (i.e., TRAPP I–II). Consequently, less core TRAPP is available for assembly of TRAPP complexes needed for autophagy (right; i.e., TRAPP III–IV). This, in turn, results in a severe autophagy phenotype. ER, endoplasmic reticulum; PM, plasma membrane.
Yeast modeling
The yeast homolog of TRAPPC4 is Trs23, with approximately 45% identity at the level of amino acid sequence (excluding a PDZL domain present only in the human protein). Moreover, the 3D structures of the human and the yeast proteins are almost identical. Thus, we wanted to test the effect of limiting the Trs23 protein level in yeast cells. However, the exact TRAPPC4 variant could not be mimicked because the yeast genome contains very few introns, and TRS23 does not have one. Therefore, to model the effect of partial depletion of the Trs23 subunit we used a different approach. When testing a temperature-sensitive trs23 mutation (trs23ts), we observed that the yeast cells have a very low level of the Trs23 protein, like the patient fibroblasts with the pathogenic-TRAPPC4 variant. We showed that a lower level of this core subunit results in a lower level of the core TRAPP complex. We then tested the effect of this mutation on secretion and autophagy. Interestingly, secretion is not defective at the permissive temperature, but a secretory defect is observed only when the cells are grown at their restrictive temperature. Conversely, trs23ts mutant cells are defective in both constitutive and stress-induced autophagy even at the permissive temperature. Autophagy is a cellular recycling pathway required mostly under stress but also for clearance of excess or damaged cellular components, and harmful protein aggregates during normal growth.
Extension to human disease
Analyses of primary fibroblasts from a patient homozygous for the pathogenic-TRAPPC4 variant shows results similar to those of the yeast mutation: lower level of the core TRAPP complex, a minor defect in secretion (slower rate), and a severe defect in starvation-induced autophagy (block). Hence, in both yeast and human cells, reduction of core TRAPP complex levels result in a minor defect in secretion, which is required for cell viability and growth, and a severe defect in autophagy. We propose that the new TRAPPC4 variant causes the severe neurological disorder primarily due to the autophagy defect.
Why the severe autophagy defect?
We interpret the fact that partial depletion of core TRAPP in yeast and human mutant cells affects autophagy before it affects secretion as a hint that this complex is recruited preferentially to drive secretion, a pathway required for cell survival. While autophagy functions also during cell growth, it becomes essential only under stress and, therefore, when limited amounts of core TRAPP are available, less of it may be sent to drive this process. We propose that the mechanism by which core TRAPP coordinates secretion and autophagy is preferential assembly of TRAPP complexes that mediate secretion over those that mediate autophagy (). Further research is needed to test this idea and to determine the type of autophagy affected: constitutive or stress-induced.
Why the neurological effect?
Neuronal cells have the longest life span of all body cells, resulting in their higher reliance on autophagy than other cells for clearance of excess, damaged or harmful cellular components. Thus, while defects in secretion might result in severe growth defects of all cells, defects in autophagy might manifest themselves more readily in neurons. This rationale is probably relevant to other neurological disorders and not only to the TRAPPC4 orphan disease discussed here.
Disclosure statement
The authors declare no conflict of interests
Additional information
Funding
Reference
- Van Bergen NJ, Guo Y, Al-Deri N, et al. Deficiencies in vesicular transport mediated by TRAPPC4 are associated with severe syndromic intellectual disability. Brain. 2020;143:112–130. PMID: 31794024. .
