ABSTRACT
Treatment of glioblastoma xenografts with chloroquine results in macroautophagy/autophagy inhibition, resulting in a reduction of tumor hypoxia and sensitization to radiation. Preclinical data show that EGFRvIII-expressing glioblastoma may benefit most from chloroquine because of autophagy dependency. This study is the first to explore the safety, pharmacokinetics and maximum tolerated dose of chloroquine in combination with radiotherapy and concurrent daily temozolomide in patients with a newly diagnosed glioblastoma. This study is a single-center, open-label, dose-finding phase I trial. Patients received oral chloroquine daily starting one week before the course of chemoradiation (temozolomide 75 mg/m2/d) until the end of radiotherapy (59.4 Gy/33 fractions). Thirteen patients were included in the study (n = 6: 200 mg, n = 3: 300 mg, n = 4: 400 mg chloroquine). A total of 44 adverse events, possibly related to chloroquine, were registered including electrocardiogram QTc prolongation, irreversible blurred vision and nausea/vomiting resulting in cessation of temozolomide or delay of adjuvant cycles. The maximum tolerated dose was 200 mg chloroquine. Median overall survival was 16 months (range 2–32). Median survival was 11.5 months for EGFRvIII- patients and 20 months for EGFRvIII+ patients. A daily dose of 200 mg chloroquine was determined to be the maximum tolerated dose when combined with radiotherapy and concurrent temozolomide for newly diagnosed glioblastoma. Favorable toxicity and promising overall survival support further clinical studies.
Abbreviations: AE: adverse events; CQ: chloroquine; DLT: dose-limiting toxicities; EGFR: epidermal growth factor receptor; GBM: glioblastoma; HCQ: hydroxychloroquine; IDH1/2: isocitrate dehydrogenase (NADP(+)) 1/2; MTD: maximum tolerated dose; CTC: National Cancer Institute Common Toxicity Criteria; MGMT: O-6-methylguanine-DNA methyltransferase; OS: overall survival; po qd: per os quaque die; SAE: serious adverse events; TMZ: temozolomide; WHO: World Health Organization
Introduction
Glioblastoma (GBM) is the most common malignant primary brain tumor with an estimated incidence of 4.27 per 100.000 in Europe [Citation1]. Despite extensive surgery, radiotherapy and temozolomide (TMZ), the prognosis remains poor with a median overall survival (OS) of approximately 15 months and a five-year OS of 5–10% [Citation2]. None of the attempts to improve the prognosis, including radiation dose escalation, targeted agents and immunotherapy, have changed the dismal outlook [Citation3]. GBM are known to be one of the most heterogeneous tumors in humans with both major inter- and intratumoral variation [Citation4]. Molecular biomarkers such as MGMT (O-6-methylguanine-DNA methyltransferase) promotor methylation, 1p/19q co-deletion and IDH1/2 (isocitrate dehydrogenase (NADP(+)) 1/2) mutation significantly predict patient survival [Citation5]. EGFRvIII (epidermal growth factor receptor mutation type III) expression, a genotype observed in 50–60% in tumors with amplification of the EGFR gene, is associated with a poor prognosis [Citation6–8]. This is in part through enhanced repair of DNA double-strand breaks and an elevated hypoxic fraction [Citation9,Citation10]. Tissue hypoxia has been shown to correlate with enhanced tumor cell invasion and poor outcome, independent of treatment modality [Citation11].
Macroautophagy (hereafter described as “autophagy”) is a lysosomal degradation pathway, which allows the cell to recycle amino acids and other nutrients to maintain energy levels, protein synthesis and essential metabolic processes during hypoxic conditions and metabolic stress [Citation12]. During this process, a double-membrane structure is formed in which cellular content is engulfed (autophagosome). The autophagosome fuses with a lysosome to expose its content to the lysosomal degradative enzymes. Resistance to autophagy is one of the major reasons why GBM stem cells are known to be highly resistant to both radiotherapy and chemotherapy [Citation13–16]. In EGFRvIII-mutated tumors, autophagy is essential to maintain a viable hypoxic fraction and, when targeted, results in elevated response of the tumor to irradiation [Citation10].
The lysosomotropic drug chloroquine (CQ) is a 4-aminoquinoline commonly used for the prophylaxis and treatment of malaria, rheumatoid arthritis, liver amoebiasis, sarcoidosis and lupus erythematodes. CQ accumulates in the lysosome and thereby raises the intralysosomal pH, preventing the autophagosome-lysosome fusion essential for autophagy [Citation17,Citation18]. There is mounting pre-clinical evidence that hypoxic cells depend on autophagy for survival and that inhibiting autophagy with CQ can enhance both radiotherapy and chemotherapy cytotoxicity in GBM [Citation19–22]. Moreover, there is evidence that CQ reduces tumor hypoxia by improving the structural and functional features of the intratumoral blood vessels through vessel normalization via NOTCH1 signaling [Citation23]. CQ can penetrate into the central nervous system and the potential added value of CQ has been demonstrated in a small randomized controlled trial in GBM treated with radiotherapy and carmustine, which showed a trend toward increased OS [Citation24]. Based on these pre-clinical and early clinical results CQ has received an orphan drug designation for the treatment of glioma in the EU in November 2014 (EU/3/14/1377) and the US in May 2015 (Request number: 15–4750). We propose that combining CQ with radiotherapy and TMZ might increase OS significantly. Taking into account that the intracellular effects of CQ are potentially dose-dependent and the effects of CQ in combination with TMZ have not yet been investigated, the recommended phase two dose of CQ in combination with radiotherapy and TMZ needs to be established. This phase I study explores the safety, pharmacokinetics and maximum tolerated dose (MTD) of CQ in combination with radiotherapy and daily TMZ in patients with a newly diagnosed GBM.
Results
Thirteen patients were enrolled in the study between August 2016 and December 2018. The patient and tumor characteristics are summarized in .
Table 1. Patient and tumor characteristics. Methylation of the MGMT (O-6-methylguanine-DNA methyltransferase) promoter, IDH1/2 (isocitrate dehydrogenase (NADP(+)) 1/2) mutation, loss of heterozygosity of 1p and 19q (1p/19q co-deletion), EGFRvIII (epidermal growth factor receptor, mutation type III). Amount of expression detected: negative (-), less than 1% (+), between 1% and 60% (++) or more than 60% (+++) of the cells positive; World Health Organization (WHO) Performance status
The adverse events ≥ CTCAE grade II are presented in . Fatigue and nausea were the most common reported side effects. No toxic deaths occurred. One patient died due to a thrombotic event shortly after chemoradiation, which was considered unlikely due to CQ. All patients with reported toxicities related to study treatment recovered except for one patient with blurred vision. In 8 patients, eleven Serious Adverse Events (SAE) were reported (), of which 5 were considered unlikely or not related to CQ. One patient was admitted to the emergency department after a seizure. During hospitalization, he sustained a minor head injury after a fall, which was possibly caused by an increased sedative effect of midazolam after co-administration with CQ, due to reduced metabolism through CYP/cytochrome P450.
Table 2. Adverse events grade II–V. Events are listed in alphabetical order and were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03) grade criteria. All counts represent a patient; multiple occurrences of the same adverse event in one individual are counted only once at the highest grade. All grade 2–5 events are shown
Table 3. Serious adverse events. Serious Adverse Events (SAEs) presented per cohort CQ. The adverse events were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4.03)
At a daily dose of 400 mg CQ, 3 out of 4 patients experienced a DLT, after which the study medication was discontinued. In the final week of study treatment, two patients showed a significant prolongation of the electrocardiogram QT corrected interval (CTCAE grade III). The QT-corrected interval recovered to normal within 1 week after cessation of CQ in one patient but required four weeks to recover for the other. Neither of the two patients experienced any physical complaints due to the cardiac conduction disturbances. Another patient in the 400 mg CQ cohort developed blurred vision in the second week of chemo-radiation. Ophthalmic examination showed no abnormalities. As an adverse effect from CQ could not be excluded, CQ was stopped. The vision did not recover after cessation of CQ at the end of follow-up 9 months after inclusion. At a daily dose of 300 mg CQ, 2 patients experienced CTCAE grade II and III nausea and vomiting, which resulted in hospital admission in one patient and discontinuation of TMZ in both and was therefore considered a DLT in both cases. At a daily dose of 200 mg CQ, 1 patient developed nausea, vomiting and liver function abnormalities resulting in hospital admission, which was considered a DLT. A daily dosage of 200 mg CQ was therefore considered the MTD.
Valid pharmacokinetic measurements were available from the 200–300 mg cohorts. Median duration of CQ exposure was 52 d. Median CQ plasma concentrations at the expected steady-state (after 2 weeks of CQ) were 192.3 μg/L (200 mg CQ) and 391.9 μg/L (300 mg CQ). At the end of radiotherapy, median CQ plasma concentrations were 199.5 μg/L (200 mg CQ) and 482.4 μg/L (300 mg CQ).
Adherence to the study medication was high, with only 2 patients missing 3 and 6 dosages of CQ, respectively. At data cutoff (February 2020), 1 patient had discontinued treatment including chemoradiation at his own request in the first week of treatment due to complaints of nausea and vomiting, without the use of anti-emetics. This patient was replaced by another patient, after which follow-up ended. No other patients were lost to follow-up. One patient misunderstood the instructions regarding TMZ and only took it during weekdays.
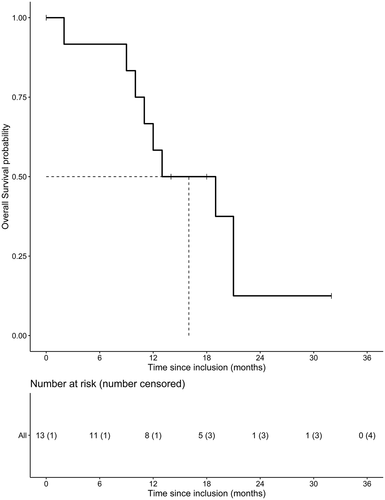
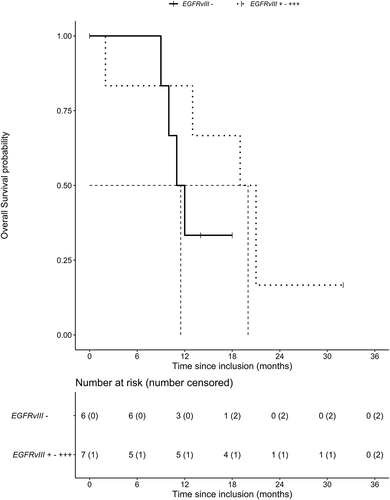
At the time of analysis, the median follow-up was 32 months. The median OS was 16 months, 95% CI [6.5–25.5] (). The median OS time was 21 months, 95% CI [12.5 −29.5], for patients with an MGMT methylated promoter and 12.5 months, 95% CI [8.9–16.1], for patients with an unmethylated promoter. An EGFRvIII mutation was present in 7 out of 13 patients. These patients had a median OS of 20 months, 95% CI [13.6–26.4], compared to 11.5 months, 95% CI [9.1–13.9], in non-EGFRvIII-mutated patients ().
Discussion
Pre-clinical evidence has shown that patients with a GBM may benefit from adding CQ to chemoradiation, resulting in autophagy inhibition and thereby sensitizing tumor treatment. This phase I dose-escalation trial was conducted to evaluate the safety of CQ when combined with radiotherapy and TMZ. This trial is the first to show that co-administration of CQ in combination with radiotherapy and TMZ is feasible. We have established 200 mg CQ daily as the recommended phase two dose in combination with chemoradiation in GBM.
Although CQ is administered in multiple conditions, and the side effect profile is well established, a significant number of adverse events were observed in this trial. At 400 mg CQ, DLTs included grade III blurred vision and grade III ECG QT-corrected interval prolongation. The complaints of severe blurred vision developed after a cumulative dose of 7.2 g. Retinopathy could not be objectified with ophthalmological examination. Retinal toxicity is a well-known complication due to CQ and its derivatives, which usually occurs at high daily doses, taken to high cumulative doses for a prolonged period of ingestion (years) [Citation25]. Cardiac conduction disturbances, including QT-corrected interval prolongation, are also a recognized adverse effect of CQ-use. Risk factors for cardiotoxicity include renal insufficiency, older age, preexisting cardiac disease, elevated per-kilogram daily dose and prolonged CQ treatment [Citation26]. Neither of the patients had prior cardiac disease or renal insufficiency and therefore the only risk factor was an elevated CQ-dose. Nausea and vomiting have been well-established side effects of CQ. Moreover, the tumor, postoperative complications and the chemoradiation may have aggravated these complaints.
Several other groups have published clinical trials adding CQ to glioma treatment. Bilger et al treated 5 patients with a recurrent GBM with 250 mg CQ daily and re-irradiation without TMZ, which was well-tolerated and no CQ-related toxicity was observed [Citation27]. Sotelo et al. combined CQ (150 mg po qd) with radiotherapy and carmustine after surgery [Citation24]. In this single-center trial, 30 patients receiving surgery, chemotherapy and radiotherapy were randomized to receive CQ or placebo for 12 months. The median OS was 24 months for the CQ-treated patients and 11 months for controls. No significant CQ-related toxicity was observed. The difference in survival time was statistically significant when compared to control subjects in their later published institutional experience [Citation28]. Rosenfeld et al published a phase I–II trial in which resected GBM patients received the CQ derivative hydroxychloroquine (HCQ) in doses of 200–800 mg po qd with radiation therapy and concurrent TMZ followed by adjuvant TMZ [Citation29]. Pharmacokinetic analysis showed a significant therapy-associated increase in autophagic vacuoles and LC3B in the peripheral blood mononuclear cells, which was correlated with higher HCQ exposure. The MTD was found to be 600 mg HCQ po qd as sustained hematological toxicity prevented further dose escalation. When comparing the clinical outcome to historical data, the OS was not significantly different. HCQ was preferred over CQ based on a more favorable toxicity profile with a lower risk of retinopathy and cardiotoxicity. This is reflected in the significantly higher MTD in comparison to the MTD of 200 mg CQ found in this study. However, the authors could not exclude the possibility that the combination of CQ with radiotherapy and TMZ would have been more potent, as CQ crosses the blood-brain barrier more easily and can potentially accumulate in brain tissue to a higher level than the blood plasma concentration [Citation30].
In addition to glioma, CQ is currently being investigated in a wide range of cancer types, with 3 studies currently recruiting including a large phase III trial in glioma (NCT03243461) (clinicaltrials.gov). In a phase I study, the combination of 300 mg CQ per day with gemcitabine in patients with metastatic or unresectable pancreatic cancer was well-tolerated [Citation31]. A recent randomized window-of-opportunity trial in breast cancer patients has evaluated single-agent 500 mg CQ per day in a preoperative setting [Citation32]. No significant effect of CQ on tumor proliferation was established. Nearly 15% of patients discontinued treatment due to adverse events, mainly nausea and/or abdominal cramps.
Induction of autophagy by radiation and TMZ results in radioresistance of glioma stem cells, which can be inhibited with CQ [Citation13,Citation21,Citation33–35]. Several studies have demonstrated that inhibition of autophagy caused by radiotherapy and TMZ can ameliorate apoptosis in GBM cells and thereby increase therapeutic outcome [Citation20,Citation36–38]. There is evidence that CQ exerts its therapeutic effects also by autophagy-independent mechanisms such as promotion of lysosomal membrane permeabilization inducing apoptosis [Citation39,Citation40] and tumor vessel normalization [Citation23]. Autophagy has been described as an important regulator of cellular immune response, inhibiting the tumor-specific immune response through suppression of ATP release attracting dendritic cells and T-lymphocytes [Citation41,Citation42]. Moreover, it can lead to accumulation TLR4 (toll like receptor 4) on the cell surface, thereby increasing the tumor-specific immune response [Citation43,Citation44].
EGFRvIII is a potential predictive biomarker for the addition of CQ to concurrent chemoradiation. Even though a formal statistical analysis was not possible, shows that CQ may increase OS in EGFRvIII-positive tumors. Overexpression of EGFRvIII has been shown to increase cell proliferation and survival under stress conditions such as hypoxia or nutrient deprivation by an increase in autophagic activity [Citation9,Citation45,Citation46]. A retrospective analysis of patients with a GBM treated with radiotherapy and carmustine demonstrated that the addition of CQ improved OS from 5 to 10 months in the EGFRvIII-negative GBM and improved survival from 3 to 15 months in the EGFRvIII-positive GBM [Citation10].
Although the pharmacokinetic properties of CQ alone are known, the pharmacokinetic properties of CQ in combination with TMZ have not yet been investigated. The required dose for autophagy inhibition has not yet been established. The trough level was chosen as it is directly measurable and the most reliable assessment of the CQ plasma concentration. The CQ plasma T1/2 is ca 2 weeks, meaning a steady-state plasma concentration was expected to be reached in the second week of chemoradiation. In this trial, a near steady-state was observed after 2 weeks (data not shown). Rosenfeld et al have previously demonstrated a dose-dependent increase in autophagy inhibition for HCQ [Citation29]. Although we did not reach the planned 600 mg CQ dose level in this study, pharmacokinetic studies have shown that patients will reach a steady-state concentration of approximately 10 μM CQ with a daily intake of 250 mg, which has been shown to be an effective dose for autophagy inhibition [Citation36,Citation47]. Yet other studies suggest that higher doses of CQ are required for a therapeutic effect [Citation48,Citation49]. This possibly explains an apparent lack of effect on OS in our overall patient cohort.
This study has several limitations. Although this study’s traditional 3 + 3 design is considered safe, it does have several disadvantages, e.g., the low likelihood of selecting the true MTD due to pre-defined dose levels, uncertainty in detecting all DLTs because of low patient numbers and no option to re-escalate the CQ dose. CQ concentrations vary significantly between different types of tissue, e.g., CQ accumulates in red blood cells, and the concentration within red blood cells may be > 4–10 x higher than the plasma concentration [Citation50]. However, the serum fraction of CQ is in direct contact with the possible target tissue. Therefore, we hypothesize this concentration to be more relevant with regards to both the effects and side effects of CQ, and therefore chose to quantify CQ based on serum concentration. This should be further investigated in future trials. CQ was initiated one week before the start of chemo- and radiotherapy in order to reach a steady-state early into treatment. However, it is unclear if a steady state is necessary to reach optimal autophagy inhibition. Unlike previous clinical trials [Citation24,Citation29], CQ was stopped at the end of concurrent chemoradiation in order to avoid potential long-term toxicity caused by CQ’s extensive plasma terminal elimination half-life of 40–60 d and its active metabolites. We, however, cannot exclude that CQ concurrent to the adjuvant cycles of TMZ is a more effective combination. Further research into the timing of adjuvant CQ at the time of chemotherapy resistance is necessary [Citation19]. LC3B-II within peripheral blood mononuclear cells currently serves as a surrogate to measure the inhibitory effect of CQ in tumor cells [Citation51]. There currently is no technique available to directly measure autophagy inhibition within the tumor. Further development of a biomarker or tracer to evaluate autophagy inhibition is highly desirable to evaluate the clinical effect in vivo.
In conclusion, this study has shown that despite CQ’s well-known toxicity profile, it may still elicit significant adverse effects in combination with TMZ and radiotherapy in doses commonly described for rheumatoid arthritis. It is anticipated that more potent and less toxic autophagy inhibitors such as Lys05 or Verteporfin will become clinically available throughout the years to come, but these are currently still under investigation in preclinical models. Combination treatment with autophagy inhibitors has the potential to become the new standard of care for GBM as it may significantly impact survival, especially for a subset of patients.
Materials and methods
Eligibility
The main inclusion criteria were histologically confirmed newly diagnosed GBM, age 18–70 y/o and a World Health Organization (WHO) performance status ≥ 2. The blood samples of these patients required a neutrophil count of ≥ 1.5 x 109/L, platelets ≥ 100 x 109/L, a serum creatinine ≤1.5 x upper limit of normal, total bilirubin ≤ 1.5 x upper limit of normal and GPT/alanine transaminase or GOT1/aspartate aminotransferase and ALPL/alkaline phosphatase ≤3 x upper limit of normal. The main exclusion criteria included prior radio- or chemotherapy, recent severe cardiac disease, a history of cardiac arrhythmia, cardiac conduction disturbances or medication potentially causing them, retinal or visual field changes unrelated to the tumor location and the use of concurrent CYP/cytochrome P450 enzyme-inducing drugs.
Study design
This study was a single-center, open-label, dose-finding phase I trial. Eligible patients received radiotherapy and TMZ (Sun Pharmaceutical Industries Ltd, 15913384) according to the standard clinical protocol. This consists of 33 daily fractions of 1.8 Gy to the tumor which included the region of enhancement plus the resection cavity (if resected) with a 2 cm margin on magnetic resonance imaging in combination with TMZ 75 mg/m2 per os quaque die (po qd) and six adjuvant cycles of TMZ 150–200 mg/m2 po qd. CQ (Basic Pharma Manufacturing B.V, IMP10153) was taken daily starting one week before the start of chemo-radiation and ending on the last day of radiotherapy. CQ was initially planned to be escalated in 3 dose-levels (200 mg, 400 mg and 600 mg po qd), each dose level containing a minimum of 3 and a maximum of 6 patients (3 + 3 design) [Citation52]. Adverse events (AEs) were assessed using the National Cancer Institute Common Toxicity Criteria (CTC) version 4.03. Dose-limiting toxicities (DLTs) were defined as an absolute neutrophil count ≤ 500/µL for 7 d; febrile neutropenia (neutrophil count less than 500 cells per microliter and fever of ≥ 38.5°C); any grade 4 thrombocytopenia (< 25 x 109/L), and/or requiring platelet transfusion or bleeding requiring medical intervention; any ≥ grade 3 non-hematological toxicity except fatigue, nausea, fever or skin reactions; interruption of radiotherapy or TMZ due to toxicity during concurrent chemo-radiation or delay of adjuvant cycles of TMZ. Toxicity was considered a DLT if it was at least possibly related to CQ. If DLT was observed in one out of three initial patients, an additional 3 patients were enrolled at that dose level. In case more than one out of the initial three had DLT, no additional patients were required. The MTD for CQ was defined as one dose level below that at which two or more patients experienced a DLT, or if no DLTs were observed, the highest evaluated dose. Once the recommended phase two dose was identified, 3 additional patients were enrolled in order to confirm treatment tolerability. Safety was assessed by an independent data safety monitoring committee four weeks after treatment of every third subject.
Study assessments
Baseline assessment included a history and physical examination, WHO performance status, laboratory analysis, electrocardiogram, ophthalmological examination and a tone audiogram. Laboratory analyses were performed weekly during concurrent treatment and monthly thereafter. An electrocardiogram was repeated in the second and final week of study treatment for the first 7 patients. The protocol was amended to weekly ECG surveillance during CQ-treatment for the remainder of patients due to observed toxicity. Ophthalmological examination and a tone audiogram were repeated 4 weeks after the end-of-study treatment and if clinically indicated. Assessment of adverse events and physical and neurological examinations were performed monthly during the adjuvant cycles of TMZ. The blood concentration of CQ was monitored on four time points throughout the regimen and before the adjuvant cycles of TMZ (first and second week and end of chemoradiation and 4 weeks after chemoradiation). CQ was analyzed in serum samples using validated liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) method. The chromatographic separation was achieved on a reversed-phase Thermo Fisher Scientific Hypurity C18 (50x2.1 mm, 3μ) column (22103–052130), with gradient elution using ammonium formate 0.02 mol/L pH 3.5 and methanol as mobile phase at a flow rate of 0.5 mL/min. Protein precipitation with 10% trichloroacetic acid and D4-CQ was utilized for extraction of CQ from the matrix. CQ was quantitated using MS/MS detection with an electrospray ionization source in positive multiple reaction monitoring (MRM) mode. The MS/MS response was linear over a concentration range from 10 to 2000 μg/L with a correlation coefficient (R2) of 0.999 or better. The within- and between-day precision (% relative standard deviation) and accuracy were within the acceptable limits as per EMA and FDA guidelines [Citation53,Citation54]. Profile parameters included blood trough level, Area Under the Curve and elimination half-life. Magnetic resonance imaging was performed within 72 h after brain tumor surgery and at least within 6 weeks prior to registration. Follow-up imaging was performed according to standard clinical protocol on a three-monthly basis after treatment.
Molecular profiling
IDH1 or IDH2 mutations were detected with polymerase chain reaction and pyrosequencing, MGMT promoter methylation status was analyzed by bisulfite treatment and methylation-specific polymerase chain reaction followed by pyrosequencing. FISH analysis of 1p/19q status was performed using the LSI 1p36/1q25 and 19q13/19p13 FISH Probe kit (Abbott Molecular Inc., 04N60-020). To determine EGFRvIII expression, formalin-fixed paraffin-embedded biopsies were probed with antibodies directed against EGFRvIII (clone L8A4, absolute antibody, Ab00184-1.1.) and visualized using HRP-linked anti-mouse (Cell Signaling Technology, 7076S) antibodies) in combination with DAB-staining. Tumor biopsies were scored either negative, less than 1% (+), between 1% and 60% (++) or more than 60% (+++) of the cells positive in EGFRvIII-expression.
Statistical analysis
The primary objective of the study was to determine the recommended phase two dose. Secondary objectives included evaluation of safety, tolerability, pharmacokinetics and preliminary changes in treatment efficacy. All evaluable patients were included in the analyses. Analysis was limited to presentation of descriptive statistics (e.g., median and range) and frequencies in cross-tabs. Statistical analysis was performed using the statistical program SPSS for Windows (version 22. 0.0, 2013) and R Statistical Software (version 4.0.1). OS was defined as the time from enrollment until death. The survival analyses were performed using the Kaplan–Meier method.
The study was approved by the Medical Review Ethics Committee at Maastricht University Medical Center +. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. This trial is registered on clinicaltrials.gov (NCT02378532).
Acknowledgments
The authors sincerely thank Monique Bessems, Nadja Drummen, John Paulissen and Claudia Offermans for their support with data collection, patient inclusion and patient guidance. Moreover, the authors would like to thank the members of the Data Safety Monitoring Board, Filip Y.F.L. De Vos MD, PhD, prof. Daan J. Touw, Pharm D, PhD, Ruud M.A. Houben, MSc, Iverna Nijsten, MSc, and Kim M. Smits, PhD for their expertise and recommendations. The study protocol was written during the 16th joint ECCO-AACR-EORTC-ESMO Workshop Methods in Clinical Cancer Research – Flims, Switzerland, 21-27.6.2014
Disclosure statement
Chloroquine has received an Orphan Drug Designation by the European Committee (EU/3/14/1377) and US Food and Drug Administration (#15-4750) from the treatment of glioma.
Data availability statement
The dataset will be made publicly available on https://cancerdata.org
Additional information
Funding
References
- de Robles P, Fiest KM, Frolkis AD, et al. The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro Oncol. 2015;17(6):776–783.
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466.
- Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329.
- Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science (New York, NY). 2014;344(6190):1396–1401.
- Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820.
- Maire CL, Ligon KL. Molecular pathologic diagnosis of epidermal growth factor receptor. Neuro Oncol. 2014;16(Suppl 8):viii1–6.
- Brennan CW, Verhaak Rg Fau - McKenna A, McKenna A Fau - Campos B, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477.
- Shinojima N, Tada K, Shiraishi S, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63(20):6962–6970.
- Theys J, Jutten B, Dubois L, et al. The deletion mutant EGFRvIII significantly contributes to stress resistance typical for the tumour microenvironment. Radiother Oncol. 2009;92(3):399–404.
- Jutten B, Keulers TG, Peeters HJM, et al. EGFRvIII expression triggers a metabolic dependency and therapeutic vulnerability sensitive to autophagy inhibition. Autophagy. 2018;14(2):283–295.
- Wouters BG, van den Beucken T, Magagnin MG, et al. Targeting hypoxia tolerance in cancer. Drug Resist Updat. 2004;7(1):25–40.
- Mizushima N. The pleiotropic role of autophagy: from protein metabolism to bactericide. Cell Death Differ. 2005;12(Suppl 2):1535–1541.
- Kanzawa T, Germano IM, Komata T, et al. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11(4):448–457.
- Paglin S, Hollister T, Delohery T, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61(2):439–444.
- Ito H, Daido S, Kanzawa T, et al. Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells. Int J Oncol. 2005;26(5):1401–1410.
- Yao KC, Komata T, Kondo Y, et al. Molecular response of human glioblastoma multiforme cells to ionizing radiation: cell cycle arrest, modulation of the expression of cyclin-dependent kinase inhibitors, and autophagy. J Neurosurg. 2003;98(2):378–384.
- Rouschop KM, van den Beucken T, Dubois L, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120(1):127–141.
- Savarino A, Boelaert JR, Cassone A, et al. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3(11):722–727.
- Golden EB, Cho HY, Jahanian A, et al. Chloroquine enhances temozolomide cytotoxicity in malignant gliomas by blocking autophagy. Neurosurg Focus. 2014;37(6):E12.
- Hori YS, Hosoda R, Akiyama Y, et al. Chloroquine potentiates temozolomide cytotoxicity by inhibiting mitochondrial autophagy in glioma cells. J Neurooncol. 2015;122(1):11–20.
- Firat E, Weyerbrock A, Gaedicke S, et al. Chloroquine or chloroquine-PI3K/Akt pathway inhibitor combinations strongly promote gamma-irradiation-induced cell death in primary stem-like glioma cells. PLoS One. 2012;7(10):e47357.
- Rouschop KM, Ramaekers CH, Schaaf MB, et al. Autophagy is required during cycling hypoxia to lower production of reactive oxygen species. Radiother Oncol. 2009;92(3):411–416.
- Maes H, Kuchnio A, Peric A, et al. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell. 2014;26(2):190–206.
- Sotelo J, Briceno E, Lopez-Gonzalez MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144(5):337–343.
- Marmor MF, Kellner U, Lai TY, et al. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):415–422.
- White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7(8):549–558.
- Bilger A, Bittner MI, Grosu AL, et al. FET-PET-based reirradiation and chloroquine in patients with recurrent glioblastoma: first tolerability and feasibility results. Strahlenther Onkol. 2014;190(10):957–961.
- Briceno E, Calderon A, Sotelo J. Institutional experience with chloroquine as an adjuvant to the therapy for glioblastoma multiforme. Surg Neurol. 2007;67(4):388–391.
- Rosenfeld MR, Ye X, Supko JG, et al. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy. 2014;10(8):1359–1368.
- Adelusi SA, Salako LA. Tissue and blood concentrations of chloroquine following chronic administration in the rat. J Pharm Pharmacol. 1982;34(11):733–735.
- Samaras P, Tusup M, Nguyen-Kim TDL, et al. Phase I study of a chloroquine-gemcitabine combination in patients with metastatic or unresectable pancreatic cancer. Cancer Chemother Pharmacol. 2017;80(5):1005–1012.
- Arnaout A, Robertson SJ, Pond GR, et al. A randomized, double-blind, window of opportunity trial evaluating the effects of chloroquine in breast cancer patients. Breast Cancer Res Treat. 2019;178(2):327–335.
- Sui X, Chen R, Wang Z, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838.
- Wurstle S, Schneider F, Ringel F, et al. Temozolomide induces autophagy in primary and established glioblastoma cells in an EGFR independent manner. Oncol Lett. 2017;14(1):322–328.
- Ye H, Chen M, Cao F, et al. Chloroquine, an autophagy inhibitor, potentiates the radiosensitivity of glioma initiating cells by inhibiting autophagy and activating apoptosis. BMC Neurol. 2016;16(1):178.
- Lee SW, Kim HK, Lee NH, et al. The synergistic effect of combination temozolomide and chloroquine treatment is dependent on autophagy formation and p53 status in glioma cells. Cancer Lett. 2015;360(2):195–204.
- Zanotto-Filho A, Braganhol E, Klafke K, et al. Autophagy inhibition improves the efficacy of curcumin/temozolomide combination therapy in glioblastomas. Cancer Lett. 2015;358(2):220–231.
- Lin CJ, Lee CC, Shih YL, et al. Inhibition of mitochondria- and endoplasmic reticulum stress-mediated autophagy augments temozolomide-induced apoptosis in glioma cells. PLoS One. 2012;7(6):e38706.
- Enzenmuller S, Gonzalez P, Debatin KM, et al. Chloroquine overcomes resistance of lung carcinoma cells to the dual PI3K/mTOR inhibitor PI103 by lysosome-mediated apoptosis. Anticancer Drugs. 2013;24(1):14–19.
- Maycotte P, Aryal S, Cummings CT, et al. Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy. 2012;8(2):200–212.
- Michaud M, Xie X, Bravo-San Pedro JM, et al. An autophagy-dependent anticancer immune response determines the efficacy of melanoma chemotherapy. Oncoimmunology. 2014;3(7):e944047.
- Ko A, Kanehisa A, Martins I, et al. Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ. 2014;21(1):92–99.
- Husebye H, Halaas O, Stenmark H, et al. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. Embo J. 2006;25(4):683–692.
- Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059.
- Golding SE, Morgan RN, Adams BR, et al. Pro-survival AKT and ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer Biol Ther. 2009;8(8):730–738.
- Weppler SA, Li Y, Dubois L, et al. Expression of EGFR variant vIII promotes both radiation resistance and hypoxia tolerance. Radiother Oncol. 2007;83(3):333–339.
- Augustijns P, Geusens P, Verbeke N. Chloroquine levels in blood during chronic treatment of patients with rheumatoid arthritis. Eur J Clin Pharmacol. 1992;42(4):429–433.
- Weyerhauser P, Kantelhardt SR, Kim EL. Re-purposing chloroquine for glioblastoma: potential merits and confounding variables. Front Oncol. 2018;27(8):335.
- Pascolo S. Time to use a dose of Chloroquine as an adjuvant to anti-cancer chemotherapies. Eur J Pharmacol. 2016;15(771):139–144.
- Titus EO. Recent developments in the understanding of the pharmacokinetics and mechanism of action of chloroquine. Ther Drug Monit. 1989;11(4):369–379.
- Mizushima N, Yamamoto A Fau - Matsui M, Matsui M Fau - Yoshimori T, et al. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15(3):1101–1111.
- Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101(10):708–720.
- European Medicines Agency (EMA). Guideline on bioanalytical method validation. 2019. Accessed on 2020 May 26. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf
- U.S. Department of Health and Human Services Food and Drug Administration. Bioanalytical method validation. Guidance for industry. 2018. Accessed on 2020 May 26 https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf