ABSTRACT
Macroautophagy/autophagy is a highly conserved catabolic process by which cytoplasmic constituents are delivered to the vacuole/lysosome for degradation and recycling. To maintain cellular homeostasis and prevent pathologies, the induction and amplitude of autophagy activity are finely controlled through regulation of ATG gene expression. Here we report that the Ccr4-Not complex in Saccharomyces cerevisiae has bidirectional roles in regulating autophagy before and after nutrient deprivation. Under nutrient-rich conditions, Ccr4-Not directly targets the mRNAs of several ATG genes in the core autophagy machinery to promote their degradation through deadenylation, thus contributing to maintaining autophagy at the basal level. Upon starvation, Ccr4-Not releases its repression of these ATG genes and switches its role to promote the expression of a different subset of ATG genes, which is required for sufficient autophagy induction and activity. These results reveal that the Ccr4-Not complex is indispensable to maintain autophagy at the appropriate amplitude in both basal and stress conditions.Abbreviations: AID, auxin-inducible degron; Ape1, aminopeptidase I; Atg, autophagy related; Cvt, cytoplasm-to-vacuole targeting; DMSO, dimethyl sulfoxide; IAA, indole-3-acetic acid; PA, protein A; RIP, RNA immunoprecipitation.
Introduction
Autophagy is an evolutionarily conserved intracellular degradation and recycling process. During autophagy, cytoplasmic components, including long-lived proteins, protein aggregates, damaged or superfluous organelles and invading pathogens are sequestered within double-membrane vesicles termed autophagosomes and delivered to the vacuole (in yeast) or lysosomes (in mammals) for degradation and recycling [Citation1]. Autophagy operates constitutively even under basal, non-stressful conditions, albeit at low levels, as part of the constant turnover system and is one of the major quality control guardians in the cell. Upon stress, in particular nutrient starvation, autophagy is highly upregulated as a fundamental adaptation and survival strategy.
Dysregulated autophagy is associated with many human diseases, including cancer, immune disorders, liver, heart, kidney and lung diseases, and neurodegeneration [Citation2]. Therefore, autophagy needs to be stringently regulated at appropriate levels in response to different stimuli. From a molecular perspective, autophagy is executed and mediated by a group of autophagy-related (Atg) proteins, thus regulation of autophagy is mostly carried out through regulating ATG gene expression including processes that affect the amount and stability of mRNA transcripts. The cellular mRNA levels are determined by the rates of mRNA synthesis and degradation. Although tremendous research has focused on how mRNA levels are controlled through transcription (synthesis, less is known about post-transcriptional modulation including mRNA degradation [Citation3].
Shortening or removal of mRNA poly(A) tails (deadenylation), the rate-limiting step in mRNA degradation, plays a central role in post-transcriptional regulation. Deadenylation releases poly(A)-binding proteins and disrupts the circularized mRNP translation module, thus suppressing translation and leading to subsequent mRNA decay through either 3' to 5' degradation by the cytosolic exosome or 5' decapping followed by 5' to 3' degradation by the Xrn1 exonuclease [Citation4]. Targeted deadenylation of specific substrates is essential for various biological processes, such as germline stem cell maintenance, embryogenesis, and maintaining cardiac homeostasis [Citation5–7]. Recently our lab reported that under nutrient-rich conditions a subset of ATG mRNAs is repressed by the mRNA decapping enzyme Dcp2 and the exonuclease Xrn1 [Citation8,Citation9]. However, what determines the substrate specificity and whether deadenylation is involved in post-transcriptional regulation of ATG mRNAs remain elusive.
Deadenylation is mediated by the evolutionarily conserved Ccr4-Not and Pan2-Pan3 complexes. The Ccr4-Not complex is considered to play the major role in yeast and contains two poly(A)-selective deadenylases, Ccr4 and Pop2/Caf1 [Citation10]. Ccr4-Not can initiate both generic decay of mRNAs and selective induced mRNA decay by tethering specific mRNAs to direct rapid deadenylation in response to cellular signaling [Citation11]. Two recent studies have shown that ATG mRNAs can be selectively targeted by the Ccr4-Not complex. In Drosophila germline, Atg12 mRNA is directly repressed by orb/CPEB-directed twin/CCR4-Not/NOT through deadenylation to prevent autophagic cell death in oocytes [Citation12]. In mice, deadenylation of Atg7 mRNA through the CCR4-NOT complex is essential for cardiac homeostasis [Citation7]. Nonetheless, the regulation of autophagy through deadenylation in many other different cell types, nutritional conditions, and developmental stages needs further investigation. Although initially the primordial function of the Ccr4-Not complex is thought to be deadenylation, additional studies have uncovered its roles in transcription initiation, elongation, mRNA export and nuclear surveillance, and translation [Citation11].
In this study, we identified bidirectional roles of the Ccr4-Not complex in regulating autophagy. Under nutrient-rich conditions, Ccr4-Not directly binds to and deadenylates ATG1, ATG7 and ATG9 mRNA to repress their expression. Deletion or conditional knockdown of CCR4 or POP2 led to an increase in these ATG mRNAs, and subsequent protein levels, which correlate with elevated autophagy activity. Upon nitrogen starvation, Ccr4-Not no longer associates with these ATG mRNAs and releases its repression. In contrast to its role as an autophagy repressor when nutrients are replete, Ccr4-Not positively regulates the expression of a slightly different subset of ATG genes encoding the core machinery of autophagy, and the complex is required for sufficient autophagy activity. These findings advance our understanding of how ATG gene expression is finely regulated by different functions of the same complex under different physiological conditions.
Results
The Ccr4-Not complex represses ATG gene expression under nutrient-rich conditions
To explore whether deadenylation plays a role in regulating ATG gene expression, we generated strains deleted for the gene CCR4 or PAN2, which are the catalytic subunits of the Ccr4-Not and Pan2-Pan3 complexes, respectively, and examined their ATG mRNA levels compared with wild-type (WT) cells by quantitative reverse transcription PCR (RT-qPCR). We focused on nutrient-rich conditions because when nutrients are replete both autophagy and ATG gene expression are kept at basal levels, and deadenylation is a negative regulator for mRNA stability and translation. In nutrient-rich conditions, CCR4 deletion caused a significant increase in ATG1, ATG4, ATG7, ATG8, ATG9, ATG18, ATG19, ATG40 and ATG41 transcripts ( and Fig. S1). Conversely, there was no significant difference between the pan2∆ and WT cells, indicating that the Ccr4-Not complex, rather than Pan2-Pan3 complex, might selectively regulate ATG mRNA stability.
Figure 1. Ccr4-Not is a post-transcriptional repressor of autophagy during growing conditions. (A) WT, ccr4∆ and pan2∆ cells were grown in YPD until mid-log phase. Total RNA was extracted, and the mRNA levels were quantified by RT-qPCR. The mRNA levels of individual ATG genes were normalized to WT cells (set to 1). Mean ± SEM, n ≥ 3 independent experiments. Student’s t-test; *p < 0.05, **p < 0.01, ****p < 0.0001. (B) Ccr4-AID cells were grown in YPD to mid-log phase and treated with either DMSO or 300 μM IAA for 1 and 3 h. Cell lysates were prepared at different time points, and the level of Ccr4-AID-9MYC was analyzed by western blot with anti-MYC antibody. (C) Ccr4-AID cells were grown in YPD to early log phase and treated with either DMSO or 300 μM IAA for 3 h. The mRNA levels of individual ATG genes were quantified by RT-qPCR and normalized to the DMSO treatment group (set to 1). Mean ± SEM, n = 3 independent experiments. Student’s t-test; **p < 0.01, ***p < 0.001, ****p < 0.0001. (D) Atg1, Atg7-PA, and Atg9 protein levels were measured by western blot in WT and ccr4∆ strains under growing conditions and after 1 and 3 h of nitrogen starvation; representative images are shown. (E) WT and pop2∆ cells were grown in YPD until mid-log phase. The mRNA levels were quantified and shown as in Figure 1A. Student’s t-test; *p < 0.05, **p < 0.01, ****p < 0.0001.
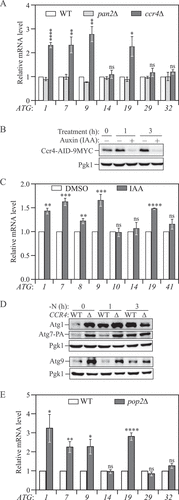
The Ccr4-Not complex controls gene expression at multiple levels; deleting CCR4 impairs the function of the complex and causes cellular stress and slow growth. To ensure that the transcriptional modulations we observed result from direct effect of compromised deadenylation instead of chronic stress caused by the loss of Ccr4, we took advantage of the auxin-inducible degron (AID) technology to conditionally knock down Ccr4 [Citation13]. Addition of indole-3-acetic acid (IAA) induced rapid poly-ubiquitination and degradation of Ccr4-AID and maintained Ccr4-AID at a minimal level after 1-h treatment (). Consistent with the results from ccr4∆ cells, we observed an approximately 50% increase of the amount of ATG1, ATG7, ATG9 and ATG19 mRNAs in cells with IAA treatment compared to those treated with dimethyl sulfoxide (DMSO) (). In contrast, ATG8 and ATG41, which show the greatest increase in mRNA level under autophagy-inducing conditions, failed to show substantial upregulation following Ccr4 depletion, suggesting that these two genes are not targets of the Ccr4-Not complex even though they are sensitive to cellular stress and growth defects.
We further investigated whether the increase in ATG mRNA levels observed in ccr4∆ cells translated to higher Atg protein levels by measuring Atg1, Atg7, and Atg9 proteins using estern blot. Under nutrient-rich and short-term starvation conditions, we found markedly higher levels of these proteins in ccr4∆ cells compared to WT (), in line with the RT-qPCR experiments. After longer-term starvation (3 h), the difference in Atg protein levels between WT and ccr4∆ cells was strongly reduced or even abolished, indicating that nitrogen starvation releases the repression of Ccr4 on ATG gene expression.
The Ccr4-Not complex contains two evolutionarily conserved poly(A)-specific exonucleases: Ccr4, a member of the endonuclease-exonuclease-phosphatase family, and Pop2/Caf1, which has similarities to the RNase D family [Citation14,Citation15]. Both exonucleases can actively carry out deadenylation, and the Pop2 protein is physically sandwiched between Ccr4 and Not1, forming the nuclease core of the complex [Citation16]. Despite the fact that the components of the nuclease module work coordinately in mRNA decay, Ccr4 and Pop2 can display different preferences for substrates, and individual knockout of the two corresponding genes can cause different phenotypes [Citation17–19]. To determine whether the increase in ATG mRNA levels is specific to Ccr4 depletion, we measured the same transcripts in pop2∆ cells. Deletion of POP2 led to changes in ATG mRNA expression profiles very similar to those observed in ccr4∆ cells compared to WT: we observed an upregulation of the same genes and the changes occurred at similar amplitudes (). Together, these results show that the Ccr4-Not complex is a negative regulator for ATG mRNA accumulation and Atg protein expression.
The Ccr4-Not complex negatively regulates autophagy activity under nutrient-rich conditions
The process of autophagy is precisely controlled by Atg protein expression. For example, with regard to the three essential ATG genes we found highly upregulated in Ccr4-depleted cells (): the Atg1 level is correlated with the on-rate of autophagy, the amount of Atg7 directly modulates autophagy amplitude, and the level of Atg9 correlates with the frequency of autophagosome formation [Citation20–22]. Furthermore, previous studies have demonstrated that the levels of these proteins directly correlate with autophagy activity [Citation21–27]. Therefore, we tested whether Ccr4-Not can regulate autophagy activity using the GFP-Atg8 processing assay. During autophagy, a portion of Atg8 is covalently conjugated to phosphatidylethanolamine on the phagophore membrane; the population that ends up on the inner membrane of the mature autophagosome is exposed to vacuolar hydrolases following autophagosome-vacuole fusion. Compared to Atg8, GFP is more resistant to vacuolar hydrolysis; thus, the conversion of GFP-Atg8 to free GFP can be used as a readout for nonselective autophagic activity [Citation28]. We overexpressed GFP-Atg8 using a CUP1 promoter-driven plasmid to eliminate minor Ccr4-dependent effects on Atg8 expression. As expected, autophagy flux was significantly induced in ccr4∆ and pop2∆ cells in nutrient-rich conditions and occurred at a higher extent upon short-term nitrogen starvation, as indicated by the level of free GFP:total GFP compared with WT cells (). The upregulated autophagy activity in Ccr4-Not-deficient cells was attenuated after a longer period of starvation, consistent with the change in Atg protein levels (, and B), indicating that the Ccr4-Not complex acts as a repressor of autophagy in nutrient-rich conditions.
Figure 2. The Ccr4-Not complex acts to downregulate autophagy under basal conditions. (A) WT and different clones of ccr4∆ cells with an integration plasmid expressing CUP1 promoter-driven GFP-ATG8 were grown to mid-log phase in YPD (-N, 0 h) and shifted to SD-N for the indicated times. Autophagy activity was measured using the GFP-Atg8 processing assay. ∆1 and ∆2 indicate two independent deletion colonies. The ratio of free GFP to total GFP (free GFP plus GFP-Atg8) was quantified. Mean ± SEM of n ≥ 3 independent experiments are indicated. Student’s t-test; *p < 0.05, **p < 0.01. (B) WT and pop2∆ cells with an integration plasmid expressing CUP1 promoter-driven GFP-ATG8 were grown to mid-log phase in YPD (-N, 0 h) and shifted to SD-N for the indicated times. Autophagy activity was measured using the GFP-Atg8 processing assay. (C) Ccr4-AID cells expressing a CUP1 promoter-driven GFP-ATG8 plasmid were grown to mid-log phase in YPD, diluted to OD600 = 0.005, treated with either DMSO or 300 μM IAA and grown for an additional 6 h, allowing the cultures reach mid-log phase. Autophagy activity was measured using the GFP-Atg8 processing assay. (D) WT (WLY176, Ccr4-AID) cells were grown to mid-log phase in YPD, diluted, then treated with either DMSO or 300 μM IAA for 3 and 8 h. Autophagy activity was monitored by the Pho8∆60 assay. Pho8∆60 activity was normalized to the sample with 8 h of DMSO treatment (set to 100%). Mean ± SEM of n ≥ 3 independent experiments are indicated. Student’s t-test; *p < 0.05, ***p < 0.001. (E) Ccr4-AID cells were grown to early-log phase (OD600 = 0.1) in YPD for over 15 doublings and treated with either DMSO or 300 μM IAA for 3 h. Autophagy activity was measured with the prApe1 processing assay using anti-Ape1 antiserum. Representative images and quantification of the data are shown. Mean ± SEM, n = 3 independent experiments. Student’s t-test; *p < 0.05.
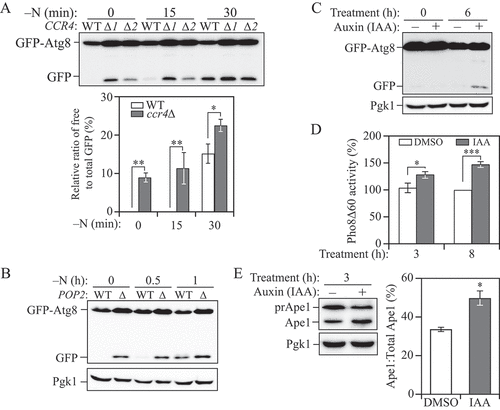
To exclude the possible accumulative effects on autophagy resulting from the growth defect caused by CCR4 deletion, we used the AID system to conditionally knock down CCR4 expression. Compared to the cells treated with DMSO, we observed higher autophagy activity when Ccr4 was temporally depleted based on the GFP-Atg8 processing assay (). We extended our analysis by taking advantage of the quantitative Pho8∆60 assay. Pho8Δ60 is a mutant form of the vacuolar phosphatase whose activation is completely dependent on its delivery to the vacuole through autophagy. Therefore, the Pho8∆60-dependent phosphatase activity can be a measurement for autophagy flux. In agreement with the GFP-Atg8 processing assay, a significant increase in autophagy activity was observed in cells treated with IAA for either 3 or 8 h ().
To further validate our results that the loss of Ccr4-Not enhances autophagy, we examined whether the cytoplasm-to-vacuole targeting (Cvt) pathway, a form of selective autophagy that overlaps extensively with nonselective autophagy, was affected in Ccr4-depleted cells by measuring the processing of the precursor form of the vacuolar hydrolase aminopeptidase I (prApe1). prApe1 is constitutively delivered into the vacuole through the Cvt pathway, where it is matured by cleaving its propeptide [Citation29]. We again utilized the Ccr4-AID system to avoid the accumulation of mature Ape1 from long-term stress. Similar to other autophagy activity assays, we observed a greater than 40% increase in prApe1 processing after acute temporal Ccr4 depletion in growing conditions (), further demonstrating that the Ccr4-Not complex negatively regulates autophagy. The increased autophagy activity in ccr4∆ or pop2∆ cells is not seen when the decapping factor Dcp2 or exonuclease Xrn1 is depleted [Citation8,Citation9], suggesting a more predominant role of the Ccr4-Not complex in regulating autophagy, possibly because Ccr4-Not not only affects transcript stability through mRNA decay but also represses translation [Citation11].
Ccr4 binds to select ATG mRNAs and controls their stability only under nutrient-rich conditions
The Ccr4-Not complex interacts with targeted mRNAs to shorten their poly(A) tails. To address whether Ccr4-Not directly targets ATG mRNAs to promote their degradation in vivo, we performed an RNA immunoprecipitation (RIP) assay [Citation30]. To this end, we tagged Ccr4 with protein A (PA) and affinity isolated the Ccr4-PA. Next, we purified and quantified co-precipitated mRNAs using RT-qPCR. As a control, we used an untagged Ccr4 strain to normalize background RNA levels. Our RIP analysis showed significant enrichment of ATG1, ATG7 and ATG9 mRNA in Ccr4-PA cells under nutrient-rich conditions, compared to the negative control PGK1 mRNA, whose expression is not affected by Ccr4 (). This finding confirmed that the Ccr4-Not complex directly associates with select ATG mRNAs. It is important to note that the enrichment was only observed in growing conditions but not nitrogen-starvation conditions, in accordance with the effects we found for ATG gene expression and autophagy activity, suggesting that starvation releases the binding of Ccr4 to these mRNAs to switch on their expression ().
Figure 3. Ccr4-Not binds to ATG mRNAs and negatively regulates their stability in growing conditions. (A) WT and CCR4-PA cells were grown in YPD medium to mid-log phase (+N) and then shifted to SD-N for 2 h (-N). Cells were subjected to RNA immunoprecipitation as described in Materials and Methods. qRT-PCR experiments were performed to show the enrichment of ATG mRNAs based on the Ccr4-PA RIP assay. Mean ratios ± SEM of n = 3 independent experiments of ATG mRNA levels in Ccr4-PA:non-tag RIP are indicated. PGK1 mRNA served as a negative control. Student’s t-test, *p < 0.05, ns, not significant. (B and C) WT and ccr4∆ cells were grown in YPD medium until mid-log phase, and then (B) treated with 200 µg/ml 1,10-phenanthroline for 50 and 100 min, or (C) shifted to SD-N for 50 and 100 min in the presence of 200 µg/ml 1,10-phenanthroline. The mRNA levels of individual ATG genes were first normalized to the reference gene SCR1 and then normalized to WT cells with no treatment (set to 1). Mean ± SEM, n ≥ 3 independent experiments. Student’s t-test; *p < 0.05, **p < 0.01, ns, not significant. (D) WT (CCR4-PA) and the indicated Ccr4 catalytic mutant cells were grown in YPD until mid-log phase. The mRNA levels of individual ATG genes were measured, quantified, and shown as in . Student’s t-test; **p < 0.01, ***p < 0.001.
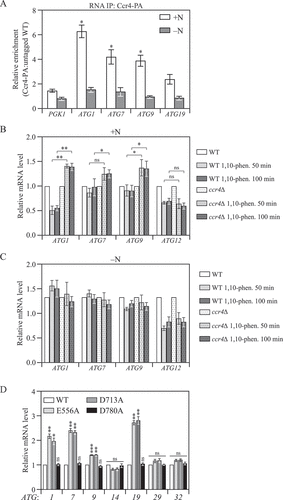
Besides mediating mRNA decay through deadenylation of targeted transcripts, the Ccr4-Not complex also interacts with transcription factors and the elongating polymerase to either promote or repress transcription initiation and elongation [Citation31–33]. To directly demonstrate that the phenotypes we observed were not due to interference with ATG mRNA transcription, we measured ATG mRNA levels in the presence of the mRNA synthesis inhibitor 1,10-phenanthroline. RT-qPCR analysis showed that during nutrient-rich conditions, deleting CCR4 appeared to stabilize the ATG1, ATG7 and ATG9 transcripts resulting in elevated levels, whereas ATG12 mRNA that was not bound by Ccr4 or affected by CCR4 deletion, showed similar time-dependent degradation in WT and ccr4∆ cells (). We note that there was a mild increase in the level of ATG1, ATG7 and ATG9 transcripts in the presence of 1,10-phenanthroline. However, it was recently reported that 1,10-phenanthroline inhibits TORC1 [Citation34], which would cause an upregulation of ATG gene expression. In addition, the inhibitory effect requires a certain amount of time, and is not likely to result in a complete block. In addition, nitrogen starvation typically results in an approximately 10-fold increase in ATG1, ATG7 and ATG9 transcripts [Citation22]; however, there was essentially no change in the levels of these transcripts in nitrogen-starvation conditions in the presence of 1,10-phenanthroline (), indicating that the inhibitor did block transcription. In contrast to the results seen under basal conditions, when cells were starved for nitrogen, we no longer observed any difference in ATG transcript levels between WT and ccr4∆ cells following 1,10- phenanthroline treatment (). These results indicate that the increased ATG mRNA level caused by CCR4 deletion is due to reduced mRNA degradation rather than enhanced transcription.
To further validate our conclusion that the purpose of Ccr4-Not binding to select ATG mRNAs is to initiate targeted mRNA decay through its exonuclease activity, we mutated conserved catalytic residues E556 and D713 that are functionally critical for Ccr4 exonuclease activity [Citation35]. Mutations in either one of these two residues led to significant upregulation of the same set of ATG mRNAs as in ccr4∆ cells (). For comparison, we also made the mutation D780A, which corresponds to a previously predicted catalytic residue; subsequent studies revealed that this mutant still retains a significant level of catalytic activity [Citation35]. In contrast to the E556A and D713A mutants, D780A did not alter the ATG expression profile. Together, these findings indicated that the exonuclease activity of Ccr4 is essential for maintaining ATG mRNA transcripts at the basal level. Collectively, our data showed that the Ccr4-Not complex directly binds to ATG mRNA during nutrient-rich conditions to induce their degradation through deadenylation, thus repressing their expression.
The Ccr4-Not complex positively regulates ATG gene expression during nitrogen starvation
During nutrient deprivation, the expression of Atg1, Atg7, and Atg9 was highly upregulated compared to growing conditions in WT cells, whereas ccr4∆ cells did not display such an upregulation (). However, our data indicated that the Ccr4-Not complex targets ATG mRNA for degradation only under growing conditions, which prompted us to propose that the Ccr4-Not complex plays a different role during starvation. To address this possibility, we first quantified ATG mRNA levels in ccr4∆ cells after 2 h of nitrogen starvation. Surprisingly, in contrast to growing conditions, we observed a significant decrease in the mRNA levels of several genes encoding the core machinery of autophagy, including ATG1, ATG7, ATG8, ATG9 and ATG13, in ccr4∆ cells compared to WT (), suggesting that Ccr4-Not complex was required for the induction and high expression of a subset of ATG mRNAs. CCR4 deletion led to accumulation of several ATG transcripts in the cell during growing conditions (). To prevent such an accumulation from confounding the quantification of ATG mRNAs during starvation, we used the Ccr4-AID cells and induced depletion of Ccr4 30 min prior to starvation. Degradation of Ccr4 by IAA treatment led to a similar but stronger phenotype in this subset of core ATG mRNAs, with the exception being ATG7 (). Because inhibition of transcription diminished the difference between WT and ccr4 deletion strains (), we speculated that Ccr4-Not contributes to ATG gene upregulation through enhancing transcription initiation or elongation during starvation.
Figure 4. Ccr4-Not is required for sufficient autophagy under nitrogen-starvation conditions. (A) WT and ccr4∆ cells were grown in YPD until mid-log phase, and then starved for nitrogen for 2 h. The mRNA levels were quantified and shown as in . Mean ± SEM, n ≥ 3 independent experiments. Student’s t-test; *p < 0.05, **p < 0.01, ****p < 0.0001, ns, not significant. (B) Ccr4-AID cells were grown in YPD to mid-log phase and pre-treated with either DMSO or 300 μM IAA for 30 min, then they were shifted to SD-N for 1 h in the presence of either DMSO or IAA. The mRNA levels of individual ATG genes were quantified by RT-qPCR and normalized to the DMSO treatment group (set to 1). Mean ± SEM, n = 4 independent experiments. Student’s t-test; **p < 0.01, ***p < 0.001, ns, not significant. (C) Ccr4-AID cells were grown in YPD medium to mid-log phase and pre-treated with either DMSO or 300 μM IAA for 30 min (-N, 0 h), then they were shifted to SD-N for the indicated times in the presence of either DMSO or IAA. Atg1, Atg8, and Atg9-PA protein levels were measured by western blot; representative images are shown. (D and E) WT and ccr4∆ cells in which either PGI1 or FBA1 was chromosomally tagged with GFP were grown in YPD to mid-log phase (-N, 0 h) and shifted to SD-N for the indicated times. Autophagy activity was measured by the (D) Pgi1-GFP processing assay or (E) Fba1-GFP processing assay; representative images are shown. (F) Ccr4-AID Pgi1-GFP cells were grown in YPD to mid-log phase and pre-treated with either DMSO or 300 μM IAA for 30 min (-N, 0 h), then they were shifted to SD-N for the indicated times in the presence of either DMSO or IAA. Autophagy activity was measured by the Pgi1-GFP processing assay; a representative image is shown. (G) WT and ccr4∆ cells were grown in YPD to mid-log phase and then shifted to SD-N for the indicated times. The indicated dilutions were grown on YPD plates for 3 days.
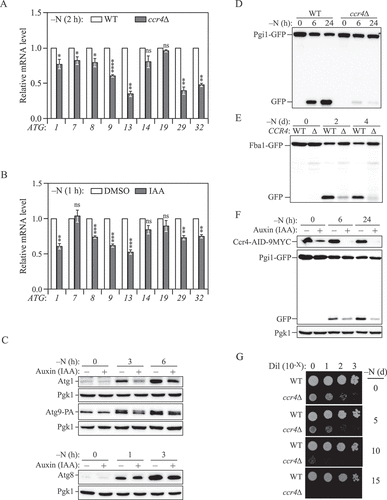
We further examined whether the decrease in ATG mRNA levels seen in the Ccr4 knockdown cells correlated with lower protein levels. To measure Atg8 protein levels, we deleted the PEP4 gene, which encodes a primary protease involved in initiating the activity of many vacuolar hydrolases, to avoid rapid turnover of Atg8 in the vacuole. Upon starvation, we observed a strong upregulation of Atg1, Atg8 and Atg9 in both DMSO- and IAA-treated cells (). However, the amounts of these Atg proteins were significant lower in Ccr4-depleted cells after 3 and 6 h of starvation, suggesting a weaker induction, which was consistent with what we observed by RT-qPCR (). Therefore, in starvation conditions, the Ccr4-Not complex switches its role to that of a positive factor involved in upregulating the expression of a subset of ATG genes.
The Ccr4-Not complex is required for sufficient autophagy activity under nitrogen-starvation conditions
Because the Ccr4-Not complex positively regulates the expression of a subset of core Atg proteins during starvation, we tested whether it is a positive regulator of autophagy. We first measured autophagy activity using the GFP-Atg8 processing assay with longer-term starvation. Significant decreases in autophagy activity were observed in ccr4∆ cells after 3 and 6 h of nitrogen starvation (Fig. S2A). However, processing of the GFP-Atg8 chimeric protein in the WT strain became saturated after 3 h of starvation, meaning that the assay may not accurately reflect the magnitude of the defect in the ccr4∆ strain. To avoid exhaustion of the substrate, we repeated the analysis using a similar Pgi1-GFP processing assay. Pgi1 is a long-lived cytosolic glycolytic enzyme, whose degradation is dependent on autophagy [Citation36]. After prolonged starvation, the ccr4∆ strain showed a markedly lower level of Pgi1-GFP processing as indicated by the conversion of Pgi1-GFP to GFP (), suggesting that long-term autophagy was impaired. Similar results were obtained when we analyzed the processing of two additional long-lived cytosolic GFP fusion proteins, Fba1 (fructose-1,6-bisphosphate aldolase 1)-GFP, and Pgk1 (3-phosphoglycerate kinase)-GFP ( and S2B) [Citation37,Citation38]. To extend our analysis, we also measured autophagy activity in a pop2∆ strain and following Ccr4 temporal knockdown, both of which showed decreased processing of GFP fusion proteins, suggesting decreased autophagy flux in Ccr4-Not-deficient cells ( and S2C). Collectively, these results indicate that Ccr4-Not is necessary for maximal autophagy activity during nitrogen starvation.
Autophagic degradation is critical to maintain cell viability during starvation, and defects in autophagy activity are associated with increased cell death, which can lead to a loss in viability. To examine the physiological importance of the Ccr4-Not-dependent upregulation of autophagy, we monitored the survival phenotype of cells harboring a CCR4 deletion after prolonged nitrogen starvation. The ccr4∆ cells exhibited a slower growth rate when nutrients were replete and showed a strong reduction in viability after 10 days of starvation compared to WT cells (). Taken together, our data uncovered a new role for the Ccr4-Not complex to positively regulate autophagy during nitrogen starvation possibly through increasing ATG mRNA and protein levels.
Discussion
Under growing conditions, the majority of yeast genes are positively regulated, whereas the opposite is true during starvation. However, ATG genes typically display the opposite regulation, being downregulated during vegetative growth and upregulated when most genes are turned off. The transcriptional and post-transcriptional repressors are essential for maintaining ATG gene expression and autophagy activity at the basal level by either inhibiting mRNA synthesis or inducing transcript degradation [Citation3]. The major pathway for mRNA degradation is initiated by deadenylation, which can be either nonselective or selective [Citation11]. So far, it remains poorly understood what cellular processes are particularly regulated by targeted deadenylation. Here we present data characterizing the Ccr4-Not complex as a post-transcriptional repressor of autophagy during nutrient-rich conditions.
Chromosomal deletion or temporal knockdown of the deadenylase CCR4 or POP2 led to significant accumulation of a subset of ATG mRNAs including ATG1, ATG7, andATG9 transcripts, along with a similar upregulation in their protein levels, which is likely the primary cause of elevated autophagy activities. We further showed that Ccr4 directly bound ATG transcripts and mediated their degradation rather than inhibiting transcription. In contrast, an increase in select ATG mRNA but not autophagy flux is seen in dcp2∆ and xrn1∆ cells under growing conditions [Citation8,Citation9]; the possible reasons include that deadenylation is the major determinant of substrate selection and the fate of mRNAs bound by Ccr4-Not is not limited to 5' to 3' degradation but also translation repression [Citation11,Citation39,Citation40]. An unaddressed question is how Ccr4-Not is directed to this subset of ATG transcripts. Considering that the substrate specificity for targeted deadenylation is usually determined by RNA-binding proteins or microRNAs that tether the Ccr4-Not complex to specific sequences at the 3' UTR of the mRNA [Citation41], future work will be required to identify the RNA binding protein(s) and possibly the consensus motif being recognized.
The ability of cells to adjust autophagy activity to proper levels in response to changes in nutrient availability is essential for maintaining cellular homeostasis. The negative regulatory components that inhibit ATG gene expression during growth are usually quickly switched off under stress conditions allowing for efficient induction of autophagy. For example, the decapping enzyme Dcp2 undergoes TOR-dependent phosphorylation during nutrient-rich conditions leading to ATG mRNA decapping followed by degradation and autophagy suppression, but gets rapidly dephosphorylated and thus inactivated upon starvation resulting in the accumulation of ATG mRNA [Citation41]. Likewise, Ccr4-Not no longer associated with ATG mRNA and released its repression of their expression during starvation.
In previous studies, our lab and others reported the function of the nutrient-dependent bidirectional regulator Dhh1, which acts to inhibit autophagy when cells are actively growing, in part by downregulating genes such as ATG8, but then actively promotes Atg1 and Atg13 translation when cells are shifted to medium lacking nitrogen [Citation8,Citation36]. Accordingly, we examined the possibility that the Ccr4-Not complex acted in a positive manner for ATG gene expression under stress conditions. We found that during nitrogen starvation Ccr4-Not is required for efficient expression of a different subset of ATG genes including ATG1, ATG8, ATG9 and ATG13, as well as a robust autophagy response. Our data further suggest that this regulation is likely through promoting transcription initiation or elongation. A possible mechanism could be through the interaction with transcription regulatory factors; for example, the Ccr4-Not subunits can functionally and physically associate with the SAGA (Spt-Ada-Gcn5 acetyltransferase) complex, which is required for the efficient activation of autophagy [Citation42,Citation43].
The binding with ATG mRNA and the role of Ccr4-Not in regulating autophagy are completely reversed after nitrogen starvation. Therefore, we speculated that there is a switch to alternate the function of Ccr4-Not in response to changes in cellular signaling. One common mechanism that controls functions of autophagy regulators is posttranslational modifications. Previous phosphoproteome analyses showed that rapamycin treatment induces decreased phosphorylation of Ccr4 at sites S281, T282 and T285 [Citation44]. However, neither non-phosphorylatable nor phosphomimetic mutations of these sites affected levels of ATG transcripts (Fig. S3A and S3B), indicating that the phosphorylation status of these sites on Ccr4 is not the molecular switch we were looking for. It is possible that such a switch is turned on and off through other components of the Ccr4-Not complex and its interacting proteins.
Dysregulation of Ccr4-Not components is implicated in a wide-range of human diseases including cancer, neurodegenerative disorders, and heart disease [Citation45–48], which is similar to dysregulated autophagy. Here we showed the bidirectional roles of the Ccr4-Not complex in finely tuning the magnitude of autophagy in both nutrient-rich and -deprivation conditions, which provides a novel perspective in understanding the possible mechanisms underlying Ccr4-Not-related pathogenesis. The present study also expands our knowledge about the transcriptional and post-transcriptional regulation of autophagy.
Materials and Methods
Yeast strains, media, and growth conditions
All yeast Saccharomyces cerevisiae strains used in this study are listed in Table S1. Gene deletions, chromosomal tagging and point mutations were performed using standard methods in the SEY6210 genetic background. Under growing (nutrient-rich) conditions, yeast cells were grown in YPD (1% yeast extract, 2% peptone and 2% glucose). To induce autophagy, cells in mid-log phase were shifted from YPD to nitrogen starvation medium (SD-N; 0.17% yeast nitrogen base without ammonium sulfate or amino acids, containing 2% glucose) for the indicated times.
Auxin-inducible degron system
Yeast SEY6210 cells were first transformed with the plasmid pNHK53 (ADH1p-OsTIR1-9MYC). CCR4 was then tagged with AID-9MYC by homologous recombination using a DNA fragment amplified from pKAN-AID-9MYC (Addgene, 99522; deposited by Dr. Helle Ulrich). To conditionally deplete the protein of interest (Ccr4-AID-9MYC), the final concentration of 300 μM 3-indoleacetic acid (IAA; Sigma, I2886) in DMSO, or just DMSO (vehicle, 0.2%) were added to the growth media. For samples collected under nitrogen-starvation conditions, the cells were pre-treated with IAA or DMSO for 30 min in YPD medium prior to inducing starvation.
RNA isolation and RT-qPCR
Yeast cells were grown in YPD to mid-log phase and then shifted to SD-N medium for the indicated times. Total RNAs were extracted using the NucleoSpin RNA kit (Takara, 740955.250) and reverse-transcribed using the High-capacity cDNA Reverse Transcription kit (Fisher, 4368814). The cDNA levels were then analyzed by real-time PCR using the Power SYBR Green PCR Master Mix (Applied Biosystems, 4367660). The transcript abundance in samples was determined using the CFX Manager Software regression method as previously described [Citation8]. The primers used for the RT-qPCR analysis were the same as previously listed [Citation8].
RNA immunoprecipitation
Ccr4-PA and Ccr4 untagged (control) strains were cultured in YPD to mid-log phase. An aliquot was collected as the nutrient-rich sample (+N), and the remainder of the culture was shifted to SD-N medium for 2 h (-N). The RNA IP assay was performed as previously described [Citation37].
Yeast viability assay
Yeast cells were grown in YPD to mid-log phase and then shifted to SD-N medium and starved for the indicated times. At each time point, an aliquot was removed from each culture and then adjusted to OD600 = 1.0 before being subjected to serial dilution. An aliquot (2 µl) of each dilution was spotted on YPD plates; the cells were grown at 30°C for 3 days before being imaged.
Autophagic flux assays and western blotting
GFP-Atg8, Pgi1-GFP, Fba1-GFP, Pgk1-GFP, and prApe1 processing assays were performed as previously described [Citation37,Citation49]. Antisera were from the following sources: Atg1 [Citation50], Atg8 [Citation51], Atg9 [Citation52], Pgk1 (a generous gift from Dr. Jeremy Thorner, University of California, Berkeley), monoclonal YFP (Clontech, 632381), antibody to PA (Jackson ImmunoResearch, 323–005-024), and anti-MYC antibody (Sigma, M4439). The blot was imaged using a ChemiDoc Touch imaging system (Bio-Rad) and quantified using Bio-Rad Image Lab software.
Statistical analyses
The two-tailed Student’s t test was used to determine statistical significance in all cases for at least 3 independent biological replicates. For all figures, p value < 0.05 were considered significant. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; ns, not statistically significant.
Supplemental Material
Download MS Word (884.1 KB)Disclosure statement
No potential conflicts of interest were reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721.
- Klionsky DJ, Petroni G, and Amaravadi RK, et al. Autophagy in major human diseases. EMBO J. 2021;40:e108863.
- Feng Y, Yao Z, Klionsky DJ. How to control self-digestion: transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol. 2015;25:354–363.
- Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8:113–126.
- Yamaji M, Jishage M, Meyer C, et al. DND1 maintains germline stem cells via recruitment of the CCR4-NOT complex to target mRNAs. Nature. 2017;543:568–572.
- Subtelny AO, Eichhorn SW, Chen GR, et al. Poly(A)-tail profiling reveals an embryonic switch in translational control. Nature. 2014;508:66–71.
- Yamaguchi T, Suzuki T, and Sato T, et al. The CCR4-NOT deadenylase complex controls Atg7-dependent cell death and heart function. Sci Signal. 2018;11:eaan3638.
- Hu G, McQuiston T, Bernard A, et al. A conserved mechanism of TOR-dependent RCK-mediated mRNA degradation regulates autophagy. Nat Cell Biol. 2015;17:930–942.
- Delorme-Axford E, Abernathy E, Lennemann NJ, et al. The exoribonuclease Xrn1 is a post-transcriptional negative regulator of autophagy. Autophagy. 2018;14:898–912.
- Parker R. RNA degradation in Saccharomyces cerevisae. Genetics. 2012;191:671–702.
- Collart MA. The Ccr4-Not complex is a key regulator of eukaryotic gene expression. Wiley Interdiscip Rev RNA. 2016;7:438–454.
- Rojas-Rios P, Chartier A, Pierson S, et al. Translational control of autophagy by Orb in the Drosophila Germline. Dev Cell. 2015;35:622–631.
- Morawska M, Ulrich HD. An expanded tool kit for the auxin-inducible degron system in budding yeast. Yeast. 2013;30:341–351.
- Daugeron MC, Mauxion F, Seraphin B. The yeast POP2 gene encodes a nuclease involved in mRNA deadenylation. Nucleic Acids Res. 2001;29:2448–2455.
- Tucker M, Staples RR, Valencia-Sanchez MA, et al. Ccr4p is the catalytic subunit of a Ccr4p/Pop2p/Notp mRNA deadenylase complex in Saccharomyces cerevisiae. EMBO J. 2002;21:1427–1436.
- Basquin J, Roudko VV, Rode M, et al. Architecture of the nuclease module of the yeast Ccr4-not complex: the Not1-Caf1-Ccr4 interaction. Mol Cell. 2012;48:207–218.
- Temme C, Zhang L, Kremmer E, et al. Subunits of the Drosophila CCR4-NOT complex and their roles in mRNA deadenylation. RNA. 2010;16:1356–1370.
- Nousch M, Techritz N, Hampel D, et al. The Ccr4-Not deadenylase complex constitutes the main poly(A) removal activity in C. elegans. J Cell Sci. 2013;126:4274–4285.
- Webster MW, Chen YH, Stowell JAW, et al. mRNA deadenylation is coupled to translation rates by the differential activities of Ccr4-Not nucleases. Mol Cell. 2018;70:1089–1100 e1088.
- Nazio F, Carinci M, Valacca C, et al. Fine-tuning of ULK1 mRNA and protein levels is required for autophagy oscillation. J Cell Biol. 2016;215:841–856.
- Jin M, He D, Backues SK, et al. Transcriptional regulation by Pho23 modulates the frequency of autophagosome formation. Curr Biol. 2014;24:1314–1322.
- Bernard A, Jin M, Gonzalez-Rodriguez P, et al. Rph1/KDM4 mediates nutrient-limitation signaling that leads to the transcriptional induction of autophagy. Curr Biol. 2015;25:546–555.
- Bjedov I, Cocheme HM, Foley A, et al. Fine-tuning autophagy maximises lifespan and is associated with changes in mitochondrial gene expression in Drosophila. PLoS Genet. 2020;16:e1009083.
- Scott RC, Juhasz G, Neufeld TP. Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr Biol. 2007;17:1–11.
- Nagy P, Karpati M, Varga A, et al. Atg17/FIP200 localizes to perilysosomal Ref(2)P aggregates and promotes autophagy by activation of Atg1 in Drosophila. Autophagy. 2014;10:453–467.
- Pattison JS, Osinska H, Robbins J. Atg7 induces basal autophagy and rescues autophagic deficiency in CryABR120G cardiomyocytes. Circ Res. 2011;109:151–160.
- Bhuiyan MS, Pattison JS, Osinska H, et al. Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest. 2013;123:5284–5297.
- Shintani T, Klionsky DJ. Cargo proteins facilitate the formation of transport vesicles in the cytoplasm to vacuole targeting pathway. J Biol Chem. 2004;279:29889–29894.
- Lynch-Day MA, Klionsky DJ. The Cvt pathway as a model for selective autophagy. FEBS Lett. 2010;584:1359–1366.
- Selth LA, Gilbert C, Svejstrup JQ. RNA immunoprecipitation to determine RNA-protein associations in vivo. Cold Spring Harb Protoc. 2009;2009: pdb prot5234.
- Collart MA, Struhl K. CDC39, an essential nuclear protein that negatively regulates transcription and differentially affects the constitutive and inducible HIS3 promoters. EMBO J. 1993;12:177–186.
- Zwartjes CG, Jayne S, van den Berg DL, et al. Repression of promoter activity by CNOT2, a subunit of the transcription regulatory Ccr4-not complex. J Biol Chem. 2004;279:10848–10854.
- Teupe B, Bergis K. Epidemiological evidence for “double diabetes”. Lancet. 1991;337:361–362.
- Eshleman N, Luo X, Capaldi A, et al. Alterations of signaling pathways in response to chemical perturbations used to measure mRNA decay rates in yeast. RNA. 2020;26:10–18.
- Chen J, Chiang YC, Denis CL. CCR4, a 3’-5’ poly(A) RNA and ssDNA exonuclease, is the catalytic component of the cytoplasmic deadenylase. EMBO J. 2002;21:1414–1426.
- Liu X, Yao Z, Jin M, et al. Dhh1 promotes autophagy-related protein translation during nitrogen starvation. PLoS Biol. 2019;17:e3000219.
- Yin Z, Liu X, Ariosa A, et al. Psp2, a novel regulator of autophagy that promotes autophagy-related protein translation. Cell Res. 2019;29:994–1008.
- Welter E, Thumm M, Krick R. Quantification of nonselective bulk autophagy in S. cerevisiae using Pgk1-GFP. Autophagy. 2010;6:794–797.
- Wilczynska A, Gillen SL, Schmidt T, et al. eIF4A2 drives repression of translation at initiation by Ccr4-Not through purine-rich motifs in the 5ʹUTR. Genome Biol. 2019;20:262.
- Cooke A, Prigge A, Wickens M. Translational repression by deadenylases. J Biol Chem. 2010;285:28506–28513.
- Inada T, Makino S. Novel roles of the multi-functional CCR4-NOT complex in post-transcriptional regulation. Front Genet. 2014;5:135.
- Benson JD, Benson M, Howley PM, et al. Association of distinct yeast Not2 functional domains with components of Gcn5 histone acetylase and Ccr4 transcriptional regulatory complexes. EMBO J. 1998;17:6714–6722.
- Picazo C, Orozco H, Matallana E, et al. Interplay among Gcn5, Sch9 and mitochondria during chronological aging of wine yeast is dependent on growth conditions. PLoS One. 2015;10:e0117267.
- Holt LJ, Tuch BB, Villen J, et al. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science. 2009;325:1682–1686.
- Shirai YT, Suzuki T, Morita M, et al. Multifunctional roles of the mammalian CCR4-NOT complex in physiological phenomena. Front Genet. 2014;5:286.
- De Keersmaecker K, Atak ZK, Li N, et al. Exome sequencing identifies mutation in CNOT3 and ribosomal genes RPL5 and RPL10 in T-cell acute lymphoblastic leukemia. Nat Genet. 2013;45:186–190.
- Elmen L, Volpato CB, and Kervadec A, et al. Silencing of CCR4-NOT complex subunits affects heart structure and function. Dis Model Mech. 2020;13:dmm044727.
- Chartier A, Klein P, Pierson S, et al. Mitochondrial dysfunction reveals the role of mRNA poly(A) tail regulation in oculopharyngeal muscular dystrophy pathogenesis. PLoS Genet. 2015;11:e1005092.
- Cheong H, Klionsky DJ. Biochemical methods to monitor autophagy-related processes in yeast. Methods Enzymol. 2008;451:1–26.
- Abeliovich H, Zhang C, Dunn WA Jr., et al. Chemical genetic analysis of Apg1 reveals a non-kinase role in the induction of autophagy. Mol Biol Cell. 2003;14:477–490.
- Huang WP, Scott SV, Kim J, et al. The itinerary of a vesicle component, Aut7p/Cvt5p, terminates in the yeast vacuole via the autophagy/Cvt pathways. J Biol Chem. 2000;275:5845–5851.
- Noda T, Kim J, Huang WP, et al. Apg9p/Cvt7p is an integral membrane protein required for transport vesicle formation in the Cvt and autophagy pathways. J Cell Biol. 2000;148:465–480.
