ABSTRACT
Macroautophagy/autophagy is a multi-step process that leads to cargo degradation via the fusion of hydrolases-containing lysosomes with cargo-loaded autophagosomes. For this process to occur, autophagosomes are directionally transported by molecular motors toward the nucleus, where they fuse with lysosomes for cargo degradation. The molecular basis for this regulation, including the cell machinery required for this directional transport, has not been fully identified. Using a combination of proteomic and live-imaging approaches in mammalian cells, including primary neurons, we describe that the phosphorylation of the autophagosome protein Atg8/LC3B by the Hippo kinase STK4/MST1, an event we previously reported to be required for autophagy completion, reduces the binding of the transport-related protein FYCO1 to MAP1LC3B/LC3B. This event in turn allows the proficient microtubule-based transport of autophagosomes toward the perinuclear area, thus facilitating the contact of autophagosomes with lysosomes. In the absence of LC3B phosphorylation, autophagosomes undergo aberrant transport including increased movement toward the cell periphery resulting in reduced autophagosome-lysosome colocalization. Thus, LC3B phosphorylation modulates the directional transport of autophagosomes to meet with lysosomes in the perinuclear area, a crucial event in ensuring autophagic degradation of cargo.
The Atg8 family of proteins, including LC3B, interact with various proteins on the phagophore and autophagosomal membranes to support key steps of the autophagy process, including the binding to cargo receptors to drive cargo recruitment. LC3B bound to the outer member of the autophagosome also regulates the association of autophagosomes with proteins that mediate autophagosome transport and autophagosome-lysosome fusion. How this array of protein-protein interactions is regulated remains unclear, but post-translational modification of Atg8-family proteins is emerging as an important event in the regulation of the autophagy process.
Our lab previously described that phosphorylation of LC3B at threonine 50 (T50) by the Hippo kinase STK4 is required for autophagy via unknown mechanisms. In our recent article [Citation1], we characterize the molecular consequences of this phosphorylation. We speculated that this post-translational modification may be required to regulate key protein-protein interactions established by LC3B. Proteomic analysis of LC3B phosphorylation-dependent interactors in mammalian 293T cells revealed that the association of the transport-related protein FYCO1 to LC3B is controlled by LC3B phosphorylation status. Specifically, FYCO1 binding to LC3B strongly decreases upon LC3B phosphorylation on T50, and FYCO1 associates preferentially with autophagosomes containing a phospho-deficient LC3BT50A mutant and less so with a phospho-mimetic LC3BT50E mutant. Notably, the effect of LC3B phosphorylation on FYCO1 interaction with LC3B appears to be specific, as the binding of other LC3B interactors such as SQSTM1/p62, or KEAP1 is not significantly affected by this post-translational modification.
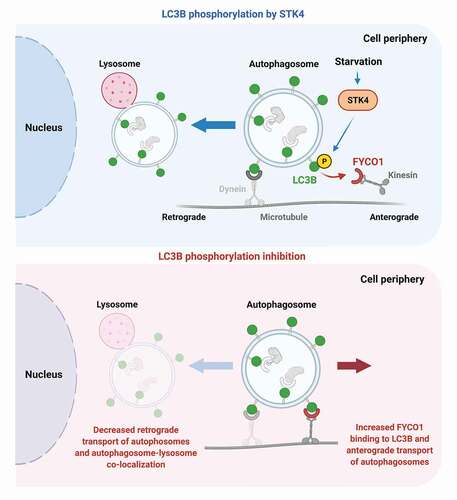
FYCO1 recruits the molecular motor kinesin via its interaction with LC3B, thus driving the directional transport of autophagosomes in the anterograde direction toward the cell periphery. We, therefore, tested whether LC3B phosphorylation regulates FYCO1 recruitment to autophagosomes to direct their movement and positioning within cells. Consistent with this hypothesis, lack of LC3B phosphorylation correlates with increased peripheral localization of autophagosomes within cells, whereas autophagosomes cluster in the perinuclear area of the cell in the presence of an LC3BT50E phospho-mimetic mutant, and this differential phenotype is FYCO1 dependent. Nutrient starvation-induced perinuclear positioning of autophagosomes is also dependent on LC3B phosphorylation and on FYCO1 function, suggesting a nutrient-sensing capacity of this regulatory axis. Finally, live-imaging analysis of autophagosome transport in HeLa cells and mouse primary hippocampal neurons, a highly polarized cell type ideal for transport studies, showed that LC3B phosphorylation is required for proper retrograde transport of autophagosomes toward the perinuclear area. Thus, autophagosomes in cells or neurons expressing LC3B with a phosphorylation block display impaired retrograde transport and instead move more toward the cell periphery. Consistent with these observations, LC3B phosphorylation inhibition leads to a deficient autophagosome-lysosome association in the proximity of the perinuclear area, in both cell types. Overall, our data demonstrate an LC3B-dependent regulatory axis that controls the transport of autophagosomes toward the perinuclear area via association with FYCO1, to promote autophagy.
Based on our results, we hypothesize that LC3B phosphorylation may be a trigger to coordinate the retrograde transport of fully formed cargo-loaded autophagosomes to meet and fuse with lysosomes. FYCO1 and kinesin association to autophagosomes may be required during the initial steps of autophagosome formation, but once autophagosomes are ready for lysosome fusion, LC3B phosphorylation may permit the release of the FYCO1-kinesin complex and allow dynein-mediated retrograde transport and autophagy process completion. STK4 may sense the maturation status of autophagosomes and trigger LC3B phosphorylation at a given time, via yet unknown mechanisms. In favor of LC3B phosphorylation being a regulated process, we observed that the LC3BT50E phospho-mimetic mutant causes frequent directional switches of autophagosomes that may reflect the permanent pseudo-phosphorylation state of this condition. This raises the question of whether phosphatases could be involved in the dynamic regulation of LC3B phosphorylation, and the potential role of other kinases, such as NEK9, recently described by Terje Johansen’s group, to also phosphorylate LC3B T50. While LC3B phosphorylation may happen during basal autophagy, this process may be enhanced by other stressors such as nutrient starvation, as shown in our data, to facilitate enhanced autophagic demands.
A number of questions remain, including how STK4 may sense nutrient deficiencies, and the identity of potential upstream factors that regulate the STK4-LC3-FYCO1 axis. Moreover, it would be of interest to investigate if LC3B phosphorylation could potentially function as part of a regulatory “switch” mechanism to define the degradative vs. a potential secretory fate of LC3B-positive vesicles, an emerging concept in the autophagy field. Irrespective of function, it will be important to test the relevance of LC3B phosphorylation in health and in conditions where autophagy is impaired.
Acknowledgments
was created with BioRender.com. J.N.T. was supported by a Fundación Ramon Areces Postdoctoral Fellowship, and an NIH K99/R00 pathway to independence grant (K99AG062774); S.E.E was supported by NIH grant R01AG049483; The Glenn Foundation for Medical Research Glenn Award for Research in Biological Mechanisms of Aging; a New Scholar in Aging Award from the Lawrence Ellison Foundation; and The Baxter Family Foundation; M.H. was supported by NIH grant GM117466.
Figure 1. LC3B phosphorylation decreases FYCO1 binding to LC3B and promotes retrograde transport of autophagosomes. Top panel: STK4-mediated LC3B phosphorylation, an event further induced by nutrient starvation, leads to a decrease of FYCO1 binding to LC3B and a putative diminished association of kinesin with autophagosomes. In turn, this promotes the retrograde transport of autophagosomes. Bottom: When LC3B phosphorylation is blocked, FYCO1 shows increased association with LC3B, and autophagosome transport toward the nucleus is reduced, compromising autophagosome-lysosome colocalization. The increased FYCO1 association correlates with higher transport of autophagosomes toward the cell periphery, as reflected by their increased time and distance in anterograde motion, among other transport parameters
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Nieto-Torres JL, Shanahan S-L, Chassefeyre R, et al. LC3B phosphorylation regulates FYCO1 binding and directional transport of autophagosomes. Curr Biol. 2021. DOI:https://doi.org/10.1016/j.cub.2021.05.052
