ABSTRACT
Macroautophagy/autophagy functions to degrade cellular components and intracellular pathogens. Autophagy receptors, including SQSTM1/p62, target intracellular pathogens. Staphylococcus aureus is a significant pathogen of humans, especially in immunocompromise. S. aureus may use neutrophils as a proliferative niche, but their intracellular fate following phagocytosis has not been analyzed in vivo. In vitro, SQSTM1 can colocalize with intracellular Staphylococcus aureus, but whether SQSTM1 is beneficial or detrimental in host defense against S. aureus in vivo is unknown. Here we determine the fate and location of S. aureus within neutrophils throughout zebrafish infection. We show Lc3 and Sqstm1 recruitment to phagocytosed S. aureus is altered depending on the bacterial location within the neutrophil and that Lc3 marking of bacterial phagosomes within neutrophils may precede bacterial degradation. Finally, we show Sqstm1 is important for controlling cytosolic bacteria, demonstrating for the first time a key role of Sqstm1 in autophagic control of S. aureus in neutrophils.
Abbreviations: AR: autophagy receptor; CFU: colony-forming unit; CHT: caudal hematopoietic tissue; GFP: green fluorescent protein; hpf: hours post-fertilization; hpi: hours post-infection; LWT: london wild-type: lyz: lysozyme; Map1lc3/Lc3: microtubule-associated protein 1 light chain 3; RFP: red fluorescent protein; Sqstm1/p62: sequestosome 1; Tg: transgenic; TSA: tyramide signal amplification; UBD: ubiquitin binding domain.
Introduction
Autophagy (macroautophagy) is a process of cellular self-degradation, in which damaged or redundant cellular components are taken into an autophagosome and subsequently trafficked to the lysosome for degradation; these degraded components can then be recycled for alternative uses by the cell [Citation1,Citation2]. During infection, autophagy is used by host cells to degrade invading pathogens, a process termed xenophagy [Citation3,Citation4].
Autophagy is considered largely nonselective of the cargo to be degraded, classically being induced by starvation conditions. However, selective autophagy is a process that enables specific cargo to be directed into the autophagy pathway, which can be used to target invading pathogens. Selective autophagy uses autophagy receptors (ARs), proteins that interact with both autophagy machinery and the cargo to be degraded [Citation5,Citation6]. Many ARs are involved in targeting invading pathogens, including SQSTM1/p62 (sequestosome 1), NBR1 (NBR1 autophagy cargo receptor), OPTN (optineurin) and CALCOCO2/NDP52 (calcium binding and coiled-coil domain 2) [Citation7].
Loss of autophagy function, for example, through mutations in key autophagy genes, can increase the risk of infection with intracellular pathogens [Citation8]. It is well established that pathogen presence can induce host cell autophagy and that pathogens can be degraded by this pathway. Intracellular pathogens such as Mycobacterium marinum, Shigella flexneri and Listeria monocytogenes [Citation9,Citation10] can be targeted by ARs for degradation. Conversely, pathogens have evolved to be able to block or subvert immune defenses, and autophagy is no exception. Indeed, many bacterial pathogens are able to inhibit the induction of autophagy or to reside within the autophagy pathway by preventing lysosomal fusion, or even avoid making any contact with autophagic machinery [Citation11]. In some cases, it is beneficial to the pathogen to up-regulate the autophagy pathway, for example, Legionella pneumophila, Coxiella burnetii and Salmonella enterica serovar Typhimurium [Citation12–14]. The outcome of host-cell autophagy, therefore, differs between various invading pathogens.
Staphylococcus aureus is a bacterial pathogen that can reside within neutrophils as an intracellular niche [Citation15,Citation16]. Autophagy has been implicated in S. aureus infection, but there are conflicting reports suggesting autophagy might be either beneficial [Citation17] or detrimental for S. aureus [Citation18]. Intracellular pathogens, including S. aureus, can escape the phagosome into the cytosol [Citation19], likely through toxins secreted by the bacteria or membrane rupture due to bacterial growth. Once in the cytosol, bacteria can be ubiquitinated and targeted by ARs [Citation7]. Indeed, Sqstm1 in fibroblasts and epithelial cells has been shown to localize to cytosolic S. aureus leading to autophagosome formation in vitro [Citation18]. Therefore, we investigated whether Sqstm1 recruitment is employed by neutrophils in S. aureus infection and what influence selective autophagy has on infection outcome in vivo.
In order to examine the role of neutrophil autophagy in S. aureus infection, we compared the fate of bacterial cells following Map1lc3/Lc3 (microtubule-associated protein 1 light chain 3) and Sqstm1 recruitment. We tested the role of Sqstm1 in pathogen handling in vivo, using the genetic tractability of the zebrafish to create a neutrophil-specific Sqstm1-GFP transgenic reporter and an sqstm1 activity-deficient mutant. With this approach, we show that Sqstm1 is recruited to cytosolic S. aureus and disruption of Sqstm1 expression or function adversely affects S. aureus infection outcome.
Results
Staphylococcus aureus location within neutrophils changes throughout infection
Autophagy responses have been demonstrated to change throughout the progression of the infection. Targeting of pathogens by autophagy receptors is likely to occur at later time points in infection. Therefore, to determine the fate and location of S. aureus in neutrophils during infection, S. aureus expressing mCherry was inoculated and imaged at early (2 to 5 h post-infection [hpi]) and late (24 to 28 hpi) time points. Initially, the well-established Tg(mpx:eGFP)i114 line that specifically marks neutrophils with eGFP [Citation20] was used to analyze the fate of intracellular S. aureus throughout infection. Imaging throughout whole organisms demonstrated a marked reduction in the number of bacterial cells within individual neutrophils, and that the number of neutrophils containing S. aureus, between 2 and 24 h post-infection (). This result suggested to us that neutrophils could degrade intracellular S. aureus effectively throughout infection. Indeed, video timelapse of Tg(mpx:eGFP)i114 larvae infected with mCherry S. aureus demonstrated that bacteria could be effectively degraded by the host neutrophils (), although in other cases the bacterial infection is not controlled (Fig. S1A).
Figure 1. Staphylococcus aureus location within neutrophils changes from vesicular to cytosolic throughout infection. (A-B) Tg(mpx:eGFP)i114 larvae were injected at 1 dpf with 1500 cfu SH1000 mCherry S. aureus, and imaged at early (1–5 hpi) and late (24–28 hpi) time points. (A) Number of bacteria contained in neutrophils, with maximum 100 bacterial cells counted (whole larvae imaged, n = 11–13, Mann-Whitney test, ****p < 0.0001, ± SD). (B) Proportion of neutrophils containing bacteria (whole larvae imaged, n = 11–12, unpaired t-test, ****p < 0.0001, ± SEM) (C) Tg(mpx:eGFP)i114 larvae were injected at 1 dpf with 1500 cfu SH1000 mCherry S. aureus, and imaged at 3 h post-infection. Images were captured every 5 min for 12 h at multiple z planes to follow infected neutrophils over time (scale: 5 µm). (D-G) Tg(lyz:RFP-GFP-lc3)sh383 larvae were injected at 2 dpf with GFP S. aureus, and imaged in the CHT at 2 hpi, and ~26 hpi. (D) The proportion of infected or non-infected neutrophils at 2 hpi and 26 hpi (****p < 0.0001 Chi-Square test, n = 3, 17 2 hpi larvae, 11 26 hpi larvae). (E) S. aureus with Lc3 marking the entire vesicle (scale: 9 µm), demonstrating a vesicle. (F) S. aureus in the cytosol (scale: 9 µm). (G) Proportion S. aureus events observed within vesicles or cytosol at 2 hpi and 26 hpi (***p < 0.001, Fisher’s exact test, n = 3, 17 larvae at 2 hpi, and 11 larvae at 26 hpi)
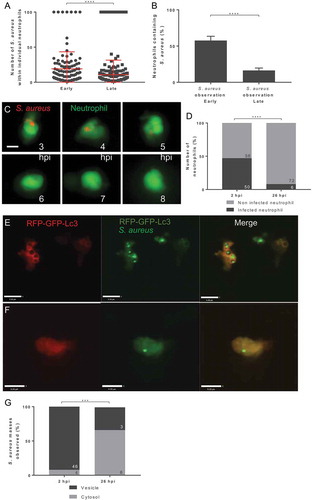
We next sought to determine the location of bacteria and their association with the autophagic machinery within neutrophils. To do this, we used fluorescently tagged Lc3, as has been demonstrated previously in zebrafish and other models [Citation21–23]. We used a newly generated Tg(lyz:RFP-GFP-lc3)sh383 [Citation23], a double fusion of RFP and GFP, both linked to Lc3, allowing visualization of Lc3 within neutrophils. We first confirmed that in the caudal hematopoietic tissue (CHT), the infection dynamics were similar to the Tg(mpx:eGFP)i114 line, with a significant reduction in intracellular bacteria by 26 hpi, indicating bacteria are efficiently controlled and a significant reduction in infected neutrophils was observed (). Importantly, the number of neutrophils counted in the CHT, used for analyzes throughout this study, did not significantly change between 2 dpf and 3 dpf (Fig. S1B), demonstrating that the change in proportions of infected neutrophils is not due to a large increase in neutrophil number between these time points. The labeling of S. aureus-containing vesicles enabled the identification of intracellular bacteria that were within a vesicle () or free in the cytosol (), as well as non-labeled vesicles, or vesicles marked with Lc3 puncta (Fig. S1C and S1D). We found that the proportion of bacteria within vesicles was significantly reduced over time post-injection, whereas the number of bacteria within the cytosol remains relatively constant at a low level, despite becoming proportionally higher relative to vesicular bacteria (). Thus, S. aureus phagocytosed by a neutrophil are initially located in a phagocytic vesicle and are subsequently degraded. However, a smaller proportion of S. aureus could survive to later infection time points, and these predominantly resided in the cytosol.
Generation and characterization of an in vivo neutrophil GFP-Sqstm1 reporter line
a previous study identified the co-localization of Sqstm1 with S. aureus in nonimmune cells [Citation18]. Our findings demonstrated a small but significant population of bacteria that were cytosolic, and therefore a possible target for Sqstm1 binding. Accordingly, we generated a transgenic neutrophil-specific Sqstm1 reporter zebrafish line to examine whether Sqstm1 and intracellular pathogens are co-localized in vivo. We used GFP fused via a small linker region to the N-terminus of sqstm1 in order to produce a fluorescently marked fusion protein expressed within neutrophils via the lyz (lysozyme) promoter [Citation24]. Using larvae with double-labeled neutrophils, we were able to identify GFP-expressing cells from the Tg(lyz:eGFP-sqstm1)i330 reporter line (hereafter called GFP-Sqstm1 reporter) also expressing mCherry (Tg[lyz:nfsB-mCherry]sh260) [Citation25] in 98% of neutrophils observed (Fig. S2A-C).
We next examined whether the GFP-Sqstm1 protein is able to function as expected. Interestingly, in the double-labeled larvae, GFP puncta but not mCherry puncta were seen (Fig. S2D). Similar Sqstm1 puncta that required ubiquitin-binding domain (UBD) to function have been observed in vitro for endogenous Sqstm1 [Citation26]. To test whether the [Citation27] **GFP-Sqstm1 puncta observed in the GFP-Sqstm1 reporter line respond as expected, GFP-Sqstm1 reporter larvae were treated with autophagy inhibitor Bay K8644: known to block autophagy in zebrafish [Citation28]. As expected, there was a significant increase in the number of neutrophils which contained GFP-Sqstm1 puncta following Bay K8644 treatment in comparison to non-treated controls (Fig. S2E), as well as a significant increase in the number of GFP-Sqstm1 puncta within individual neutrophils as expected for endogenous Sqstm1 (Fig. S2F). This result suggests that the GFP-Sqstm1 puncta are not being processed through autophagy and accumulate within the cell, as reported for endogenous Sqstm1 [Citation28]. As we had done for neutrophils and Lc3-positive vesicles, we examined the location of S. aureus throughout infection with our GFP-Sqstm1 reporter for consistency with Tg(mpx:eGFP)i114 and Tg(lyz:RFP-GFP-lc3)sh383 (). We found that there was a comparable reduction in the number of bacteria observed within neutrophils at 26 hpi in comparison to 2 hpi (Fig. S3A) and a reduction in the number of infected neutrophils from 2 hpi to 26 hpi (Fig. S3B). This result suggested that neutrophils were efficiently degrading these bacteria, in agreement with .
Cytosolic bacteria are a possible target for Sqstm1 and S. aureus has previously been visualized within the cytosol of a neutrophil from murine infection studies [Citation29].To identify S. aureus in the cytosol in our in vivo experiments in zebrafish, we looked for regions of the cytosol that co-localized with S. aureus but without a reduction of GFP signal, indicating a vacuole excluding the surrounding cytosol (containing GFP). We first confirmed that we could clearly observe phagosomes containing bacteria with low GFP fluorescence consistent with S. aureus-containing vacuoles, where host cell cytoplasm containing GFP, was excluded (Sqstm1 GFPlow, Fig. S3C). As further evidence for this analysis, we determined that vesicles containing S. aureus, visualized by TEM, were empty of cellular components, in comparison to the cytosol (Fig. S3D), suggesting GFPlow areas represent vesicles. Finally, we looked for functional differences consistent with the presence of a phagosomal membrane in GFPlow regions by examining pH differences using the pH-sensitive dye pHrodo. We found examples of low pH in vesicles correlating with low cytoplasmic fluorescence (Fig. S3E), again suggesting GFPlow areas represent vesicles. Having characterized features consistent with an S. aureus-containing vacuoles, we were able to assign a subset of bacteria as being in either a damaged phagosome or located in the cytosol (Sqstm1GFPhigh, Fig. S3F). For the purpose of this study, we are defining these bacteria as cytosolic, as they are accessible to cytosolic proteins. We then assigned the cellular location of S. aureus by these features at 2 hpi and 26 hpi. We determined that the proportion of S. aureus within vesicles was significantly reduced by 26 hpi (Fig. S3G) and that the number of bacteria within the cytosol is similar at both time points, in agreement with our Tg(lyz:RFP-GFP-lc3)sh383 data ().
Lc3 and Sqstm1 are recruited to Staphylococcus aureus within neutrophils
We determined that GFP-Sqstm1 puncta co-localize with S. aureus either marking a vesicle containing S. aureus ( and Video S1) or directly in contact with S. aureus located in the cytosol ( and Video S2). For puncta marking S. aureus in vesicles, no difference in the proportion of vesicles marked was observed at 2 or 26 hpi, although the actual number of puncta-marking vesicles was dramatically reduced by 26 hpi () as most bacteria had already been degraded. GFP-puncta-marking bacteria in the cytosol were decreased at 26 hpi (), as expected, given that Sqstm1 is degraded with the cargo targeted for degradation [Citation28]. We previously showed cytosolic GFP-Sqstm1 puncta were modulated by autophagy machinery-targeting drugs (Fig. S2E and S2F). In further agreement with this, comparison between infected and uninfected neutrophils showed there was no difference in the number of cytoplasmic GFP-Sqstm1 puncta at 2 hpi but a significant reduction by 26 hpi (), indicating these puncta are modulated by S. aureus infection.
Figure 2. In vivo recruitment of GFP-Sqstm1 puncta during S. aureus infection. (A) Representative image of S. aureus observed within a likely “vesicle” with GFP-Sqstm1 puncta localization, (scale: 7 µm) (B) representative image of S. aureus observed within the cytosol with GFP-Sqstm1 puncta localization, (scale: 9 µm) (C) S. aureus within vesicles, co-localized with GFP-Sqstm1 at 2 hpi and 26 hpi (CHT imaged, ns, Fisher’s exact test, n = 3, 14 larvae at 2 hpi, and 12 larvae at 26 hpi) (D) S. aureus in the cytosol, co-localized with GFP-Sqstm1 at 2 hpi and 26 hpi (CHT imaged, *p < 0.05, Fisher’s exact test, n = 3, 14 larvae at 2 hpi, and 12 larvae at 26 hpi) (E) GFP-Sqstm1 puncta in the cytosol of infected and non-infected at 2 hpi (CHT imaged, ns, Mann-Whitney test, n = 3, error bars ± SD, 14 larvae) (F) GFP-Sqstm1 puncta in the cytosol of infected and non-infected at 26 hpi (CHT imaged, **p < 0.01, Mann-Whitney test, n = 3, error bars ± SD, 12 larvae) (G-I) 2500 cfu of GFP S. aureus injected into Tg(lyzC:RFP-GFP-lc3)sh383, larvae imaged in the CHT at 2 hpi and 26 hpi. (G) Lc3 association to the entire S. aureus vesicle at 2 hpi and 26 hpi (ns, Fisher’s test, n = 3, 17 2 hpi larvae, 11 26 hpi larvae) (H) The number of S. aureus vesicles with Lc3 puncta (*p < 0.05, Fisher’s test, n = 3, 17 2 hpi larvae, 11 26 hpi larvae) (I) The number of S. aureus events in the cytosol with Lc3 puncta at 2 hpi and 26 hpi (ns, Fisher’s test, n = 3, 17 larvae at 2 hpi, 11 larvae at 26 hpi)
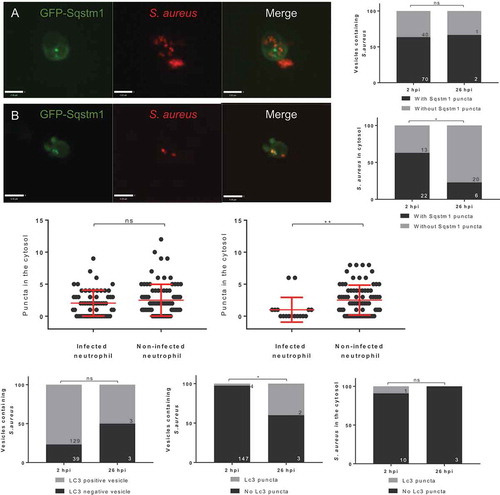
We next examined whether Lc3 can localize to vesicular and cytosolic S. aureus. At 2 hpi and 26 hpi, there was no difference in the proportion of vesicles marked by Lc3, but most vesicular bacteria are degraded by 26 hpi (), showing that a rapid Lc3 response to S. aureus infection occurs. In contrast, vesicles containing S. aureus are significantly more likely to have Lc3 puncta associated at 2 hpi ( and S1D). However, most bacteria are still cleared by 26 hpi, and there was no significant change in the association of Lc3 puncta to S. aureus in the cytosol over time ().
Loss of Sqstm1 reduces zebrafish survival following S. aureus infection
We had demonstrated the steps of Lc3 and the autophagy receptor Sqstm1 recruitment in vivo in the degradation of S. aureus by neutrophils, suggesting a function for Sqstm1 in immunity to S. aureus infection by targeting the degradation of bacteria that escaped the phagosome. To test this prediction, we examined the role of Sqstm1 in S. aureus zebrafish infection using a morpholino-modified antisense oligonucleotide (morpholino) targeting sqstm1 [Citation30] to knockdown sqstm1 expression in the zebrafish larvae. Knockdown of sqstm1 resulted in a significant reduction in zebrafish survival following S. aureus infection, compared to control larvae, supporting a requirement for sqstm1 in the control of S. aureus infection (). Knockdown of sqstm1 did not reduce larval survival for heat-killed S. aureus or the non-virulent but closely related bacterium Micrococcus luteus (Fig. S3I and S3J), suggesting Sqstm1 is important for restriction of pathogenic bacteria that escape the phagosome. To further support this conclusion, we generated an sqstm1 mutant zebrafish (sh558) that lacked a functional UBD domain in sqstm1, inhibiting the ability of Sqstm1 to bind to ubiquitinated cargo (). In agreement with our knockdown study, the sqstm1 mutant zebrafish (sh558) larvae were significantly more susceptible to S. aureus infection than wild-type control zebrafish (). Thus, in addition to demonstrating how Lc3 and Sqstm1 were localized during intracellular handling of S. aureus by neutrophils, we could independently show the requirement of Sqstm1 in the outcome of infection.
Figure 3. Zebrafish survival is reduced following infection with Staphylococcus aureus in the absence of Sqstm1. (A-B) Zebrafish survival following S. aureus infection, larvae were injected with 1500 cfu of SH1000 at 30 hpf. (A) sqstm1 morphants or control morphants survival (n = 3, 74–80 larvae per group, p = 0.004, Log-rank, Mantel-Cox test) (B) sqstm1 mutant or wild-type sibling survival (n = 3, 57–60 larvae per group, p = 0.0168, Log-rank, Mantel-Cox test) (C) Electropherograms showing the sequence of wild type and sh558 mutant Sqstm1. Dashed vertical lines show the location of the 5-bp deletion. The position of the frameshift in the Sqstm1 protein is illustrated. Since this frameshift is located in the final coding exon, we predict translation of a truncated Sqstm1 protein lacking the UBD domain. (D-E) Number of infected neutrophils at 26 hpi following S. aureus infection, larvae were injected with 1500 cfu of SH1000 mCherry (D) or GFP (E), imaging completed in CHT at 30 hpf (D) sqstm1 mutant or wild-type sibling (n = 3, 19–36 larvae per group, p = 0.0168, p = 0.1039, Mann-Whitney test, error bars ± SEM) (E) sqstm1 morphants or control morphants in Tg(mpx:eGFP)i114 larvae (n = 3, 32–34 larvae per group, p = 0.115, Mann-Whitney test, error bars ± SEM) (F) Number of neutrophils containing cytosolic S. aureus in sqstm1 morphants or control morphants Tg(mpx:eGFP)i114 larvae (n = 3, 32–34 larvae per group, **p < 0.01, Mann-Whitney test, error bars ± SEM)
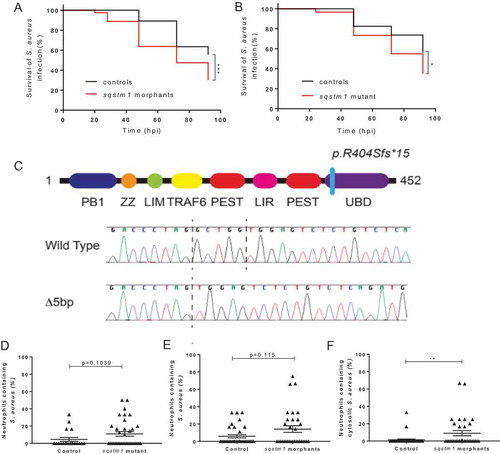
Both sqstm1 morpholino and sqstm1 mutant zebrafish (sh558) techniques do not block Sqstm1 function in neutrophils specifically; therefore, we next aimed to determine whether the loss of Sqstm1 was important in neutrophils during S. aureus infection. Interestingly, there was no difference between the survival of our GFP-Sqstm1 reporter and wild-type controls (Fig. S3H), suggesting that endogenous sqstm1 expression is sufficient for restriction of the small proportion of bacteria which reside in the cytosol. First, using tyramide signal amplification (TSA) staining of 1 dpi larvae to visualize neutrophils within sqstm1 mutant (sh558) and control larvae, we found a non-significant (p = 0.1039) increase in neutrophils containing S. aureus (). a small effect was expected due to the small proportion of cytosolic bacteria, which are likely targeted by Sqstm1 during infection. It was, therefore, likely that showing a difference in the number of infected neutrophils would have required a very large number of infections. We were able to calculate that the observed differences would require a group size of 270.
Next, using sqstm1 morphants and control larvae, a comparison of the number of bacteria present within neutrophils at 1 dpi was completed in the Tg(mpx:eGFP)i114 larvae. In agreement with the Sqstm1-UBD mutant data, a non-significant (p = 0.115) increase of neutrophils containing S. aureus was observed in sqstm1 morphants in comparison to wild-type controls (). Again, we had calculated that the observed differences would require a large group size of 219. However, the examination of the bacterial location revealed a significant increase in the number of cytosolic S. aureus in the sqstm1 morphants in comparison to control fish (), suggesting loss of Sqstm1 is important for the control of cytosolic S. aureus by neutrophils. Thus, we could show that loss of sqstm1 leads to an increase in bacterial burden within neutrophils and that Sqstm1 is likely targeting the small proportion of bacteria that escape to the cytosol.
Discussion
Using the unique attributes of long-term high-resolution imaging and genetic manipulation of zebrafish larvae, we have shown the dynamics of Lc3 and Sqstm1 on the S. aureus-containing vacuoles, their relation to bacterial degradation, and how Sqstm1 recognizes cytosolic bacteria, meaning that loss of Sqstm1 activity is sufficient to increase mortality following S. aureus infection.
Loss of zebrafish sqstm1, through morpholino-mediated knockdown, significantly increased susceptibility to the infection to S. aureus. This result is the first in vivo evidence that Sqstm1 is important in the outcome of intracellular handling of S. aureus. To confirm the sqstm1 knockdown data, we generated a zebrafish sqstm1 mutant lacking the UBD domain, which confirmed a significant increase in the susceptibility of zebrafish to S. aureus infection. This result suggests that for S. aureus infection control, the Sqstm1 UBD, which can bind to ubiquitinated S. aureus [Citation18], is important for host control of infection. In addition to its role as an autophagy receptor, Sqstm1 can aid in the killing of pathogens through the delivery of anti-microbial peptides [Citation31]. Thus, it is possible that anti-microbial peptides delivered by Sqstm1 are important in neutrophil control of S. aureus infection. The sqstm1 zebrafish mutant represents a valuable tool in the analysis of selective autophagy in infection, which may also be useful for the study of other intracellular pathogens or in other diseases, where autophagy is implicated in pathology, for example in neurodegenerative disorders.
Although in vitro studies have described co-localization of Sqstm1 and autophagy in pathogen handling, until now, no evidence of direct Sqstm1 interactions with these pathogens has been shown in neutrophils or in vivo. Interaction of Sqstm1 with S. aureus has been demonstrated through in vitro studies using fibroblasts and epithelial cells [Citation18]. In vitro data shows S. aureus can be targeted for autophagic degradation by Sqstm1 [Citation18], where puncta appear to be co-localized with S. aureus. Our new zebrafish GFP-Sqstm1 reporter shows cytosolic puncta formation, which has also been observed in other cell culture studies, both endogenous expression and using similar GFP-Sqstm1 reporter systems [Citation26,Citation32,Citation33]. By comparing GFP-Sqstm1 puncta marking of intracellular S. aureus with the location of bacteria over time, it is interesting to note that Sqstm1 marking is reduced over time for cytosolic bacteria, which appear to be a small population that persists throughout infection. This result may indicate that cytosolic bacteria marked with Sqstm1 are degraded. Furthermore, at later time points in S. aureus infection, the number of GFP-Sqstm1 puncta is reduced within infected cells, suggesting that when bacteria escape the phagosome, Sqstm1 becomes important in controlling cytosolic bacteria.
We show that most S. aureus is contained within a vesicle soon after infection, and by 26 hpi, most S. aureus are absent from neutrophils. Of note, some images show bacteria outside the neutrophils that have been phagocytosed by macrophages, which has previously been described [Citation34]. The large reduction of neutrophils containing bacteria from 2 hpi to 26 hpi, leaving a small population at 26 hpi, may be representative of a niche for bacterial persistence and/or proliferation. The role of neutrophils as an intracellular niche has previously been described to be important in determining the outcome of S. aureus infection [Citation15,Citation16,Citation35]. Interestingly, it appears that Lc3 marks the majority of vesicles containing bacteria. Lc3 localization to S. aureus may represent Lc3 recruitment to autophagosomes; however, since recruitment is observed at early infection time points, it may represent Lc3-associated phagocytosis, which is also observed in Listeria monocytogenes infection of macrophages [Citation36]. Since most bacteria are degraded, it appears that Lc3 marking of vesicles could lead to bacterial degradation in the zebrafish.
Thus, we demonstrate that host Sqstm1 is beneficial for the host outcome following S. aureus infection and that Sqstm1-mediated control of cytosolic bacteria within neutrophils may represent one of many mechanisms employed by the host in immunity to this versatile pathogen.
Materials and methods
Ethics statement
Animal work was carried out according to guidelines and legislation set out in UK law in the Animals (Scientific Procedures) Act 1986, under Project License PPL 40/3574 or P1A4A7A5E). Ethical approval was granted by the University of Sheffield Local Ethical Review Panel. Animal work completed in Singapore was completed under the Institutional Animal Care and Use Committee (IACUC) guidelines under the a*STAR Biological Resource Center (BRC) approved IACUC Protocol #140977.
Zebrafish husbandry
Zebrafish strains were maintained according to standard protocols [Citation37]. For animals housed in the Bateson Center aquaria at the University of Sheffield, adult fish were maintained on a 14:10-h light/dark cycle at 28°C in UK Home Office approved facilities. For animals housed in IMCB, Singapore, adult fish were maintained on a 14:10-h light/dark cycle at 28°C in the IMCB zebrafish facility. London wild-type (LWT) and AB wild-type larvae were used in addition to transgenic lines, Tg(lyz:eGFP-sqstm1)i330 created in this study, Tg(lyz:RFP-GFP-Lc3)sh383 [Citation23], Tg(lyz:nfsB-mCherry)sh260 [Citation25] (these fish encode nitroreductase gene nsfB within neutrophils which allows ablation of cells following metronidazole treatment, which was not used in this study) and Tg(mpx:eGFP)i114 [Citation20]. Generation of Sqstm1 sh558 mutant zebrafish is described below. Larvae were maintained in E3 (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4) plus methylene blue (Sigma-Aldrich, 50,484) at 28°C until 5 dpf.
S. aureus culture
The Staphylococcus aureus strain SH1000 [Citation38] was used in this study. a single bacterial colony was placed in 10 ml brain heart infusion medium (Thermo Fisher Scientific, OxoidCM1135B) overnight at 37°C, 250 rpm. 500 µl of this overnight culture was then added to 50 ml of brain heart infusion medium and incubated at 37°C, 250 rpm until OD600 1. The bacteria were then pelleted at 5445 x g, 4°C for 15 min. The bacteria were then resuspended in PBS (Oxoid, BR0014 G), using a volume to dilute to the required dose, with 1500 colony-forming units (cfu)/nL being standard. Bacteria were incubated on ice for a short period, until use. Strains used: SH1000 wild-type strain [Citation38], SH1000-pMV158-mCherry [Citation39], SH1000-pMV158-GFP [Citation39].
Zebrafish micro-injection
For sqstm1 morpholino microinjections: Larvae were injected immediately after fertilization using an sqstm1 morpholino [Citation30]. a standard control morpholino (Genetools) was used as a negative control. For injection of S. aureus, zebrafish larvae were injected at 1 dpf (for survival analysis [Citation35],) or 2 dpf (for microscopy analysis) and monitored until a maximum of 5 dpf. Larvae were anesthetized by immersion in 0.168 mg/mL tricaine (Pharmaq Ltd, ATC QN01AX93) in E3 and transferred onto 3% methyl cellulose (Sigma-Aldrich, M0387) in E3 for injection. For S. aureus 1 nl of bacteria, containing 1500 cfu, was injected into the yolk sac circulation valley. Larvae were transferred to fresh E3 to recover from anesthetic. Any zebrafish injured by the needle/micro-injection were removed from the procedure. Zebrafish were maintained at 28°C.
Generation of Tg(lyz:eGFP-sqstm1)i330 transgenic line
The generation of the Tg(lyz:eGFP-sqstm1)i330 line was performed using the GatewayTM system in combination with Tol2 transgenesis [Citation40]. To make the required expression clone, pDest(lyz:eGFP-sqstm1), the p5E-lyz entry clone [Citation41] and the pME-eGFP-nostop [Citation40] middle entry vectors were used. The destination vector pDesttol2 CG [Citation40], was chosen, which included tol2 sites for integration into the genome, in addition to a GFP heart marker. The required sqstm1 3ʹ entry vector and expression clone pDest(lyz:eGFP-sqstm1) were constructed following the Multisite GatewayTM three-fragment vector construction kit (Invitrogen, 12537–023). To generate tol2 mRNA, a pCS2 FA-transposase plasmid [Citation40] was used. The DNA plasmid was linearized through a restriction site digest. tol2 mRNA was generated by a transcription reaction (Ambion T3 mMessage Machine). tol2 mRNA and pDest(lyz:eGFP-sqstm1) were co-injected into a single cell (at the single cell stage) of wild-type AB larvae. a 1 nl injection contained 30 pg of tol2 mRNA and 60 pg of pDest(lyz:eGFP-sqstm1).
Microscopy of infected zebrafish
Larvae were anesthetized 0.168 mg/mL tricaine in E3 and mounted in 0.8% low melting agarose (Affymetrix, 32,830) onto glass-bottom microwell dishes (MatTek, P35 G-1.5–14 C). An UltraVIEW VoX spinning disk confocal microscope (Perkin Elmer, Cambridge, UK) was used for imaging neutrophils within larvae. 405-nm, 445-nm, 488-nm, 514-nm, 561-nm and 640-nm lasers were available for excitation. Most cellular level imaging was completed in the caudal hematopoietic tissue (CHT) using a 40x oil objective (UplanSApo 40x oil [NA 1.3]). In some cases, a 20x objective was used for whole larvae imaging. GFP, TxRed emission filters were used and bright-field images were acquired using a Hamamatsu C9100-50 EM-CCD camera. Volocity software was used. Between early and late time points, zebrafish larvae were placed back into E3 and maintained at 28°C.
pHrodo staining of S. aureus
Bacterial strains were prepared for injected (as above) and resuspended into PBS pH 9. pHrodo (Thermo Fisher Scientific, P36600) was added at a ratio of 1:200 and incubated at 37°C for 30 min, shaking, in the dark. The bacteria were suspended in PBS pH 8 and washed through a series of solutions (Tris, pH 8.5, PBS pH 8) and finally resuspended into PBS pH 7.4 for injection.
Tyramide signal amplification (TSA) staining
Following S. aureus infection, larvae were fixed in paraformaldehyde (Thermo Fisher Scientific, 28908) diluted to 4% in PBS, overnight at 4°C. Once fixed, larvae were washed in PBS thrice. Staining of neutrophils (specifically myeloperoxidase activity) in LWT larvae was completed using TSA staining kit (Cy5-TSA Cyanine Kit; PerkinElmer, NEL705A001KT). Fish were incubated in a 1:100 ratio of Cy5-TSA:amplification diluent at 28°C for 10 min in the dark. Larvae were washed thrice in PBS before imaging.
TEM of infected zebrafish
Specimens were fixed in 2.5% glutaraldehyde (Agar Scientific, AGR1010), in 0.1 M sodium cacodylate (Agar Scientific, AGR1105) and post-fixed 2% aqueous osmium tetroxide, dehydrated through graded series of ethanol, and cleared in propylene oxide (Agar Scientific, AGR1080) and then infiltrated in 50:50 Araldite resin (Araldite resin made up of a 50:50 dodecenyl succinic anhydride (Agar Scientific, AGR1051) and Araldite resin CY212 (Agar Scientific, AGR1040) mix plus 1 drop/ml benzyl dimethylamine (Agar Scientific, AGR1060) and propylene oxide (Agar Scientific, AGR1080) mixture overnight on a rotor. This mixture was replaced with two changes over 8 h of fresh Araldite resin mixture before being embedded in fresh resin and cured in a 60°C oven for 48–72 h. Ultrathin sections, approximately 85-nm thick, were cut on a Leica UC6 ultramicrotome onto 200-mesh copper grids (Agar Scientific, G2200 C). These were stained for 10 min with saturated aqueous uranyl acetate followed by Reynolds lead citrate [Citation42] for 5 min. Sections were examined using a FEI Tecnai Transmission Electron Microscope at an accelerating voltage of 80 kV. Electron micrographs were recorded using Gatan Orius 1000 digital camera and Gatan Digital Micrograph software.
Image analysis
Image analysis was performed using ImageJ software [Citation43] to quantify the number of S. aureus cells within neutrophils and to quantify GFP-Sqstm1 puncta and Lc3 co-localization to these pathogens.
Drug treatment of zebrafish
Larvae were treated with an autophagy inhibitor through immersion in E3 medium. Bay K8644 (Sigma-Aldrich, B2112) was added to the E3 to the required concentration of 1 µM. Larvae were incubated at 28°C for 24 h before microscopy. Zebrafish were not anesthetized for immersion drug treatments.
Generation of sqstm1 mutant
a zebrafish sqstm1 mutant was generated using CRISPR-Cas9 mutagenesis. a guide RNA targeting exon 8 of zebrafish sqstm1 (ACAGAGACTCCACCAGCCTA) was inserted into a published oligonucleotide scaffold [Citation44] and injected together with recombinant Cas9 protein (New England Biolabs) into 1–2 cell stage zebrafish (AB strain). Efficiency of mutagenesis was confirmed using high-resolution melt curve analysis as previously described [Citation45] and several founders were identified. sqstm1sh558 carries a 10-base pair deletion resulting in a frameshift and premature truncation of Sqstm1 in the ubiquitin-associated (UBA) domain.
Statistical analysis
Statistical analysis was performed as described in the results and figure legends. We used Graph Pad Prism 7 (v7.04) for statistical tests and plots. Fisher’s exact tests, which are reliable with very small group sizes, were used to analyze data sets that have uneven group sizes. In these cases, small group sizes were unavoidable due to the nature of these experiments in which we describe only a very small proportion of bacterial cells are observed at later time points in zebrafish infection.
Supplemental Material
Download Zip (8.6 MB)Acknowledgments
JFG was supported by an award from the Singapore a*STAR Research Attachment Programme (ARAP) in partnership with the University of Sheffield, and a Medical Research Council Grant (MR/R001111/1 with SAR and SJF). TKP was supported by an individual Marie Curie fellowship (PIEF-GA-2013-625975) and by AMR cross-council funding from the MRC to the SHIELD consortium “Optimising Innate Host Defence to Combat Antimicrobial Resistance” MRNO2995X/1. RDT and AJG were supported by the University of Sheffield. JJS was a Marie Curie fellow in the Initial Training Network FishForPharma (PITN-GA-2011- 289209). Work in the PWI lab was funded by the a*STAR Institute of Molecular and Cell Biology (IMCB) and the Lee Kong Chian School of Medicine. SAJ was supported by the Medical Research Council and Department for International Development Career Development Award Fellowship MR/J009156/1 (http://www.mrc.ac.uk/). SAJ was additionally supported by a Krebs Institute Fellowship (http://krebsinstitute.group.shef.ac.uk/), and Medical Research Council Centre grant (G0700091). SAR was supported by a Medical Research Council Programme Grant (MR/M004864/1) (http://www.mrc.ac.uk/). Imaging was completed at the Wolfson Light Microscopy Facility. We thank the aquarium staff at the Bateson Centre (Sheffield) and the IMCB (Singapore) for zebrafish husbandry.
Disclosure statement
The authors have no conflict of interests
Supplementary material
Supplemental data for this article can be accessed here.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Mizushima N, Levine B, Cuervo a, et al. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075.
- Tanida I. Autophagy basics. Microbiol Immunol. 2011 Jan;55(1):1–11.
- Gatica D, Lahiri V, Klionsky DJ. Cargo recognition and degradation by selective autophagy. Nat Cell Biol. 2018 Mar;20(3):233–242.
- Sharma V, Verma S, Seranova E, et al. Selective autophagy and xenophagy in infection and disease. Front Cell Dev Biol. 2018;6:147.
- Popovic D, Dikic I. The molecular basis of selective autophagy. Biochem (Lond). 2012;34(2):24–30.
- Rogov V, Dötsch V, Johansen T, et al. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol Cell. 2014 Jan;53(2):167–178.
- Farré J-C, Subramani S. Mechanistic insights into selective autophagy pathways: lessons from yeast. Nat Rev Mol Cell Biol. 2016 Jul 6;17(9):537–552.
- Levine B, Mizushima N, Virgin H. Autophagy in immunity and inflammation. Nature. 2011;469(7330):323–335.
- Zhang R, Varela M, Vallentgoed W, et al. The selective autophagy receptors optineurin and p62 are both required for zebrafish host resistance to mycobacterial infection. Behr MA, editor. PLoS Pathog. 2019 Feb 28;15(2):e1007329.
- Mostowy S, Sancho-Shimizu V, Hamon MA, et al. p62 and NDP52 proteins target intracytosolic shigella and listeria to different autophagy pathways. J Biol Chem. 2011 Jul 29;286(30):26987–26995.
- Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5(6):527–549.
- Amer AO, Swanson MS. Autophagy is an immediate macrophage response to Legionella pneumophila. Cell Microbiol. 2005 Jun;7(6):765–778.
- Hernandez LD, Pypaert M, Flavell RA, et al. A salmonella protein causes macrophage cell death by inducing autophagy. J Cell Biol. 2003 Dec 8;163(5):1123–1131.
- Gutierrez MG, Vázquez CL, Munafó DB, et al. Autophagy induction favours the generation and maturation of the coxiella-replicative vacuoles. Cell Microbiol. 2005 May 12;7(7):981–993.
- Thwaites GE, Gant V. Are bloodstream leukocytes trojan horses for the metastasis of staphylococcus aureus? Nat Rev Microbiol. 2011 Mar 7;9(3):215–222.
- Prajsnar TK, Hamilton R, Garcia-Lara J, et al. a privileged intraphagocyte niche is responsible for disseminated infection of staphylococcus aureus in a zebrafish model. Cell Microbiol. 2012 Oct;14(10):1600–1619.
- Schnaith a, Kashkar H, Leggio SA, et al. Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J Biol Chem. 2007 Jan 26;282(4):2695–2706.
- Neumann Y, Bruns SA, Rohde M, et al. Intracellular Staphylococcus aureus eludes selective autophagy by activating a host cell kinase. Autophagy. 2016 Nov 14;12(11):2069–2084.
- Bayles KW, Wesson CA, Liou LE, et al. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun. 1998 Jan;66(1):336–342.
- Renshaw SA, Loynes CA, Trushell DMI, et al. a transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108(13):3976–3978.
- Mathai B, Meijer a, Simonsen a. Studying autophagy in zebrafish. Cells. 2017 Jul 9;6(3):21.
- Li L, Wang ZV, Hill JA, et al. New autophagy reporter mice reveal dynamics of proximal tubular autophagy. J Am Soc Nephrol. 2014 Feb;25(2):305–315.
- Prajsnar TK, Serba JJ, Dekker BM, et al. The autophagic response to Staphylococcus aureus provides an intracellular niche in neutrophils. bioRxiv. 2019;18:581223.
- Yang C-T, Cambier CJ, Davis JM, et al. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe. 2012 Sep 13;12(3):301–312.
- Buchan KD, Prajsnar TK, Ogryzko NV, et al. a transgenic zebrafish line for in vivo visualisation of neutrophil myeloperoxidase. bioRxiv. 2019;20:456541.
- Bjørkøy G, Lamark T, Brech a, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171(4):603–614.
- Williams a, Sarkar S, Cuddon P, et al. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat Chem Biol. 2008 May;4(5):295–305.
- Bjørkøy G, Lamark T, Pankiv S, et al. Chapter 12 monitoring autophagic degradation of p62/SQSTM1. Methods in Enzymology. Amsterdam: Elsevier; 2000. pp 181–197.
- Gresham HD, Lowrance JH, Caver TE, et al. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol. 2000 Apr 1;164(7):3713–3722.
- van der Vaart M, Korbee CJ, Lamers GEM, et al. The DNA damage-regulated autophagy modulator DRAM1 links mycobacterial recognition via TLR-MYD88 to autophagic defense. Cell Host Microbe. 2014 Jun 11;15(6):753–767.
- Ponpuak M, Davis AS, Roberts EA, et al. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity. 2010 Mar 26;32(3):329–341.
- Larsen KB, Lamark T, ∅vervatn a, et al. a reporter cell system to monitor autophagy based on p62/SQSTM1. Autophagy. 2010;66(6):784–793.
- Pankiv S, Clausen TH, Lamark T, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007 Aug 17;282(33):24131–24145.
- Prajsnar TK, Cunliffe VT, Foster SJ, et al. a novel vertebrate model of Staphylococcus aureus infection reveals phagocyte-dependent resistance of zebrafish to non-host specialized pathogens. Cell Microbiol. 2008 Nov;10(11):2312–2325.
- Pollitt EJG, Szkuta PT, Burns N, et al. Staphylococcus aureus infection dynamics. PLoS Pathog. 2018;14(6):e1007112.
- Gluschko a, Herb M, Wiegmann K, et al. The β2 integrin Mac-1 induces protective LC3-associated phagocytosis of listeria monocytogenes. Cell Host Microbe. 2018 Mar 14;23(3):324–337.e5.
- Nüsslein-Volhard C (Christiane), Dahm R. Zebrafish : a practical approach. Oxford (UK): Oxford University Press; 2002.
- Horsburgh MJ, Aish JL, White IJ, et al. Omega B Modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from staphylococcus aureus 8325-4. J Bacteriol. 2002;184(19):5457–5467.
- Boldock E, Surewaard BGJ, Shamarina D, et al. Human skin commensals augment Staphylococcus aureus pathogenesis. Nat Microbiol. 2018;3(8):881–890.
- Kwan KM, Fujimoto E, Grabher C, et al. The Tol2kit: a multisite gateway-based construction kit forTol2 transposon transgenesis constructs. Dev Dyn. 2007 Nov;236(11):3088–3099.
- Elks PM, van Eeden FJ, Dixon G, et al. Activation of hypoxia-inducible factor-1α (Hif-1α) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood. 2011 Jul 21;118(3):712–722.
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17(1):208–212.
- Schneider CA, Rasband WS, Eliceiri KW. “NIH Image to ImageJ: 25 years of image analysis”. Nat Method. 2012;9(7):671–675.
- Talbot JC, Amacher SL. a streamlined CRISPR pipeline to reliably generate zebrafish frameshifting alleles. Zebrafish. 2014 Dec;11(6):583–585.
- Sutton BC, Allen RA, Zhao ZJ, et al. Detection of the JAK2V617F mutation by asymmetric PCR and melt curve analysis. Cancer Biomarkers. 2007 Nov 16;3(6):315–324.
