ABSTRACT
The caspase-like vacuolar processing enzyme (VPE) is a key factor in programmed cell death (PCD) associated with plant stress responses. Growth medium lacking a carbon source and dark conditions caused punctate labeling of 35S::VPE1-GFP (StVPE1-GFP) in potato leaves. Under conditions of carbon starvation, VPE activity and PCD symptoms strongly increased in BY-2 cells, but to a much lesser extent in VPE-RNAi BY-2 cells. During extended exposure to carbon starvation, VPE expression and activity levels peaked, with a gradual increase in BY-2 cell death. Histological analysis of StVPE1-GFP in BY-2 cells showed that carbon starvation induces its translocation from the endoplasmic reticulum to the central vacuole through tonoplast engulfment. Exposure of BY-2 culture to the macroautophagy/autophagy inhibitor concanamycin A led to, along with an accumulation of autophagic bodies, accumulation of StVPE1-GFP in the cell vacuole. This accumulation did not occur in the presence of 3-methyladenine, an inhibitor of early-stage autophagy. BY-2 cells constitutively expressing RFP-StATG8IL, an autophagosome marker, showed colocalization with the StVPE1-GFP protein in the cytoplasm and vacuole. RNAi silencing of the core autophagy component ATG4 in BY-2 cells reduced VPE activity and cell death. These results are the first to suggest that VPE translocates to the cell vacuole through the autophagy pathway, leading to PCD.
Abbreviations: ATG: autophagy related; CLP: caspase-like protease; HR: hypersensitive response; PCD: programmed cell death; St: Solanum tuberosum; VPE: vacuolar processing enzyme.
Introduction
Programmed cell death (PCD) is involved in almost all stages of the plant’s life cycle and can be developmental or stress-induced [Citation1,Citation2]. During the course of their ontogenesis, plants are continuously exposed to a large variety of abiotic stress factors, which can damage tissues and jeopardize the survival of the organism unless properly countered [Citation3]. When the intensity of stress is high, one defense program employed by plants is the induction of PCD [Citation4,Citation5].
In plants, based on morphology, it has been suggested that tonoplast rupture distinguishes two broad classes of PCD, “autolytic” and “non-autolytic”. The first occurs mainly during normal plant development (developmental PCD) and after mild abiotic stress, and the second takes part mainly in response to pathogen invasion (hypersensitive response [HR]-related PCD) [Citation6].
PCD often requires the activity of serine and/or cysteine proteases (caspases) [Citation7,Citation8]. Although surveys of plant genomes have not revealed any “true” caspases or close orthologs of animal caspases, proteases with activities similar to those of animal caspases have been reported during plant PCD, termed caspase-like proteases (CLPs) [Citation9–11].
Plant CLPs have been identified as either metacaspases or vacuolar processing enzymes (VPEs) [Citation12–14]. Metacaspases have arginine/lysine-specific endopeptidase activity, unlike caspases that cleave their substrates at aspartic acid residues [Citation15,Citation16]. The VPE proteins belong to a family of cysteine proteinases that are well conserved among a variety of organisms, including many plant and animal species [Citation17–19]. VPEs were originally found to be responsible for the maturation of seed storage proteins and various other vacuolar proteins in plants [Citation13,Citation20,Citation21]. VPE, which is released into the vacuole during autolytic PCD, triggers the proteolytic processing of various vacuolar proteins [Citation16,Citation22,Citation23]. They exhibit CASP1-like activity and play important roles in plant PCD, be it developmental or in response to biotic or abiotic stress [reviewed by Citation18,Citation24]. Specifically, VPE has been characterized as a major factor in HR. By silencing the gene encoding VPE, Hatsugai et al. [Citation13] showed that vacuolar collapse, caused by VPE activity, seems to be required for virus-induced non-autolytic HR-related PCD in tobacco (Nicotiana tabacum) plants. VPEs have also been found to contribute to PCD in other HR-related systems, such as mycotoxin-induced PCD, where the knockout of GAMMA-VPE resulted in less PCD [Citation14,Citation25]. Single-silenced (NbVPE1a) or dual-silenced (NbVPE1a/b) Nicotiana benthamiana plants also failed to show HR-related PCD after treatment with the bacterial toxin harpin [Citation26]. In other examples, a mutation in GAMMA-VPE reduced non-autolytic PCD induced by the necrotrophic pathogen Botrytis cinerea in Arabidopsis [Citation14], and knockout of all four VPE genes in Arabidopsis prevented the effect of fumonisin B1, a toxin secreted by the necrotrophic fungus Fusarium moniliforme, and also prevented disappearance of the tonoplast [Citation23].
Autophagy is a conserved intracellular trafficking pathway in eukaryotes for the degradation and recycling of cellular components. In plants, autophagy is activated in response to developmental or environmental cues. It is essential for plant growth, maintenance of cellular homeostasis, and overcoming biotic and abiotic stresses [for recent reviews see Citation27,Citation28,Citation29]. Autophagy in plants can be broadly divided into microautophagy and macroautophagy [Citation30]. The former is characterized by the trapping of cytosolic material to be degraded by tonoplast invaginations, followed by tonoplast scission to release the intravacuolar vesicles. The better-characterized macroautophagy pathway (hereafter referred to as autophagy) involves the sequestration of cytoplasmic constituents within de-novo formed double-membrane organelle – the autophagosome – that is transported to the vacuole for degradation. Both processes can be either selective or nonselective with respect to the cytoplasmic material that is being degraded. Many instances of PCD in plants show typical morphological features of autophagic cell death, including an increase in vacuole and cell size, uptake of organelles into the vacuole followed by organelle degradation, and eventual lysis of the vacuole resulting in cell death [Citation31]. In plants, the involvement of autophagy in PCD in response to different developmental and environmental cues is not well understood, and autophagy has been shown to have both pro-survival and pro-death activities [Citation32,Citation33]. Autophagy is induced upon carbon and nitrogen limitation, as well as in response to multiple abiotic stresses, and mutants that are defective in core autophagy genes are hypersensitive to these stresses [Citation34]. Thus, autophagy is usually presumed to play a pro-survival role under these conditions. However, some evidence suggests that autophagy may also promote PCD in response to abiotic stress [Citation35]. This dual function of autophagy is better characterized in the plant’s innate immune system, where autophagy has been shown to act as either a survival or cell-death pathway, depending on the type of pathogen (i.e., biotrophic or necrotrophic) and the type of plant immune receptors involved in the response [Citation33,Citation36,Citation37].
The core mechanism of autophagy is mediated by an evolutionarily well-conserved set of ATG (AuTophaGy-related) genes [Citation30,Citation38,Citation39]. A central protein of both selective and nonselective autophagy is ATG8, which in plants exists as a gene family. Lipidated ATG8 is located on both the outer and inner membrane of the phagophore, the precursor to the autophagosome and is involved in autophagosome formation, as well as in recognition of specific cargo targeted for selective autophagy [Citation40]. As ATG8 is found on the autophagosome membrane from its formation to its lytic destruction in the vacuole, it is the most commonly used marker for autophagosomes.
An excess or loss of carbohydrates or their derivatives triggers various reactions in plants and significantly affects their metabolism, growth, and development. Moreover, abiotic and biotic stress responses are regulated, at least in part, by sugars [Citation41,Citation42]. During etiolated plant development, the stored carbohydrates are used, and their reserves may be greatly diminished in this non-photosynthetic condition. Potato (Solanum tuberosum) VPE1 (StVPE1) has been shown to be involved in the autolytic PCD response of the stem apical meristem to abiotic stress in etiolated stems [Citation43,Citation44]. Following stress, induction of StVPE1 in the stem meristem causes PCD in the apical meristem and a loss of apical dominance. The mature StVPE1 protein exhibits specific activity for CASP1, with optimal activity at acidic pH, consistent with its established vacuolar localization [Citation44]. Downregulation of StVPE1 by RNA interference (RNAi) or overexpression of green fluorescent protein-labeled StVPE1 (StVPE1-GFP) results in reduced or enhanced stem branching, respectively [Citation44]. However, the role of StVPE1 as a general executor of PCD is not clear. In this study, we show for the first time the importance of VPE as an executor of plant PCD during carbon starvation. Moreover, using a cell culture model system, we suggest that VPE is translocated to the cell vacuole through the autophagy pathway [Citation45].
Results
StVPE1 plays a role in the response to carbon deficiency in potato leaves
The roles of VPE in developmental PCD, as well as in plant response to pathogen attack, are well documented [for a recent review, see 45,46]. However, although VPE has also been implicated in participating in response to several abiotic stresses [Citation46], much less is known about this aspect of its activity.
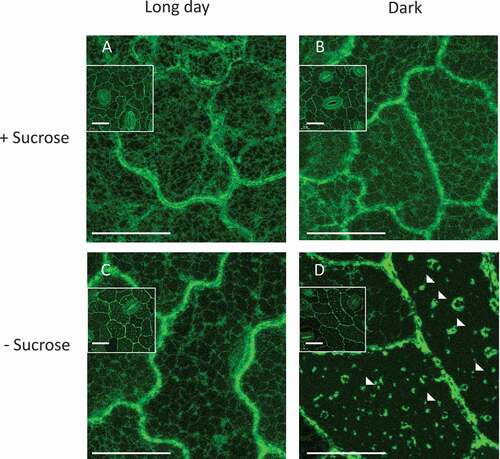
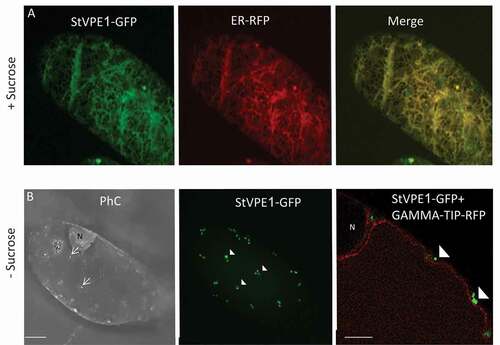
To look into the possible roles of VPE and PCD in the response to carbon starvation, transgenic potato plants expressing StVPE1-GFP were grown with or without sucrose under light (long day) or dark growth conditions. GFP fluorescence was detected in the peripheral part of the cell (probably the cytoplasm) in leaves grown under long-day conditions, regardless of whether sucrose was added to the medium (). Similar StVPE1-GFP localization was observed in plants grown under dark conditions with the addition of sucrose (). Surprisingly, combining dark conditions with carbon starvation changed the fluorescence pattern of StVPE1 in the leaves markedly, with GFP labeling in multiple puncta and occasionally in larger clusters (), suggesting relocalization of VPE in the cell following carbon starvation.
Figure 1. StVPE1-GFP localizes in puncta in potato leaf cells under carbon starvation. (A) Transgenic potato plants overexpressing StVPE1-GFP were grown for 7 d at 25°C in culture medium supplemented with sucrose (+Sucrose) under long day (16 h light) conditions. (B) As in (A) but plants were grown in the dark. (C) As in (A) but culture medium did not contain sucrose (-Sucrose). (D) As in (C) but plants were grown in the dark. Inset in each picture represents zoomed-out picture of the epidermal tissue, showing the stomata location. Arrowheads indicate StVPE1-GFP puncta formed under carbon starvation and dark conditions. Bar: 20 µm
Silencing VPE activity in BY-2 cells prevents PCD induced by carbon starvation
VPE is considered a PCD executor in plant systems in response to several biotic and abiotic stresses [reviewed by 18,24]. Phylogenetic analysis has shown that StVPE1, classified as a vegetative-type VPE, has high sequence similarity and conserved regions with tobacco NtVPE1a, NtVPE1b, NtVPE2 and NtVPE3 [44; Data Set S1A]. To study its role in PCD induction, we produced BY-2 lines expressing a VPE-RNAi construct, and compare their PCD response to that of wild-type (WT) cells. Alignment of VPE cDNA from potato and tobacco showed a 500-bp sequence of StVPE1 cDNA that was 83–90% similar to tobacco NtVPE1a, NtVPE1b, NtVPE2 and NtVPE3, and 67–72% similar to the tobacco VPEs NtPB1, NtPB2 and NtPB3, which were ligated in tandem in opposite directions to produce VPE-RNAi lines of BY-2 cells (Data Set S1B).
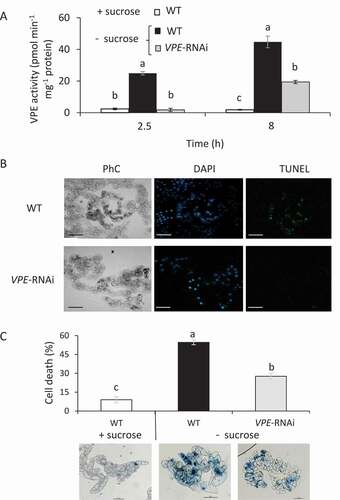
WT and VPE-RNAi BY-2 cells were transferred to a sucrose-free medium, and VPE activity was examined. After 2.5 or 8 h incubation of WT cells without sucrose, VPE activity was 11- and 21-fold higher, respectively, than that in the sucrose-containing culture (). When VPE-RNAi cells were grown under the same conditions, VPE activity was nearly undetectable after 2.5 h of exposure and only 10-fold higher than in the sucrose-containing culture after 8 h (). To determine whether VPE activity induces PCD in carbon-starved BY-2 cells, we examined cell cultures by terminal deoxynucleotidyl transferase (Tdt)-mediated deoxy-uridinetriphosphate (dUTP) nick end labeling (TUNEL) assay. Twenty-four hours after the initiation of carbon starvation, only WT cells showed TUNEL-positive labeling, whereas almost no labeling was observed in the VPE-RNAi cells ( and S1A). Staining of carbon-starved BY-2 cells with Evans blue showed 50% less cell death in the VPE-RNAi line (). Wild type and VPE-RNAi BY-2 cells grown in sucrose-supplied culture showed no PCD symptoms (Fig. S1B). The results suggest that VPE activity is involved in inducing PCD in BY-2 cells in response to carbon starvation.
Figure 2. Silencing VPE activity in BY-2 cells decreases PCD under carbon starvation. (A) VPE activity in VPE-RNAi-transgenic BY-2 cells was compared to that in WT cells in the presence (+) or absence (-) of sucrose. Ac-ESEN-MCA was used as the VPE-specific substrate. (B) Cells subjected to 24 h of carbon starvation were counterstained in situ with DAPI to label nuclei (blue), followed by TUNEL reagents to detect DNA fragmentation (green). Corresponding phase contrast (PhC) images of the cells are also shown. Bars: 100 μm. (C) Quantification of non-viable cells. Six-day-old tobacco BY-2 WT and VPE-RNAi cells were subjected to 24 h of carbon starvation (- sucrose) and stained with Evans blue. The percentage of dead cells was calculated using ImageJ software. Data are mean ± SE of three repeats, each with 100 cells. Different letters represent significant differences between genotypes at different time points (P < 0.005) analyzed by ANOVA followed by Tukey–Kramer HSD test. Representative images of each treatment are shown at the bottom of the figure. Bars: 100 μm
Gradual cell death in response to carbon starvation correlates with VPE expression
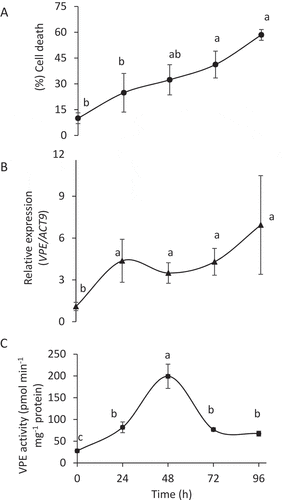
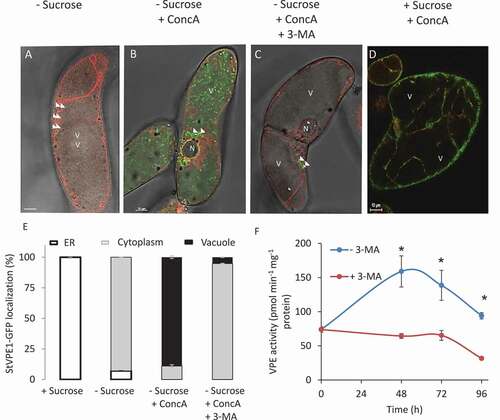
The population of BY-2 cells tended to lose their viability gradually over time following exposure to carbon starvation (). To look at the correlation between cell death and VPE, we analyzed tobacco VPEs (NtVPE2, NtVPE3, NtVPE1a, NtVPE1b) expression and activity in the course of carbon starvation (). Transcription analysis of WT BY-2 cells showed that VPE expression was upregulated during the first 24 h of carbon starvation, and then its level stabilized up to 96 h of carbon starvation (). VPE activity was upregulated during the first 48 h followed by downregulation when incubation was extended to 72–96 h (), suggesting a possible post-transcriptional regulatory mechanism of VPE activity. However, progressive cell death continued.
Figure 3. VPE activity is upregulated in the early phase of carbon starvation, inducing PCD. Six-day-old culture of BY-2 cells was exposed to 96 h of sucrose-free medium. (A) Cell death; cells were stained with Evans blue. (B) Expression levels of VPE1-like (tobacco), relative to that of ACT9 (actin 9) as analyzed by quantitative RT-PCR. (C) VPE activity, measured using the VPE-specific substrate Ac-ESEN-MCA. Data are means ± SE of three repeats. Different letters represent significant differences between time points (P < 0.005) analyzed by ANOVA followed by Tukey–Kramer HSD test
VPE1 relocalizes to vesicles under carbon starvation
To study the mechanism of VPE activation under carbon starvation, StVPE1-GFP was stably expressed in BY-2 cells. It showed a reticular pattern under standard growth conditions and colocalized with an endoplasmic reticulum (ER) marker (ER-Rb; 35s::mCherry-HDEL) [Citation47], as expected for immature VPE () [Citation48]. However, following 24–48 h of carbon starvation, StVPE1-GFP was no longer observed on the ER, but had relocalized to punctate structures with a diameter of 0.2 to 0.48 µm ( and S2). Under these conditions, the ER remained intact, suggesting that the cell was still viable. As VPE needs to be mobilized from the ER to the vacuole to exert its proteolytic pro-PCD activity, the vesicles containing VPE-labeled puncta are likely to be its means of transport. Coexpression of StVPE1-GFP and a tonoplast-red fluorescent protein (RFP) marker [Citation47] in the transgenic BY-2 cell line suggested that the visualized StVPE1-containing puncta localized to the cytoplasm ().
Figure 4. Carbon starvation induces StVPE1-GFP relocalization in BY-2 cells. Confocal images of BY-2 cells incubated in sucrose-supplemented (+ Sucrose) or sucrose-free medium (- Sucrose) for 48 h expressing: (A) StVPE1-GFP (in green) + ER-RFP (in red). (B) StVPE1-GFP (in green) + GAMMA-TIP-RFP (tonoplast marker, in red). Arrows indicate cytoplasmic vesicles; arrowheads indicate punctate StVPE1-GFP. Images are shown as Z-stack projection or single optic section (B, right picture). Bars: 10 µm in (A) and (B), 5 µm in the right picture of B. PhC, phase contrast; N, nucleus
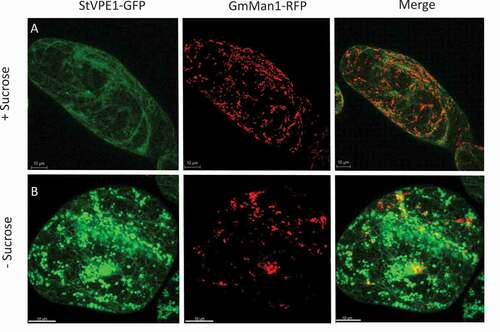
To shed more light on the StVPE1-GFP-labeled vesicle type, we used a Golgi-RFP marker (GmMan1-RFP) [Citation47]. No colocalization was detected between StVPE1-GFP puncta and the Golgi-RFP marker following 48 h of carbon starvation ( and S3). These results suggested that the transport of StVPE1 does not involve the Golgi apparatus upon carbon starvation.
VPE1 is transported to the central vacuole following carbon starvation
To determine whether the StVPE1-GFP puncta eventually translocate to the vacuole, a BY-2 cell line stably expressing StVPE1-GFP and a tonoplast-RFP marker was exposed to carbon starvation. The formation of vesicles containing StVPE1-GFP-labeled bodies in the cytoplasm was observed after 72 h of starvation (). Concanamycin A (ConcA) is a specific inhibitor of vacuolar-type H+-ATPase (V-ATPase) activity, resulting in an increase in vacuolar pH and inhibition of vacuolar enzyme activity [Citation49–51]. Thus, ConcA treatment facilitates detection of the pH-sensitive fluorescence of GFP in the vacuole and prevents the degradation of autophagosomes in the vacuolar lumen, resulting in the accumulation of autophagic bodies [Citation52–55]. In BY-2 cells under carbon starvation and treated with ConcA, StVPE1-labeled puncta clearly accumulated in the vacuole (). Similar results were obtained with the acid-insensitive fluorescent tag RFP, fused to StVPE1 (Fig. S4), suggesting that StVPE1 might be transported to the vacuole by autophagy. In contrast, no signal could be detected in the vacuole after exposure to ConcA treatment in a medium that contained sucrose (). To verify the involvement of autophagy in StVPE1 transport to the vacuole, 3-methyladenine (3-MA), a phosphoinositide 3-kinase (PI3K) inhibitor, was used. PI3K plays an essential role in the formation of autophagosomes [reviewed by Citation56], and 3-MA has been shown to inhibit autophagy in eukaryotic cells, including BY-2 cells [Citation57]. Exposure of a BY-2 cell line stably expressing StVPE1-GFP and GAMMA-TIP-RFP to ConcA and 3-MA under carbon starvation greatly reduced the accumulation of StVPE1-GFP-labeled puncta inside the vacuole (), confirming that these puncta are autophagic bodies. The application of 3-MA caused a significant reduction in VPE activity but, surprisingly, no significant change in cell death ( and S5). This suggested that StVPE1 accumulation in the cell vacuole as a result of carbon starvation is facilitated by an autophagy-like pathway.
Figure 6. StVPE1-GFP translocates to the vacuole during carbon starvation. Six-day-old BY-2 cells coexpressing StVPE1-GFP (in green) and GAMMA-TIP-RFP (tonoplast marker, in red) were incubated for 72 h in various media. (A) – Sucrose: sucrose-free medium. (B) – Sucrose + ConcA: sucrose-free medium with 1 μM concanamycin A (ConcA). (C) – Sucrose + ConcA + 3-MA: sucrose-free medium with 1 μM ConcA, and 5 mM 3-methyladenine (3-MA) for the last 48 h of incubation. (D) + Sucrose + ConcA: control – BY-2 cells were exposed to sucrose-containing medium for 72 h with 1 μM ConcA added for the last 48 h. (A-D) Arrowheads indicate StVPE1-GFP puncta. Bars: 10 µm. N, nucleus; V, vacuole. Images are shown in a single optic section. (E) Quantitative analysis of StVPE1-GFP localization in treated cells after 72 h. Data are mean ± SE of three repeats, each with 100 cells. (F) Effect of 3-MA on VPE activity during carbon starvation. Asterisk represents significant differences between treatments in each time point (P < 0.05) determine by t-test analysis. Data are means ± SE of three experiments
VPE1 colocalizes with ATG8IL under carbon starvation
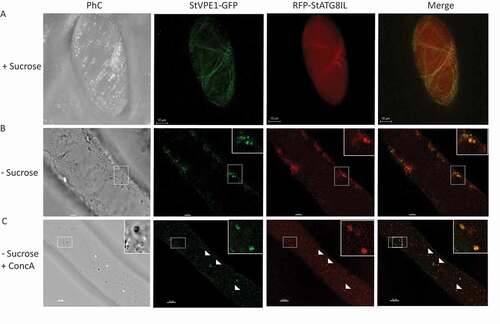
ATG8 is localized to autophagosomal membranes during autophagy [Citation58,Citation59]. An increase in ATG8-labeled puncta is widely used as a functional readout of autophagic activity in tobacco BY-2 cells and plants [Citation49,Citation60]. To further verify the involvement of autophagy in the relocation of StVPE1-GFP to the vacuole under carbon starvation, we stably expressed StVPE1-GFP and either StATG8CL or StATG8IL, both fused to RFP in BY-2 cells [Citation61]. As expected, under standard growth conditions, the fluorescence signal of RFP-StATG8IL was mostly uniformly distributed in the cytoplasm and autophagosomes were rarely seen, whereas StVPE1-GFP primarily localized to the ER (). After 48 and 72 h of carbon starvation, RFP-StATG8IL had accumulated in autophagosomes in the cytoplasm, and RFP-StATG8IL-labeled autophagic bodies could be clearly seen in the vacuole following ConcA treatment ( and S6). Interestingly, colocalization of StVPE1-GFP with RFP-StATG8IL-labeled puncta was observed in both the cytosol and the vacuole ( and ). Quantitative analysis showed that after 72 h of carbon starvation, 23.8 ± 2.8% and 47.7 ± 7.6% of StVPE1-GFP colocalized with RFP-StATG8IL in the cytoplasm or vacuole, respectively. Interestingly, no accumulation of RFP-StATG8CL was detected under carbon starvation (Fig. S7). StVPE1-GFP colocalization with the autophagosome marker RFP-StATG8IL supported the hypothesis that during carbon starvation, VPE1 is relocalized to autophagosomes and transported to the vacuole.
Figure 7. StVPE1-GFP colocalizes with RFP-StATG8IL under carbon starvation. Six-day-old BY-2 cells expressing StVPE1-GFP (green) and RFP-StATG8IL (red) were incubated for 72 h. (A) + Sucrose: with sucrose (shown as a 3D image view). (B) – Sucrose: under carbon starvation. Inset, magnified view of boxed area. (C) – Sucrose + ConcA: as in (B) but with concanamycin A (1 μM) added for the last 48 h. Inset, magnified view of boxed area. Arrowheads indicate colocalization of StVPE1-GFP and RFP-StATG8IL (yellow puncta)
Silencing of ATG4 downregulates VPE1 activity and reduces cell death
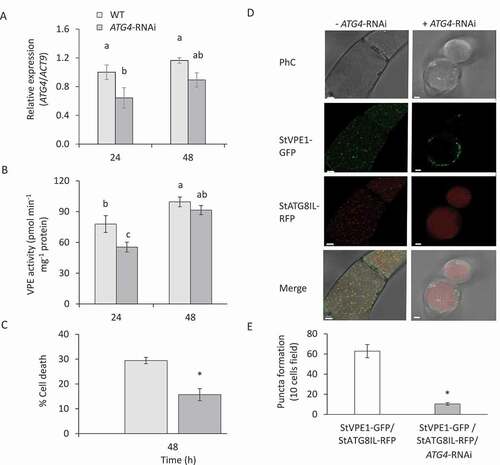
The core autophagy protein ATG4 is a cysteine protease that cleaves the C-terminal part of ATG8 to expose a C-terminal glycine residue, which is then modified by phosphatidylethanolamine for membrane insertion; it is, therefore, essential for autophagosome formation [Citation52,Citation62]. To determine whether the autophagy pathway is necessary for VPE transport to the vacuole and hence controls VPE activation under sucrose starvation, we employed RNAi to knockdown the expression level of ATG4 [Citation63]. The expression of ATG4 in ATG4-RNAi-transgenic BY-2 cells was significantly decreased in the first 24 h following carbon starvation (). Interestingly, VPE activity was reduced in parallel to the reduction in ATG4 expression (). Staining of carbon-starved BY-2 cells with Evans blue showed a decrease in cell death in the ATG4-RNAi line under carbon starvation (), giving rise to higher tolerance to carbon starvation. To verify the effect of ATG4-RNAi on VPE1 transport to the vacuole, StVPE1-GFP and RFP-StATG8IL were expressed in the background of the RNAi line. As expected, in the ATG4-RNAi background, StVPE1-GFP puncta were reduced in the vacuole (). This resulted in a concomitantly reduced number of RFP-StATG8IL-labeled autophagosomes (). Taken together, our results suggested that VPE-induced cell death is dependent on the activity of the autophagy pathway.
Figure 8. ATG4 is required for VPE activity and cell death of BY-2 cells under carbon starvation. (A) Expression level of ATG4 relative to that of ACT9 as analyzed by quantitative RT-PCR. (B) VPE activity, measured using the VPE-specific substrate Ac-ESEN-MCA. (C) Quantification of cell death by Evans blue staining in ATG4-RNAi compared to WT cells. (D) ATG4 silencing decrease StVPE1-GFP transport to the vacuole and colocalization with RFP-StATG8IL. Single optic section of 72 h carbon starved cells in the presence of 1 uM ConcA. (E) Quantification of the numbers of RFP-StATG8IL-labeled autophagosomes. Ten microscopy fields of 10 cells each were counted, in 3D view, for each treatment. Different letters and asterisk represent significant differences (P < 0.05) by ANOVA followed by Tukey–Kramer HSD and t-test, respectively. Data are mean ± SE of three repeats, each with 100 cells
Discussion
Carbon starvation induces VPE activation
During exposure of plants to prolonged growth in the absence of light, the stored carbohydrates are used, and their reserves may be greatly diminished in these low-photosynthetic conditions [Citation64,Citation65]. Understanding the stress response and the adaptive mechanisms to carbon starvation is fundamental. We performed our study in a suspension culture of tobacco BY-2 cells, instead of using a whole plant, since the cultured cells offer several advantages for autophagic studies, including their accessibility to inhibitors and small fluorescent molecules and the ability to induce autophagy by sucrose starvation [Citation57,Citation66].
The VPE-dependent PCD pathway has been shown to be involved not only in immune responses but also in responses to a variety of stress inducers [reviewed by 18]. We have previously demonstrated the involvement of StVPE1 in PCD induced by cold incubation or chemical stress under dark conditions [Citation43,Citation44]. Here, transgenic potato plants expressing StVPE1-GFP grown in the dark without sucrose formed multiple puncta in the leaf epidermal cells (). Since these puncta formation was prevented by light exposure or sugar medium, we assume it is a phenotype of carbon starvation. Low-light conditions were associated previously with autophagy. Significant differences between atg mutants and wild-type plants have been demonstrated, suggesting global effects of autophagy on central metabolism during carbon starvation [Citation67].
We could mimic this leaf phenotype in culture by carbon starvation of BY-2 cells for short periods, 24 h and 48 h. During this period of carbon starvation, VPE expression and activity were induced and accompanied by gradual PCD of the cell population (). Longer exposure to carbon starvation, up to 96 h, stabilized VPE expression and activity (). In accordance, a transient increase in VPE followed by HR-related PCD has been shown by Hatsugai et al. [Citation13], suggesting that VPE is required to initiate the first wave of the cell death process. Silencing VPE led to a higher survival rate for the cells, supporting VPE’s role in response to carbon starvation (). To the best of our knowledge, this is the first time that VPE activity has been shown to be associated with cell death as a result of carbon starvation. Carbon starvation has been associated with growth delay, accelerated degradation of cellular proteins, and an autophagic response in sycamore maple [Citation68], tobacco [Citation69], and Arabidopsis suspension-cultured cells [Citation70,Citation71]. Carbon starvation in cultures of marine pine (Pinus pinaster Ait.) was suggested to induce PCD events [Citation72,Citation73].
Carbon starvation induces autophagic transport of VPE1 to the vacuole
VPEs are synthesized as large precursor proteins and are self-catalytically converted into an active mature form under acidic conditions [Citation18,Citation22,Citation48](Kuroyanagi et al. 2002). This implies that the VPE precursor is transported to the vacuole, where it is converted into its active mature form [Citation74]. However, the transport mechanism is not known. Here, we followed StVPE1 relocalization from the ER to cytosolic vesicles and then to the vacuole under carbon starvation ( and 6). VPE transport to the vacuole did not involve the Golgi (), but rather the autophagy machinery (). Both developmental PCD and HR-related PCD require autophagy and its upstream regulator, the caspase-fold protease metacaspase [Citation75,Citation76]. Metabolic analysis of autophagy-deficient mutants, as well as their phenotypes, suggests that autophagy has global effects on the central metabolism in response to carbon starvation [Citation67]. Here, exposure of BY-2 cells to carbon starvation resulted in sequestration of StVPE1-GFP in membrane vesicles that were eventually relocalized to the vacuole (). Colocalization of StVPE1-GFP with the autophagosome marker RFP-StATG8IL, but not RFP-StATG8CL, and accumulation of double-labeled bodies in the vacuole following treatment with the ATPase inhibitor ConcA suggest the involvement of the autophagy machinery in VPE transport to the cell vacuole during carbon starvation (, S4 and S6). The involvement of direct ER-to-vacuole trafficking through the autophagy pathway was reviewed by Michaeli et al [Citation77]. This route is an important one for vacuole biogenesis, plant growth and the response to environmental stress, supporting the existence of a Golgi-independent, direct ER-to-vacuole trafficking route in plants that uses the autophagy machinery [Citation77]. ER-to-vacuole relocalization has been demonstrated for Arabidopsis GAMMA-VPE through the spindle-shaped ER body, which is considered to be the largest ER-derived body in plants [Citation78]. ER bodies were seen to fuse with the tonoplast following abiotic stress, such as salt treatment, mediating the delivery of Arabidopsis GAMMA-VPE to the vacuole [Citation79]. In addition, the accumulation of two cysteine proteases – RD21 and GAMMA-VPE – on the ER bodies have been identified in Arabidopsis seedlings to be involved in cell death induced by senescence [Citation80]. This indicates that cysteine proteases stored in ER‐derived compartments in senescing tissues reach directly to the vacuole. However, there is no clear evidence linking autophagy with ER-body pathways [Reviewed by 77]. Taken together, we show, for the first time, VPE relocalization from the ER to vesicles that are not related to the Golgi apparatus, but rather to autophagosomes. This suggests VPE transportation by the autophagy pathway following carbon starvation. Though initially defined as a bulk nonselective process, it has become clear in recent years that multiple selective autophagy processes target specific cell components for degradation in response to different environmental or developmental signals [for a recent review, see 34]. ATG8 plays a key role in the selective recruitment of autophagic cargo into autophagosomes, either directly or through cargo receptors that link ATG8 to specific cargo. ATG8 binding is often mediated by a conserved motif, the ATG8-interacting motif (AIM), also known as LC3-interacting region (LIR), on the target protein [Citation81,Citation82]. Vegetative type VPEs contain several evolutionarily well-conserved potential AIMs, as predicted by two available bioinformatics tools, iLIR and hfAIM (Data Set S2) [Citation83]. In contrast, no ATG8–ubiquitin-interacting motif has been found [Citation84], suggesting the intriguing possibility that VPE might be an ATG8 cargo.
VPE1 autophagy induces cell death under carbon starvation
We found several lines of evidence suggesting the involvement of autophagy in VPE transport to the vacuole during carbon starvation, leading to cell death: (i) exposure to starvation resulted in StVPE1-GFP relocalization from the ER to cytosolic vesicles that are transported to the vacuole ( and 6); (ii) an increase in StVPE1-GFP puncta in the vacuole after ConcA treatment in both StVPE1-GFP- and StVPE1-RFP -transgenic cells ( and S4), which were (iii) clearly inhibited in the presence of 3-MA in the culture media (); (iv) StVPE1-GFP colocalized with the autophagy marker RFP-StATG8IL in BY-2 cells in the cytoplasm and vacuole ( and S7); (v) downregulation of the core ATG component ATG4 reduced StVPE1-GFP translocation to the vacuole and BY-2 cell death in response to carbon starvation ().
Genetic analysis in Arabidopsis and tobacco plants has indicated a critical role for autophagy in the initiation and promotion of the HR upon infection with avirulent strains of different pathogens, including Pseudomonas syringae pv. tomato, Tobacco mosaic virus, and Hyaloperonospora arabidopsidis [Citation85–87]. Accordingly, several atg mutants (e.g., atg7, atg9) displayed considerable suppression of HR-associated cell death in Arabidopsis [Citation88]. Autophagy is also thought to contribute to developmental PCD, mostly based on microscopic morphological observations, and has a crucial role in the death of suspensor cells during normal embryogenesis in Norway spruce [Citation89]. In addition, it has been recently suggested that the autophagy pathway might promote PCD during microspore embryogenesis in barley. After a stress treatment at 4°C, autophagosome formation was visible in microspores along with PCD, and treatment with autophagy inhibitors decreased microspore cell death [Citation35]. Autolytic cell death through VPE in BY-2 cells treated with aluminum has been reported [Citation90,Citation91]. Autolytic cell death accompanied by autophagic activity involving the formation of lytic lysosome-like structures has also been described in BY-2 cells treated with cadmium or chemicals and in response to sucrose starvation [Citation92,Citation93]. Here, we show, for the first time to our knowledge, the involvement of the autophagy pathway in VPE translocation to the vacuole (, 6, 7, S4 and S6), followed by VPE activation associated with BY-2 cell death (). In agreement with this, silencing of VPE and ATG4 in BY-2 cells decreased VPE activity and cell death ( and 8). Surprisingly, inhibition of autophagy by 3-MA and prevention of VPE1 release to the vacuole decrease significantly VPE activity but did not modify cell death level ( and S5). Previous studies showed that 3-MA, as well as another autophagy inhibitor wortmannin, led to a strong increase, not decrease, in cell death levels when added to sucrose-starved cells [Citation57,Citation66]. Voitsekhovskaja et al. [Citation66], suggested that 3-MA, which inhibits class I as well as class III phosphatidylinositol 3-kinases, is not fully specific to autophagy but also reported to inhibit, for instance, cell growth. The class I enzymes generate products that inhibit autophagic sequestration, whereas the class III products generally stimulate autophagic sequestration. The overall effect of 3-MA is typically to block autophagy because the class III enzymes that are required to activate autophagy act downstream of the negative regulatory class I enzymes, although cell death may ensue in cell types that are dependent upon high levels of AKT1/protein kinase B for survival [Citation94].
VPEs are cysteine proteases that activate protein precursors functioning in the vacuole [Citation95]. VPEs are involved in cell death through the destruction of the vacuolar membrane and the release of hydrolytic enzymes to the cytoplasm [Citation95,Citation96]. Autophagy has been mainly described as a process that promotes cell survival; here, it is suggested that it can also promote PCD under carbon starvation. Dissecting the relationship between autophagy and PCD is complicated by the fact that the vacuole and its hydrolytic enzymes are needed for the pro-survival homeostasis that maintains autophagy-mediated recycling of biological macromolecules, as well as for vacuolar PCD processes [Citation97]. Much remains to be learned about the relationships between autophagy and VPE translocation and activity. The analysis of an additional tobacco RNAi line for another ATG gene could be generated to verify the conclusion, based on ATG4 RNAi, that VPE1 is transported into the vacuole via autophagy as a step in PCD induction. Clearly, a mechanistic understanding of VPE activity and its substrates in the vacuole, and its effect on cell viability, is critical to being able to better link VPE activity to autophagy.
Materials and methods
Plant material
Freshly harvested potato tubers (Solanum tuberosum L.) cv. Désirée were obtained from a potato-growing field in the northern Negev, Israel. Young plantlets excised from tubers were subjected to surface sterilization, using 10% (v/v) sodium hypochlorite (for 20 min), followed by five washes in sterile water. The apex of each plantlet was cut off and placed into a tube prefilled with Nitsch’s medium (Duchefa, N0224) [Citation98] supplemented with 2% (w:v) sucrose (Duchefa, S0809). Wild type and transgenic potato plants expressing StVPE1-GFP [Citation44] were grown on Nitsch’s medium supplemented with 2% (w:v) sucrose and 50 mg mL−1 kanamycin (Duchefa, K0126). Plants were grown under a 16-h light/8-h dark cycle (long day) at 25°C in a growth chamber. For the carbon-starvation treatment, 10 uniform plants were transferred to dark conditions or to fresh Nitch’s medium without sucrose for 7 d.
Tobacco (Nicotiana tabacum L.) suspension-cultured cells (BY-2; kindly provided by Dr. Yoram Eyal, The Volcani Center, ARO, Rishon LeZion, Israel) were agitated on a rotary shaker at 130 rpm, 26°C, and maintained by weekly dilution (400 μL culture into 20 mL fresh medium) in modified Linsmaier & Skoog (LS) medium (Duchefa, L0230), as previously reported [Citation99]. A sucrose-free culture medium was prepared by omitting sucrose from the culture medium. The pH of these culture media was adjusted to 5.8 with 1 M KOH.
Carbon starvation and viability assay of BY-2 cells
Six-day-old BY-2 cells were collected by gravity flow, and the pellet was resuspended in 30 mL sucrose-free LS medium. After three additional washing steps with 30 mL sucrose-free LS medium, the cells were resuspended in the same volume of fresh medium and kept at 26°C with rotation at 130 rpm. BY-2 cell viability was determined by incubation for 15 min with 0.012% (w:v) Evans blue dissolved in water. Unbound dye was removed by extensive washing with sucrose-free culture medium, and percentage cell death was determined using ImageJ (NIH) digital imaging software [Citation100].
DNA fragmentation assay
DNA fragmentation was evaluated by TUNEL reaction. The TUNEL method was used to detect 3′OH termini of nuclear DNA. The procedure was performed based on the method described by Jones et al. [Citation101] using the In Situ Cell Death Detection Kit, Fluorescein (Roche Applied Science, 11,684,795,910), according to the manufacturer’s instructions.
To visualize nuclei in BY-2 cells, samples were stained with 4’,6-diamidino-2-phenylindole (DAPI; Sigma, 28,718–90-3) at 1 μg mL-1 in PBS buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) for 10 min. DAPI- and TUNEL-positive staining were observed with an IX81/FV500 confocal laser-scanning microscope (Olympus) equipped with a 488-nm argon ion laser and a 405-nm diode laser. DAPI was excited with the 405-nm diode laser, and the emission was filtered with a BA 430- to 460-nm filter. TUNEL was excited with 488 nm of light, and the emission was filtered with a BA505IF filter. The transmitted light images were obtained using Nomarski differential interference contrast, and three-dimensional images were obtained using the FluoView 500 software supplied with the confocal laser-scanning microscope. For the positive control, we incubated fixed BY-2 cells with DNase I (1,500 U mL−1 [Roche, 045536282001] in 50 mM Tris–HCl, pH 7.5, 1 mg mL−1 BSA [Sigma, A6003]) for 10 min at 20°C to induce DNA strand breaks, prior to the labeling procedures. Negative and positive controls were treated identically except for omission of the enzyme solution (terminal deoxynucleotidyl transferase) or after incubation with DNase I for 30 min, respectively.
VPE activity
VPE activity was measured using the method reported by Kuroyanagi et al. [Citation48] with some modifications [Citation44]. Briefly, BY-2 cells were harvested and immediately frozen in liquid nitrogen. Ground tissue (500 mg) was homogenized in 1 mL extraction buffer (50 mM sodium acetate, pH 5.5, 50 mM NaCl, 1 mM EDTA, 100 mM DTT) under ice-cold conditions for protein extraction. The homogenate was centrifuged at 15,000 g for 15 min at 4°C, and 90 µL of the supernatant was used for the enzyme assay. Ac-ESEN-MCA (1 µL of 10 mM) dissolved in DMSO (Peptide Institute, MCA-3227-v) was used as the substrate for the reactions in a final volume of 110 µL (90 µM). The amount of 7-amino-4-methylcoumarin released was determined spectrophotometrically at an excitation wavelength of 380 nm and an emission wavelength of 460 nm (Enspire 2003 Multi Label Reader, Perkin-Elmer) after 2 h of incubation at room temperature. A known amount of 7-amino-4-methylcoumarin was used for calibration. Protein content was determined with PierceTM 660 nm Protein Assay Reagent (Thermo Fisher Scientific, 22,660) using bovine serum albumin as the standard.
Construction of plasmids
VPE-RNAi and StVPE1-GFP constructs were prepared as previously reported [Citation44].
To determine subcellular localization, a tobacco BY-2 cell line stably expressing StVPE1-GFP was coexpressed with an ER marker (HDEL), tonoplast marker (GAMMA-TIP) and Golgi marker (GmMan1) [Citation47], and with autophagosome markers StATG8IL and StATG8CL (autophagy-related proteins; a gift from Dr Tolga Bozkurt Department of Life Sciences, Imperial College London, UK) [Citation61].
For ATG4 silencing, a hairpin RNAi construct targeting a conserved region of ATG4 (Niben101Scf02450g03007.1) was kindly provided by Tolga Bozkurt [Citation63].
BY-2 cell transformation and selection
Transformation of tobacco cell suspension cultures was performed as previously reported [Citation102]. Briefly, a 4-mL aliquot of a 6-d-old exponentially growing suspension of BY-2 cells was transferred to a 250-mL Erlenmeyer flask and incubated for 30 min at 25°C with 40 mL of an overnight culture of Agrobacterium tumefaciens EHA105 (kindly provided by Dr. Yoram Eyal, The Volcani Center, ARO, Rishon LeZion, Israel) harboring the binary plasmid, and containing 500 μM acetosyringone (Sigma, D134406) and 10 mM MgSO4. After 2 d of cocultivation, the cells were washed with modified liquid LS containing 250 μg mL−1 claforan (Duchefa, C0111), 50 μg mL−1 kanamycin (Duchefa, K0126), 15 μg mL−1 hygromycin (Toku-E, 31,282–04-9) and 2 μg mL−1 Basta herbicide (Plant media, SKU# 30,632,007–5). After 2 weeks, the antibiotic-resistant cells were transferred to solid medium containing 250 μg mL−1 claforan and the appropriate antibiotic (eg. 50 μg mL−1 kanamycin). Four weeks later, the selected transformants were transferred to a modified liquid LS medium containing the appropriate antibiotic.
RNA extraction
RNA extraction was performed as described by Chen et al. [Citation103] with some modifications. Briefly, BY-2 cells were harvested and immediately frozen in liquid nitrogen. Pulverized tissue (0.5 g) was added to 1.5 mL prewarmed (65°C) extraction buffer (100 mM Tris–HCl, pH 8.0, 25 mM EDTA, 2 M NaCl, 3% [w:v] CTAB [Sigma, H5882], 4% [w:v] polyvinylpyrrolidone 40 [Sigma, 9003–39-8], 3% [w:v] beta-mercaptoethanol) and samples were incubated for 45 min at 65°C. After cooling the samples to room temperature, 1.5 mL of chloroform:isoamylalcohol (24:1, v/v) was added. Samples were vortexed and incubated for 10 min at room temperature, then centrifuged at 12,000 g for 20 min at 4°C. The upper phase was collected, and the above steps were repeated. RNA was precipitated for 2.5 h at −20°C by the addition of LiCl at a final concentration of 3 M. Following centrifugation at 12,000 g and 4°C for 20 min, the pellet was washed twice with 1.5 mL of 70% ethanol, centrifuged for 10 min, and air-dried at room temperature. Finally, the pellet was resuspended in 50 µL ultrapure water. After extraction, RNA samples were treated with the Turbo DNA-free Kit (Invitrogen, Thermo Fisher Scientific, AM1907) to remove contaminating DNA according to the manufacturer’s protocol. Concentrations of RNA samples were measured with a ND-1000 spectrophotometer (Nanodrop Technologies) and purity was verified by the ratio of optical density at 260 nm and 280 nm (OD260:OD280 between 1.80 and 2.05), and OD260:OD230 (between 2.00 and 2.30). Sample integrity was evaluated by electrophoresis on a 1% agarose gel containing 0.5 μg mL−1 SafeView Nucleic Acid Stain (NBS Biologicals, NBS-SV1). Observation of intact 18S and 28S rRNA subunits and absence of smears in the gel indicated minimal RNA degradation.
cDNA synthesis and RT-PCR analysis
cDNA was synthesized from 400 ng of total BY-2 RNA using the qPCRBIO cDNA Kit (PCR Biosystems) according to the manufacturer’s specifications. RT-PCR primers, synthesized by Hylabs (Rehovot, Israel), were designed using Primer
Express 2.0 (Applied Biosystems, Foster City, CA). Expression analysis of tobacco endogenous VPE genes, NtVPE2, NtVPE3, NtVPE1a, NtVPE1b (vegetative type VPEs) [Citation44], was performed with the primers: F 5’-GGGTACCGATCCTGCAAATG-3’ and R 5’-TGCATCACGCTGGTTGACA-3’. For ATG4 expression analysis, the primers were: F 5’-CACAGTCAGCCGCATGACC-3’ and R 5’-GACCATATGTCTTCCCGGCTTG-3’. For ACT9 (actin 9), used as the housekeeping gene, primers were: F 5’- CTATTCTCCGCTTTGGACTTGGCA-3’ and R 5’-AGGACCTCAGGACAACGGAAACG-‘3 (GenBank accession no. X69885), as previously described [Citation91]. Quantitative real‐time RT‐PCR was performed in a total volume of 10 µL including 5 µL fast SYBRTM Green Master Mix (Applied Biosystems, AB-4,385,612). The following program: 95°C for 20 min, 40 cycles of 95°C for 3 s and 60°C for 30 s was run in a StepOne Real-Time PCR machine (Applied Biosystems). The quality of the graphs, melting curves and quantitative analyses of the data were performed using StepOne software Version 2.2.2 (Applied Biosystems).
Potato plant transformation and transgenic selection
Potato leaves (cv. Désirée) were used for Agrobacterium-mediated leaf-disc infection as described previously [Citation104,Citation105]. Transgenic plants were selected on 25 mg L−1 kanamycin (Duchefa, K0126).* [Citation106–109] For transgenic plant validation, DNA extraction from potato leaves and PCR were performed as described previously [Citation43] using primers VPE-F 5’-TGGTCAAAGAGAGAACTGCCAG-3’ and GFP-R 5’-GATGTTGTGGCGGATCTT-3’, amplifying a PCR fragment of 908 bp.
Live-cell imaging by confocal laser-scanning microscopy
A Leica SP8/LAS X confocal laser-scanning microscope was used to observe fluorescently labeled cells and leaves. GFP and RFP were excited at 488 and 561 nm with an argon laser and visualized at 495–550 nm and 570–620 nm, respectively. Pearson’s correlation coefficient was calculated by selecting a region of interest in 15 repeats. Analyzed images had the same acquisition parameters and chosen thresholds. Image series (Z-stacks) and colocalization analysis between StVPE1-GFP and RFP-ATG8IL were performed using Bitplane Imaris software version 8.0.1 (Bitplane A.G.). About 100 cells in three biological replicates were analyzed per genotype.
Treatment of BY-2 cells with autophagy inhibitors
A 500-μL aliquot of 5-d-old tobacco culture was transferred to a sterile 48-well Petri dish supplemented with a final concentration of 1 μM ConcA (Sigma, C9705). ConcA was prepared as a 100 μM stock solution in DMSO. As a control, DMSO was added to the tobacco culture at the same final volume [Citation55]. 3-MA (Sigma, 5142–23-4) was added to BY-2 cells at a final concentration of 5 mM. 3-MA was solubilized in BY-2 medium without sugar, under gentle heating (45°C), as a stock of 100 mM. The cells supplemented with the inhibitors were cultured at 26°C with rotation of 130 rpm for 48 h until GFP or RFP analysis on about 100 cells in 3–4 repeats.
Statistical analysis
Statistical analysis of the data was performed with JMP-in software (version 3 for Windows; SAS Institute), using a t-test, or by two-way analysis of variance (ANOVA) followed by Tukey–Kramer HSD test. Statistical significance was set at P < 0.05. Values were expressed as mean ± standard error of the mean (SEM).
Supplemental Material
Download MS Word (16.6 MB)Acknowledgments
The authors thank Professor Robert Fluhr, from the Department of Plant Sciences, Weizmann Institute of Science, Rehovot, Israel, and Dr. Yasin F. Dagdas, from Gregor Mendel Institute, Austrian Academy of Sciences, Vienna BioCenter, Vienna, Austria for valuable suggestions and constructive criticism.
Disclosure statement
There is no potential conflict of interest to be disclosed.
Supplementary Material
Supplemental data for this article can be accessed here.
References
- Escamez S, Tuominen H. Programmes of cell death and autolysis in tracheary elements: when a suicidal cell arranges its own corpse removal. J Exp Bot. 2014;65(5):1313-1321.
- Devillard C, Walter C. Formation of plant tracheary elements in vitro–a review. N Z J Forestry Sci. 2014;44(1):22.
- Petrov V, Hille J, Mueller-Roeber B, et al. ROS-mediated abiotic stress-induced programmed cell death in plants. Front Plant Sci. 2015;6(69).
- Del Río LA. ROS and RNS in plant physiology: an overview. J Exp Bot. 2015;66(10):2827–2837.
- Suzuki N, Koussevitzky S, Mittler R, et al. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012;35(2):259–270.
- van Doorn WG, Beers EP, Dangl JL, et al. Morphological classification of plant cell deaths. Cell Death Differ. 2011;18(8):1241–1246.
- Pak C, Van Doorn WG. Delay of iris flower senescence by protease inhibitors. New Phytol. 2005;165(2):473–480.
- Schaller A, Stintzi A, Rivas S, et al. From structure to function–a family portrait of plant subtilases. New Phytol. 2018;218(3):901–915.
- Woltering EJ, van der Bent A, Hoeberichts FA. Do plant caspases exist? Plant Physiol. 2002;130(4):1764–1769.
- Belenghi B, Salomon M, Levine A. Caspase-like activity in the seedlings of Pisum sativum eliminates weaker shoots during early vegetative development by induction of cell death. J Exp Bot. 2004;55(398):889–897.
- Iakimova ET, Woltering EJ. Xylogenesis in zinnia (Zinnia elegans) cell cultures: unravelling the regulatory steps in a complex developmental programmed cell death event. Planta. 2017;245:681–705.
- Vercammen D, Van De Cotte B, De Jaeger G, et al. Type II metacaspases Atmc4 and Atmc9 of Arabidopsis thaliana cleave substrates after arginine and lysine. J Biol Chem. 2004;279(44):45329–45336.
- Hatsugai N, Kuroyanagi M, Yamada K, et al. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science. 2004;305(5685):855–858.
- Rojo E, Martın R, Carter C, et al. VPEγ exhibits a caspase-like activity that contributes to defense against pathogens. Curr Biol. 2004;14(21):1897–1906.
- Silva RD, Sotoca R, Johansson B, et al. Hyperosmotic stress induces metacaspase- and mitochondria-dependent apoptosis in Saccharomyces cerevisiae. Mol Microbiol. 2005;58(3):824–834.
- Van Durme M, Nowack MK. Mechanisms of developmentally controlled cell death in plants. Curr Opin Plant Biol. 2016;29:29–37.
- Sueldo DJ. van der Hoorn RA.Plant life needs cell death, but does plant cell death need Cys proteases? Febs J. 2017;284(10):1577–1585.
- Hatsugai N, Yamada K, Goto-Yamada S, et al. Vacuolar processing enzyme in plant programmed cell death. Front Plant Sci. 2015;6:234.
- Cai Y-M, Gallois P. Programmed Cell Death Regulation by Plant Proteases with Caspase-Like Activity. In: Gunawardena A, McCabe P, editors. Plant programmed cell death. Springer; 2015. p. 191–202.
- Hara-Nishimura I, Takeuchi Y, Nishimura M. Molecular characterization of a vacuolar processing enzyme related to a putative cysteine proteinase of Schistosoma mansoni. Plant Cell. 1993;5:1651–1659.
- Hara-Nishimura I, Inoue K, Nishimura M. A unique vacuolar processing enzyme responsible for conversion of several proprotein precursors into the mature forms. FEBS Lett. 1991;294(1–2):89–93.
- Hara-Nishimura I, Hatsugai N, Nakaune S, et al. Vacuolar processing enzyme: an executor of plant cell death. Curr Opin Plant Biol. 2005;8(4):404–408.
- Kuroyanagi M, Yamada K, Hatsugai N, et al. Vacuolar processing enzyme is essential for mycotoxin-induced cell death in Arabidopsis thaliana. J Biol Chem. 2005;280(38):32914–32920.
- Vorster BJ, Cullis C, Kunert K. Plant vacuolar processing enzymes. Front Plant Sci. 2019;10:479.
- Yamada K, Nishimura M, Hara-Nishimura I. The slow wound-response of γVPE is regulated by endogenous salicylic acid in Arabidopsis. Planta. 2004;218(4):599–605.
- Zhang H, Dong S, Wang M, et al. The role of vacuolar processing enzyme (VPE) from Nicotiana benthamiana in the elicitor-triggered hypersensitive response and stomatal closure. J Exp Bot. 2010;61(13):3799–3812.
- Avin-Wittenberg T, Baluška F, Bozhkov PV, et al. Review of autophagy-related approaches for improving nutrient use efficiency and crop yield protection. J Exp Bot. 2018;69(6):1335-1353.
- Marshall RS, Vierstra RD. Autophagy: the master of bulk and selective recycling. Annu Rev Plant Biol. 2018;69(1):173–208.
- Signorelli S, Tarkowski ŁP, Van den Ende W, et al. Linking autophagy to abiotic and biotic stress responses. Trends Plant Sci. 2019;24(5):413-430.
- Galluzzi L, Baehrecke EH, Ballabio A, et al. Molecular definitions of autophagy and related processes. Embo J. 2017;36:1811–1836.
- Bassham DC. Plant autophagy—more than a starvation response. Curr Opin Plant Biol. 2007;10(6):587–593.
- Floyd BE, Pu Y, Soto-Burgos J, et al. To live or die: autophagy in plants. In: Gunawardena A, Mc Cabe P, editors. Plant programmed cell death. Springer; 2015. p. 269–300.
- Üstün S, Hafrén A, Hofius D. Autophagy as a mediator of life and death in plants. Curr Opin Plant Biol. 2017;40:122–130.
- Avin-Wittenberg T, Baluška F, Bozhkov PV, et al. Autophagy-related approaches for improving nutrient use efficiency and crop yield protection. J Exp Bot. 2018;69:1335–1353.
- Bárány I, Berenguer E, Solís M-T, et al. Autophagy is activated and involved in cell death with participation of cathepsins during stress-induced microspore embryogenesis in barley. J Exp Bot. 2018;69(6):1387–1402.
- Zhou J, Yu JQ, Chen Z. The perplexing role of autophagy in plant innate immune responses. Mol Plant Pathol. 2014;15(6):637–645.
- Leary AY, Sanguankiattichai N, Duggan C, et al. Modulation of plant autophagy during pathogen attack. J Exp Bot. 2018;69(6):1325–1333.
- Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333(1–2):169–174.
- Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2016;12:1–222.
- Kellner R, De la Concepcion JC, Maqbool A, et al. ATG8 expansion: a driver of selective autophagy diversification?. Trends Plant Sci. 2017;22(3):204–214.
- Smeekens S, Ma J, Hanson J, et al. Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol. 2010;13(3):273–278.
- Keunen E, Peshev D, Vangronsveld J, et al. Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant Cell Environ. 2013;36(7):1242–1255.
- Teper-Bamnolker P, Buskila Y, Lopesco Y, et al. Release of apical dominance in potato tuber is accompanied by programmed cell death in the apical bud meristem. Plant Physiol. 2012;158(4):2053–2067.
- Teper‐Bamnolker P, Buskila Y, Belausov E, et al. Vacuolar processing enzyme (VPE) activates programmed cell death in the apical meristem inducing loss of apical dominance. Plant Cell Environ. 2017;40(10):2381–2392.
- Kabbage M, Kessens R, Bartholomay LC, et al. The Life and Death of a Plant Cell. Annu Rev Plant Biol. 2017;68(1):375-404.
- Shimada T, Takagi J, Ichino T, et al. Plant vacuoles. Annu Rev Plant Biol. 2018;69(1):123–145.
- Nelson BK, Cai X, Nebenführ A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. The Plant Journal. 2007;51(6):1126–1136.
- Kuroyanagi M, Nishimura M, Hara-Nishimura I. Activation of Arabidopsis vacuolar processing enzyme by self-catalytic removal of an auto-inhibitory domain of the C-terminal propeptide. Plant Cell Physiol. 2002;43(2):143–151.
- Hanamata S, Kurusu T, Okada M, et al. In vivo imaging and quantitative monitoring of autophagic flux in tobacco BY-2 cells. Plant Signal Behav. 2013;8(1):e22510.
- Tamura T, Kioi Y, Miki T, et al. Fluorophore labeling of native FKBP12 by ligand-directed tosyl chemistry allows detection of its molecular interactions in vitro and in living cells. Journal of the American Chemistry Chemical Society. 2013;135(18):6782–6785.
- Tamura K, Shimada T, Ono E, et al. Why green fluorescent fusion proteins have not been observed in the vacuoles of higher plants. Plant J. 2003;35(4):545–555.
- Yoshimoto K, Hanaoka H, Sato S, et al. Processing of ATG8s, ubiquitin-like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell. 2004;16(11):2967–2983.
- Thompson AR, Doelling JH, Suttangkakul A, et al. Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol. 2005;138(4):2097–2110.
- Xiong Y, Contento AL, Nguyen PQ, et al. Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol. 2007;143(1):291–299.
- Yano K, Yanagisawa T, Mukae K, et al. Dissection of autophagy in tobacco BY-2 cells under sucrose starvation conditions using the vacuolar H+-ATPase inhibitor concanamycin A and the autophagy-related protein Atg8. Plant Signal Behav. 2015;10(11):e1082699.
- He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Ann Rev Genet. 2009;43(1):67.
- Takatsuka C, Inoue Y, Matsuoka K, et al. 3-Methyladenine inhibits autophagy in tobacco culture cells under sucrose starvation conditions. Plant Cell Physiol. 2004;45(3):265–274.
- Kirisako T, Baba M, Ishihara N, et al. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J Cell Biol. 1999;147(2):435–446.
- Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000;19(21):5720–5728.
- Bassham DC. Methods for analysis of autophagy in plants. Methods. 2015;75:181–188.
- Dagdas YF, Belhaj K, Maqbool A, et al. An effector of the Irish potato famine pathogen antagonizes a host autophagy cargo receptor. Elife. 2016;5:e10856.
- Kirisako T, Ichimura Y, Okada H, et al. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol. 2000;151(2):263–276.
- Dagdas YF, Pandey P, Tumtas Y, et al. Host autophagy machinery is diverted to the pathogen interface to mediate focal defense responses against the Irish potato famine pathogen. Elife. 2018;7:e37476.
- Armarego-Marriott T, Sandoval-Ibañez O, Kowalewska Ł. Beyond the darkness: recent lessons from etiolation and de-etiolation studies. J Exp Bot. 2020;71(4):1215–1225.
- Salam BB, Malka SK, Zhu X, et al. Etiolated stem branching is a result of systemic signaling associated with sucrose level. Plant Physiol. 2017;175(2):734–745.
- Voitsekhovskaja OV, Schiermeyer A, Reumann S. Plant peroxisomes are degraded by starvation-induced and constitutive autophagy in tobacco BY-2 suspension-cultured cells. Front Plant Sci. 2014;5:629.
- Avin-Wittenberg T, Bajdzienko K, Wittenberg G, et al. Global analysis of the role of autophagy in cellular metabolism and energy homeostasis in Arabidopsis seedlings under carbon starvation. Plant Cell. 2015;27(2):306–322.
- Aubert S, Gout E, Bligny R, et al. Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrates. J Cell Biol. 1996;133(6):1251–1263.
- Moriyasu Y, Ohsumi Y. Autophagy in tobacco suspension-cultured cells in response to sucrose starvation. Plant Physiol. 1996;111(4):1233–1241.
- Contento AL, Kim S-J, Bassham DC. Transcriptome profiling of the response of Arabidopsis suspension culture cells to Suc starvation. Plant Physiol. 2004;135(4):2330–2347.
- Rose TL, Bonneau L, Der C, et al. Starvation‐induced expression of autophagy‐related genes in Arabidopsis. Biol Cell. 2006;98(1):53–67.
- Azevedo H, Dias A, Tavares RM. Establishment and characterization of Pinus pinaster suspension cell cultures. Plant Cell Tissue Organ Cult. 2008;93(1):115–121.
- Azevedo H, Castro PH, Gonçalves JF, et al. Impact of carbon and phosphate starvation on growth and programmed cell death of maritime pine suspension cells. In Vitro Cell Dev Biology-Plant. 2014;50(4):478–486.
- Kinoshita T, Yamada K, Hiraiwa N, et al. Vacuolar processing enzyme is up-regulated in the lytic vacuoles of vegetative tissues during senescence and under various stressed conditions. Plant J. 1999;19(1):43–53.
- Minina EA, Bozhkov PV, Hofius D. Autophagy as initiator or executioner of cell death. Trends Plant Sci. 2014;19(11):692–697.
- Minina EA, Smertenko AP, Bozhkov PV. Vacuolar cell death in plants. Autophagy. 2014;10(5):1–2.
- Michaeli S, Avin-Wittenberg T, Galili G. Involvement of autophagy in the direct ER to vacuole protein trafficking route in plants. Front Plant Sci. 2014;5:134.
- Yamada K, Hara-Nishimura I, Nishimura M. Unique defense strategy by the endoplasmic reticulum body in plants. Plant Cell Physiol. 2011;52:2039–2049.
- Hayashi Y, Yamada K, Shimada T, et al. A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiol. 2001;42(9):894–899.
- Rojo E, Zouhar J, Carter C, et al A unique mechanism for protein processing and degradation in Arabidopsis thaliana. Proc Nat Acad Sci. 2003;100(12):7389–7394.
- Michaeli S, Galili G, Genschik P, et al. Autophagy in plants–what’s new on the menu? Trends Plant Sci. 2016;21(2):134–144.
- Birgisdottir ÅB, Lamark T, Johansen T. The LIR motif–crucial for selective autophagy. J Cell Sci. 2013;126:3237–3247.
- Kalvari I, Tsompanis S, Mulakkal NC, et al. iLIR: A web resource for prediction of Atg8-family interacting proteins. Autophagy. 2014;10(5):913–925.
- Marshall RS, Hua Z, Mali S, et al. ATG8-binding UIM proteins define a new class of autophagy adaptors and receptors. Cell. 2019;177(3):e24.
- Coll N, Smidler A, Puigvert M, et al. The plant metacaspase AtMC1 in pathogen-triggered programmed cell death and aging: functional linkage with autophagy. Cell Death Differ. 2014;21(9):1399.
- Han S, Wang Y, Zheng X, et al. Cytoplastic glyceraldehyde-3-phosphate dehydrogenases interact with atg3 to negatively regulate autophagy and immunity in Nicotiana benthamiana. Plant Cell. 2015;27(4):1316–1331.
- Hackenberg T, Juul T, Auzina A, et al. Catalase and NO CATALASE ACTIVITY1 promote autophagy-dependent cell death in Arabidopsis. Plant Cell. 2013;25(11):4616–4626.
- Hofius D, Schultz-Larsen T, Joensen J, et al. Autophagic components contribute to hypersensitive cell death in Arabidopsis. Cell. 2009;137(4):773–783.
- Minina EA, Filonova LH, Fukada K, et al. Autophagy and metacaspase determine the mode of cell death in plants. J Cell Biol. 2013;203(6):917–927.
- Kariya K, Demiral T, Sasaki T, et al. A novel mechanism of aluminium-induced cell death involving vacuolar processing enzyme and vacuolar collapse in tobacco cell line BY-2. J Inorg Biochem. 2013;128:196–201.
- Kariya K, Tsuchiya Y, Sasaki T, et al. Aluminium-induced cell death requires upregulation of NtVPE1 gene coding vacuolar processing enzyme in tobacco (Nicotiana tabacum L). J Inorg Biochem. 2018;181:152–161.
- Iakimova ET, Yordanova ZP, Cristescu SM, et al. Cell death signaling and morphology in chemical-treated tobacco BY-2 suspension cultured cells. Environ Exp Bot. 2019;164:157–169.
- Kutik J, Kuthanova A, Smertenko A, et al. Cadmium‐induced cell death in BY‐2 cell culture starts with vacuolization of cytoplasm and terminates with necrosis. Physiol Plant. 2014;151(4):423–433.
- Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4(2):151–175.
- Hatsugai N, Kuroyanagi M, Nishimura M, et al. A cellular suicide strategy of plants: vacuole-mediated cell death. Apoptosis. 2006;11(6):905–911.
- Hara-Nishimura I, Hatsugai N. The role of vacuole in plant cell death. Cell Death Differ. 2011;18(8):1298–1304.
- Müntz K. Protein dynamics and proteolysis in plant vacuoles. J Exp Bot. 2007;58(10):2391–2407.
- Nitsch J, Nitsch C. Haploid plants from pollen grains. Science. 1969;163(3862):85–87.
- Nagata T, Nemoto Y, Hasezawa S. Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int Rev Cytol. 1992;132:1–30.
- Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–43.
- Jones AM, Coimbra S, Fath A, et al. Programmed cell death assays for plants. Methods Cell Biol. 2001;66:437–451.
- Frydman A, Weisshaus O, Bar‐Peled M, et al. Citrus fruit bitter flavors: isolation and functional characterization of the gene Cm1, 2RhaT encoding a 1, 2 rhamnosyltransferase, a key enzyme in the biosynthesis of the bitter flavonoids of citrus. Plant J. 2004;40(1):88–100.
- Chen L, Guo Y, Bai G, et al. Effect of 5-aminolevulinic acid and genistein on accumulation of polyphenol and anthocyanin in’Qinyang’apples. J Anim Plant Sci. 2015;25:68–79.
- Rocha-Sosa M, Sonnewald U, Frommer W, et al. Both developmental and metabolic signals activate the promoter of a class I patatin gene. Embo J. 1989;8(1):23–29.
- Horsch R, Rogers S, Fraley R. Transgenic plants. In: Cold Spring Harbor Symposia on Quantitative Biology. 1985;50:433–437.
- van der Hoorn RA, Kaiser M. Probes for activity-based profiling of plant proteases. Physiol Plant. 2012;145(1):18–27.
- Fischer J, Becker C, Hillmer S, et al. The families of papain-and legumain-like cysteine proteinases from embryonic axes and cotyledons of Vicia seeds: developmental patterns, intracellular localization and functions in globulin proteolysis. Plant Mol Biol. 2000;43(1):83–101.
- Zakharov A, Müntz K. Seed legumains are expressed in stamens and vegetative legumains in seeds of Nicotiana tabacum L. J Exp Bot. 2004;55(402):1593–1595.
- Xie Q, Tzfadia O, Levy M, et al. hfAIM: A reliable bioinformatics approach for in silico genome-wide identification of autophagy-associated Atg8-interacting motifs in various organisms. Autophagy. 2016;12(5):876–887.