ABSTRACT
The circadian clock drives daily cycles of physiology and behavioral outputs to keep organisms in tune with the environment. Cyclic oscillations in levels of the clock proteins maintain circadian rhythmicity. In our recent work, we have discovered the interdependence of the circadian clock and chaperone-mediated autophagy (CMA), a selective form of lysosomal protein degradation. Central and peripheral degradation of core clock proteins by CMA (selective chronophagy) modulates circadian rhythm. Loss of CMA in vivo disrupts physiological circadian cycling, resembling defects observed in aging, a condition with reduced CMA. Conversely, the circadian clock temporally regulates CMA activity in a tissue-specific manner, contributing to remodeling of a distinct subproteome at different circadian times. This timely remodeling cannot be sustained when CMA fails, despite rerouting of some CMA substrates to other degradation pathways.
Circadian cellular proteome changes require fine-tuned balance between protein synthesis and degradation. The cycling nature of transcription has been extensively studied but understanding the contribution of different proteolytic pathways to circadian rhythms lags behind. CMA and macroautophagy are the most studied forms of mammalian autophagy. Recent studies have shown that macroautophagy activity exhibits oscillations and regulates the circadian control of liver glucose metabolism. The connection(s) between CMA and circadian clock was not known. In our recent work [Citation1], we investigated whether CMA exhibits circadian oscillations and whether circadian clock proteins are among CMA substrates.
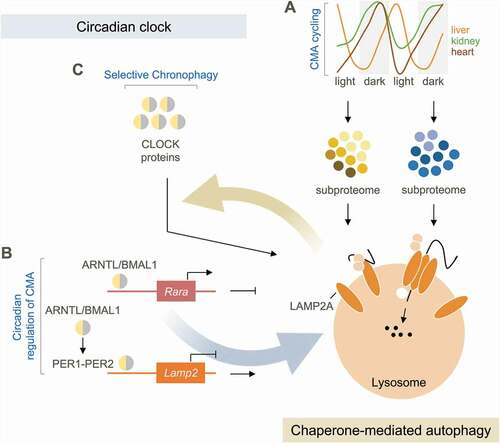
CMA degrades soluble proteins bearing a pentapeptide motif (KFERQ-like), which after being recognized by the chaperone HSPA8, delivers them for binding to LAMP2A (lysosomal-associated membrane protein 2A) on lysosomes. This binding triggers LAMP2A multimerization into a translocation complex for internalization of substrates into lysosomes for degradation. To monitor changes in CMA activity during the light/dark cycle, we used KFERQ-Dendra CMA reporter mice and tracked cargo delivery to lysosomes via CMA. CMA displays tissue-specific oscillations with liver CMA activity reaching maximal activity during the day, when kidney and heart CMA are at their lowest (). Cycling of CMA is transcriptionally regulated by the clock components that impose cycling repression of LAMP2A – the limiting CMA component – both directly (by PER1-PER2 binding to the Lamp2 locus) and indirectly (through ARNTL/BMAL1-mediated expression of RARA (retinoic acid receptor, alpha), an endogenous negative regulator of Lamp2a) (). While this transcriptional regulation seems universal, we identified some differences in the way in which tissues manage CMA cycling. For example, in liver and kidney maximal daily CMA involves recruiting a higher fraction of the cellular lysosomal pool to perform CMA, whereas in heart the number of lysosomes allocated to CMA remains constant and it is their intrinsic activity that changes cyclically. Daily CMA rhythmicity is independent of nutrition – a stark contrast to the nutrition dependence of circadian macroautophagy.
Figure 1. Reciprocal dependence of chaperone-mediated autophagy and the circadian clock. (A) Chaperone-mediated autophagy (CMA) exhibits diverging circadian oscillations in different tissues. CMA remodels the subproteome in a circadian manner by degrading distinct proteins at different times. (B) Circadian clock proteins regulate CMA, with ARNTL/BMAL1 activating a negative regulator of CMA (Rara), and by inhibiting Lamp2a expression. (C) Clock proteins are CMA substrates (selective chronophagy), and blockage of their CMA degradation leads to disruption of central and peripheral clocks and alterations in circadian outputs.
Daily changes in CMA degradation are both quantitative and qualitative. Proteomic analysis of liver lysosomes isolated at different circadian timepoints revealed that the subproteome degraded by CMA during the day and during the night are distinct (). Proteins related with glucose and lipid metabolism, protein translation and processing in the ER and with endocytosis are degraded by CMA at night (time of maximal activity in mice); whereas proteins involved in repair/quality control mechanisms, such as redox buffering, cell death by ferroptosis and the ubiquitin-proteasome and autophagy pathways are diurnally degraded. By contrast, Golgi regulatory proteins undergo CMA degradation without circadian preference. This selectivity in the time of degradation, reinforces the idea of a role for CMA beyond mere quality control of damaged proteins. Instead, by degrading fully functional proteins at specific times during the light/night cycle, CMA terminates their function and, thus, modulates the net outcome of many cellular functions at a given time. After CMA failure in liver, protein quality control dependent on CMA can be undertaken by pathways such as macroautophagy and the ubiquitin-proteasome system, but the different time of degradation of the rerouted CMA substrates by those other pathways makes them unsuitable as an effective replacement for the regulatory functions of CMA. This explains why liver-specific lamp2a knockout (KO) mice do not present abnormal protein buildup but still display major functional defects. The observed rerouting of CMA substrates to macroautophagy upon CMA blockage also challenges the idea that autophagy blockage should always be associated with substrate accumulation in order to have functional consequences. Rerouted CMA substrates upon CMA failure lose their circadian timing of degradation, thus “staying on” at circadian time-points when they are physiologically required to be “switched off”.
A good example of the importance of time of degradation by CMA is the regulation of the circadian clock itself (). The primary clock encompasses positive elements (CLOCK and ARNTL/BMAL1), negative elements (PER1 to PER3 and CRY1-CRY2) and stabilizing elements (RORA/RORα and NR1D1/REVERBα). Clock proteins reach the right levels at the right circadian time via a transcriptional-translational feedback loop and post-translational regulation. Because we noticed KFERQ-motifs in most clock proteins, we investigated their abundance in lysosomes isolated at different circadian times and confirmed their dependence on CMA for degradation using lamp2a KO mice (). ARNTL/BMAL1 and CLOCK show primarily nocturnal CMA degradation and NR1D1/REVERBα diurnal. Lysosomal degradation of ARNTL/BMAL1 and CLOCK is completely lost in lamp2a KO mice, whereas NR1D1/REVERBα is rerouted to other autophagic pathways, although at a shifted circadian time. We confirmed macroautophagy of CRY1 at night, as previously reported, and noticed a larger fraction of CRY1 degraded by CMA during the light period. RORα, despite showing cyclic lysosomal association, does not get degraded. Using these same physiological in vivo settings, we detected proteasome-dependent degradation for CRY2 (during the dark period), CLOCK (during the light period) and NR1D1/REVERBα (during the light period, but at rates one third of those detected in lysosomes). Our study highlights the cooperativity among three different proteolytic systems in the regulation of the clock under physiological conditions.
Functionally, CMA disruption in vivo leads to central and peripheral clock derangements. Lamp2a KO mice display marked changes in circadian behaviors such as activity and adaptation to light cues that phenocopy those in old wild-type mice thus supporting the idea that the decline in CMA activity in aging may contribute to circadian abnormalities in the elderly.
Disclosure statement
AMC serves as scientific advisor of Selphagy (a program under Life Biosciences), and she consults for Generian Pharmaceuticals and Cognition Therapeutics. No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Juste YR, Kaushik S, Bourdenx M, et al. Reciprocal regulation of chaperone-mediated autophagy and the circadian clock. Nat Cell Biol. 2021 Dec;23(12):1255–1270. PMID: 34876687; PMCID: PMC8688252.
