ABSTRACT
STX17 (syntaxin 17) mediates autophagosome-lysosome fusion, and the translocation of STX17 to autophagosomes is characteristic of this process. STX17 arrives at autophagosomes when they are closed, stays there for approximately 10 min to promote fusion with lysosomes, and leaves when the autolysosomes are mature. However, the mechanism of this transient visit remains largely unknown. Here, we summarize the current knowledge about this phenomenon, including a recently discovered retrieval mechanism, and discuss remaining questions.
Abbreviations: MAM: mitochondria-associated membrane; SNX: sorting nexin; STX17: syntaxin 17.
The fusion of autophagosomes with lysosomes is controlled spatiotemporally; lysosomes must fuse with completely sealed autophagosomes to ensure degradation of the engulfed material. If lysosomes fuse with unclosed autophagosomes, the introduced lysosomal enzymes will not remain inside the autophagosomes. One of the main mediators of autophagosome-lysosome fusion is the autophagosomal SNARE protein STX17 (syntaxin 17) [Citation1,Citation2]. STX17 behaves in a manner that we would view as being rational; it is not present on unclosed autophagosomes but is recruited to the autophagosomal membrane immediately before or after closure (). Then, STX17 forms a ternary complex with VAMP7 or VAMP8 in the lysosome membrane as well as SNAP29 in order to promote autophagosome-lysosome fusion. Finally, STX17 leaves the autolysosomes after fusion. The duration of STX17ʹs association with the autophagosome is approximately 10 min [Citation3]. However, it remains unclear how this transient visit of STX17 is regulated.
Figure 1. STX17ʹs recruitment to and retrieval from autophagosomes. “?” indicates processes that have not been demonstrated experimentally. MAM, mitochondria-associated membrane.
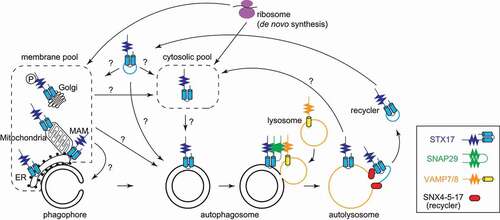
Although several mechanisms of STX17 recruitment have been reported [Citation1,Citation4–7], the release of STX17 from autolysosomes has not been thoroughly investigated. Recently, however, Zhou et al. reported an unexpected recycling mechanism of STX17 [Citation8].
STX17 is pooled in several distinct cellular compartments, including the mitochondria, endoplasmic reticulum (ER), and cytosol, but it is not yet clear from which pool STX17 is recruited to autophagosomes. STX17 is a tail-anchored protein, with two tandem transmembrane domains near the C terminus. Like other tail-anchored proteins, the localization of STX17 is regulated mainly by the C-terminal transmembrane domains and a short tail region [Citation1,Citation5]. Itakura et al. suggested that the two transmembrane domains form a hairpin-like structure via glycine-zipper motifs and are then inserted into the autophagosomal membrane. Time-lapse imaging of green fluorescent protein (GFP)-STX17 led to the expectation that STX17 in the cytosol is inserted into the autophagosomal membrane because no fusion of STX17-positive vesicles was observed, and the fluorescence intensity of GFP-STX17 increases uniformly across the entire autophagosomal membrane () [Citation3]. Kumar et al. reported that STX17 binds to IRGM (immunity related GTPase M) and Atg8-family proteins (LC3s and GABARAPs) to form trimeric complexes, called ARPs (autophagosome-recognition particles), which are recruited from the cytosol to the autophagosomal membrane [Citation7]. However, because STX17 recruitment can be observed in both Atg8-family protein-deficient cells [Citation9] and Atg8 lipidation-deficient cells [Citation3], an STX17 recruitment mechanism should exist independent of IRGM and Atg8-family proteins. The amount of the cytosolic pool of endogenous STX17 varies depending on the specific study: some reports show significant amounts of cytosolic STX17 in mouse embryonic fibroblasts [Citation1] and HeLa cells [Citation10], but others show only small amounts in HeLa cells [Citation11] and HEK293T cells [Citation4]. Thus, an even small amount of cytosolic STX17 may be sufficient to decorate autophagosomes.
STX17 is also present in mitochondria, the ER, and mitochondria-associated membranes (MAMs), raising the possibility that STX17 may be recruited to autophagosomes by vesicle transport or by diffusion through membrane contact sites (). Because STX17 binds to ATG14 at the MAM and recruits the class III phosphatidylinositol 3-kinase complex in order to promote autophagosome formation at the MAM [Citation4,Citation11], it is possible that STX17 is transported from the MAM to the autophagosomal membrane (ATG14 also acts as a tethering factor and interacts with STX17 to promote autophagosome-lysosome fusion [Citation12]). It has also been reported that phosphorylated STX17, which is pooled in the Golgi, is required for the formation of mammalian phagophore formation sites [Citation6]. In this report, phosphorylated STX17 co-localizes with the autophagy initiation complex and the omegasome marker FYVE1/DFCP. However, fluorescence and electron microscopy observations revealed that STX17 is present on closed autophagosomes but not on cup-shaped autophagosome intermediates (i.e., phagophores). Thus, even though STX17 plays a role in the early stages of autophagosome formation, it is likely that fusion-promoting STX17 is recruited once autophagosomes have closed.
Regarding the release of STX17 from autolysosomes, Tsuboyama et al. observed by time-lapse imaging that STX17 dissociates a few minutes after the inner autophagosomal membrane is degraded [Citation3]. Because of this rapid release, it was speculated that STX17 would be removed from the membrane and released into the cytosol all at once. However, Zhou et al. recently reported that STX17 retrieval is achieved via vesicle transport () [Citation8]. They found that STX17-positive autolysosomes are abundant at 2 h after starvation but are no longer observed after 5 h. Furthermore, time-lapse imaging showed that STX17-enriched buds are pinched off and released from the autolysosomes, thereby reducing the amount of autolysosomal STX17. Approximately one budding per minute occurs from a single autolysosome, and almost all the STX17 on each autolysosome is retrieved after approximately four rounds of budding. The budding of STX17-positive vesicles is carried out by a complex named “recycler,” which comprises SNX4 (sorting nexin 4), SNX5, and SNX17 (). This mechanism operates independent of the retromer and retriever complexes, which involve SNXs. Furthermore, Zhou et al. found that ATG9A, which is present in the autophagosomal membrane in small amounts, is also recycled from autolysosomes by the recycler complex, suggesting that the recycler complex selectively sorts autophagosome-derived membrane proteins such as STX17 and ATG9A from autolysosomes and prevents their accumulation on the autolysosomal surface. Recycler-deficient cells demonstrate lower autophagic flux but normal autophagosome formation. Zhou et al. named this retrieval mechanism “autophagosomal components recycling” (ACR).
Many questions about the behavior of STX17 in the autophagy pathway remain (). For example, it is still unclear whether STX17 is recruited from the cytosol or from organelles such as the mitochondria and ER. If the latter is the case, it should be clarified how STX17 comes to the autophagosomes. Is it mediated by vesicle transport or by lipid transport between the membranes at contact sites? What determines the timing of recruitment is also an important topic for future research. Regarding ACR, what is the fate of the retrieved vesicles containing STX17? Are they recycled back to the autophagosomes or perhaps to other organelles? Tracking these small vesicles in living cells would be challenging. If both STX17 and ATG9 are contained in the same retrieved vesicles, they will arrive at the same destination and from there may head to their respective destinations. Identification of other cargos that are recycled by ACR would be another interesting topic for future research. These questions need to be addressed in order to fully understand the intriguing behavior of STX17 and the regulation of autophagosome-lysosome fusion.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151(6):1256–1269.
- Takats S, Nagy P, Varga A, et al. Autophagosomal Syntaxin17-dependent lysosomal degradation maintains neuronal function in drosophila. J Cell Biol. 2013;201(4):531–539.
- Tsuboyama K, Koyama-Honda I, Sakamaki Y, et al. The ATG conjugation systems are important for degradation of the inner autophagosomal membrane. Science. 2016;354(6315):1036–1041.
- Hamasaki M, Furuta N, Matsuda A, et al. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495(7441):389–393.
- Kato S, Arasaki K, Tokutomi N, et al. Syntaxin 17, an ancient SNARE paralog, plays different and conserved roles in different organisms. J Cell Sci. 2021;134(22). DOI:https://doi.org/10.1242/jcs.258699.
- Kumar S, Gu Y, Abudu YP, et al. Phosphorylation of syntaxin 17 by TBK1 controls autophagy initiation. Dev Cell. 2019;49(1):130–144 e6.
- Kumar S, Jain A, Farzam F, et al. Mechanism of Stx17 recruitment to autophagosomes via IRGM and mammalian Atg8 proteins. J Cell Biol. 2018;217(3):997–1013.
- Zhou C, Wu Z, Du W, et al. Recycling of autophagosomal components from autolysosomes by the recycler complex. Nat Cell Biol. 2022;24(4):497–512.
- Nguyen TN, Padman BS, Usher J, et al. Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J Cell Biol. 2016;215(6):857–874.
- Matsui T, Jiang P, Nakano S, et al. Autophagosomal YKT6 is required for fusion with lysosomes independently of syntaxin 17. J Cell Biol. 2018;217(8):2633–2645.
- Arasaki K, Shimizu H, Mogari H, et al. A role for the ancient SNARE syntaxin 17 in regulating mitochondrial division. Dev Cell. 2015;32(3):304–317.
- Diao J, Liu R, Rong Y, et al. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature. 2015;520(7548):563–566.
