ABSTRACT
Temperature variations induce stressful conditions that challenge the ability of organisms to maintain cell homeostasis. The intensity and duration of heat stress affect cell response very differently, ranging from a beneficial effect – hormesis – to necrotic cell death. There is a strong interplay between the cell response to heat shock and macroautophagy/autophagy, which is induced to cope with stress. Using Caenorhabditis elegans, we developed a new paradigm to study adaptation to acute non-lethal heat-stress (aHS) during development. We found that aHS results in transient fragmentation of mitochondria, decreased cellular respiration, and delayed development. Moreover, an active autophagy flux associated with mitophagy events is triggered in many tissues, enables the rebuilding of the mitochondrial network and modulates the adaptive plasticity of the development, showing that the autophagic response is protective for C. elegans. Using genetic and cellular approaches, we showed that mitochondria are a major site for autophagosome biogenesis in the epidermis, under both standard and heat-stress conditions. We determined that DRP-1 (Dynamin-Related Protein 1) involved in mitochondrial fission, is an important player for the autophagy process and the adaptation to aHS. Our study suggests that DRP-1 is involved in coordinating mitochondrial fission and autophagosome biogenesis during stress.
Heat stress-induced adaptive response has been extensively studied at the onset of C. elegans adulthood, but much less information is available on how the worm gets along with the impact of heat-stress through its development. We experimentally tested how C. elegans can cope with environmental stress such as heat shock during its development and investigated the mechanisms underlying its adaptation.
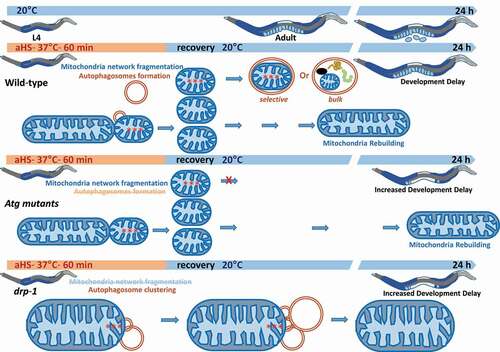
In our study [Citation1], we report that an acute non-lethal heat-stress (aHS: 37°C during 60 min) delivered to early 4th stage larvae induces organelle modifications, developmental delay and a slight reduction in the progeny number without any lethality. Specifically, aHS induces a mitochondrial network fragmentation and an alteration of its functioning, that both recover after a 24-h recovery period ().
Figure 1. Acute heat-stress (aHS), delivered to C. elegans L4 larvae, alters its development, induces mitochondrial network fission and formation of aggregates (red asterisks) within the mitochondrial matrix, and triggers an autophagic flux. Autophagy is required for stress adaptation because autophagy mutants show delay in development and mitochondrial rebuilding after aHS compared to WT animals. Moreover, both aHS-induced mitochondria fragmentation and aHS-induced autophagosome formation are dependent on DRP-1. Our data show that a DRP-1-dependent autophagy process takes place as part of the worm adaptive mechanism to aHS, probably by coordinating autophagosome biogenesis with mitochondria fission
There is a strong interplay between autophagy induction and environmental stress condition such as HS. We used transgenic C. elegans strain expressing either ATG-18::GFP or GFP::LGG-1 and GFP::LGG-2 to label and visualize autophagosome site formation and maturation during aHS. We observed an increase of ATG-18::GFP dots, and GFP::LGG-1 and GFP::LGG-2 puncta and monitored the increased ratio of the lipidated to non-lipidated form of LGG-1 after aHS. Altogether these results indicate that aHS triggers an autophagic flux.
Then, we used multiple genetic approaches to test whether autophagy contributes to aHS-induced developmental plasticity. Our study shows that autophagy mutants can survive the aHS but mitochondrial rebuilding and developmental recovery are delayed and progeny number decreased in autophagy mutants when compared to wild type. These results demonstrate that autophagic flux protects larvae from aHS and facilitates its adaptation following aHS ().
In the epidermis, we found that a large number of autophagosomes are associated with, or are in close vicinity to, mitochondria, which is suggestive of mitophagy events. We hypothesized that mitophagy pathways might be involved in aHS adaptation. Therefore, we tested various mutants known to eliminate specifically the pathways involved in selective autophagy of mitochondria in C. elegans. We found that mitophagy genes pink-1/PINK1, pdr-1/PRKN/Parkin, dct-1/BNIP3L/NIX, and fndc-1/FUNDC1 are dispensable for adaptation to aHS. This result suggests that autophagy and not mitophagy is required for aHS adaptation. Alternatively, if mitophagy is involved it may be through a yet unknown pathway/mechanism.
We then investigated how autophagosomes mature following aHS, employing a combination of reverse genetic screening, live cell imaging and electron microscopy of the epidermis. We then depleted fzo-1/MFN2 and drp-1/DNM1L to look whether contact sites between the endoplasmic reticulum and mitochondria could be involved in response to aHS. We found that animals with null mutation in drp-1 do not show mitochondrial fission upon aHS, indicating that DRP-1 is necessary for aHS-induced mitochondrial fragmentation. Moreover, the drp-1 mutant shows a similar developmental delay as Atg mutants with no additive effect, suggesting that DRP-1 and autophagy are required together for developmental recovery following aHS (). Moreover, employing a CRISPR engineered strain expressing DRP-1::GFP, we observed more DRP-1 colocalization with mitochondria and autophagy reporters upon aHS, supporting a role of DRP-1 during autophagosome biogenesis. Indeed, we found that after aHS, drp-1 depleted animals display abnormal clusters of autophagosomes that amalgamate with mitochondria, forming opened-shape membranous structure surrounding cytoplasmic cargoes. We excluded the possibility of an indirect consequence due to the mitochondria size as the double mutant fzo-1;drp-1, in which the mitochondrial network is more fragmented, displays a similar defect as observed in drp-1. Altogether, we demonstrated that DRP-1 is involved in autophagosome formation as part of the C. elegans adaptive response to acute heat stress. In mammals, DNM1L/Drp1 is not only involved in regulating mitochondrial fission but, participates together with the Zn2+ transporter SLC39A1/ZIP1 in a mitochondria control quality mechanism, and modulates ER shape promoting ER-mitochondria contact sites. Our result indicate that DRP-1 contribute to autophagosome biogenesis upon aHS.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Chen Y, Leboutet R, Largeau C, et al. Autophagy facilitates mitochondrial rebuilding after acute heat stress via a DRP-1-dependent process. J Cell Biol. 2021 Apr 5;220(4):e201909139. PMID: 33734301.
