ABSTRACT
Transitions from the early to late phagophore, which occur to engulf cytoplasmic material within an autophagosome for macroautophagic/autophagic degradation, involve dynamic ultrastructural changes that are not fully understood. A recent study combined cryo-electron tomography (cryo-ET) with extensive computational analysis to get a better insight into autophagosome biogenesis in situ within yeast cells. This approach disclosed new information on the shape of autophagic structures, their contacts with surrounding organelles, membrane sources, and mechanisms of transition. Together, these results provide new directions for autophagy research, and show the potential of cryo-ET in cell biology.
Abbreviations: Cryo-ET, cryo-electron tomography; ER, endoplasmic reticulum; IMDa, intermembrane distance in the autophagosome; IMDp, intermembrane distance in the phagophore; LD, lipid droplets
Autophagosome biogenesis involves dynamic shaping and transitions of an open cup-shaped structure, the phagophore, that eventually closes into a double-membrane organelle, the autophagosome. Ultrastructural details of this process are still not well understood because they are difficult to capture by microscopy. Cryo-electron tomography (cryo-ET) is a technique that allows for visualization of ultrastructures in situ within cells and, thereby, allows for a unique observation of cellular environment with minimal disruption. Here, we discuss a recent study by Bieber et al. [Citation1] that applied cryo-ET on yeast cells to reveal structural details during autophagosome formation. In order to capture some rare intermediates, the authors used overexpressed fluorescently tagged Atg8, a hallmark of autophagy membranes, and Ede1, as a marker for selective autophagy cargo, in nitrogen-starved cells that were subjected to focused ion beam milling and tomogram acquisition. Correlative cryo-ET captured transient autophagic structures such as open phagophores in various stages of maturation, closed autophagosomes with or without Ede1-containing cargo, and autophagic bodies in the vacuole. Ribosomes, lipid droplets (LDs), the endoplasmic reticulum (ER), small vesicles, mitochondria, the Golgi apparatus, and the nuclear membrane were also visible in the tomograms and their 3D renderings.
A closer examination of ribosomes and autophagy cargo revealed that, under starvation conditions, the majority of phagophores and autophagosomes engulf either solely ribosomes or ribosomes next to selective cargo. Only rarely is the selective cargo exclusively engulfed by autophagic structures. Comparison of the ribosome density within and outside of autophagic structures shows no difference between these two locations. Autophagic and cytosolic ribosomes also exhibit similar nearest neighbor distances, suggesting that autophagic membranes are not guided by a cargo.
In-situ detected phagophores and autophagosomes are found making contacts with other cellular organelles. The autophagic structures frequently contact the vacuole, small vesicles (average diameter of 40 nm), LDs, and the ER. Interestingly, mitochondria are predominantly in an out-of-contact distance of more than 100 µm from autophagic structures. Bieber et al. evaluated positions where various organelles interact with the phagophore. They found that the vacuole contacts the back (convex surface) and side of the phagophore, and rarely the rim (, light gray line). Half of the phagophores exhibit a membrane deformation in the contact with the vacuole, by either forming a peak () or having a tendency to follow the contour of the vacuole membrane. LDs prefer to interact with the side or rim of the phagophore, again causing deformations in the rim (, dashed gray line) or reshaping along the contour of the LDs. The nuclear membrane makes contact sites exclusively with the phagophore rim, which, in this case, is always deformed (, thick black line). The ER contacts the phagophore rim without any deformations (, dark gray line at the bottom of the phagophore) and small vesicles do not have any preferred interacting site on the phagophore. In contrast to phagophores, autophagosomes are far less prone to deformations by surrounding organelles. Some phagophore-ER contact sites are so close that the Atg2 protein can bridge the distance between these two organelles. Bieber et al. analyzed these sites and calculated that less than 100 Atg2 molecules would be sufficient to build an average-sized autophagosome.
Figure 1. Shaping and transitions of ultrastructures revealed by cryo-ET during autophagosome biogenesis. Cellular organelles contact the phagophore at different sites. The vacuole interacts predominantly with the side and back of the phagophore, and often deforms the phagophore membrane into a peak (light gray line). Lipid droplets (LD; gray spheres) interact with the side and rim of the phagophore, and cause deformations of the rim (dashed gray line). The nuclear envelope contacts exclusively the rim of the phagophore, and strongly deforms it (thick black line). The endoplasmic reticulum (ER) makes preferred contacts with the phagophore rim that is free of any deformations by the ER membrane (dark gray line). Atg9 and COPII vesicles do not have a preferred interaction site on the phagophore. The highly curved rim at the opening of the phagophore is typically swollen near the tip (dark gray line). Atg9-containing and COPII vesicles contribute their lipid membrane and lumen when they fuse with the growing phagophore. A high membrane area:lumen volume ratio for the spherical autophagosome is proposed to be a result of direct lipid transfer from the ER in a later stage of phagophore expansion. The maturing phagophore is thinning; the intermembrane distance in the phagophore (IMDp) is larger than that in the autophagosome (IMDa).
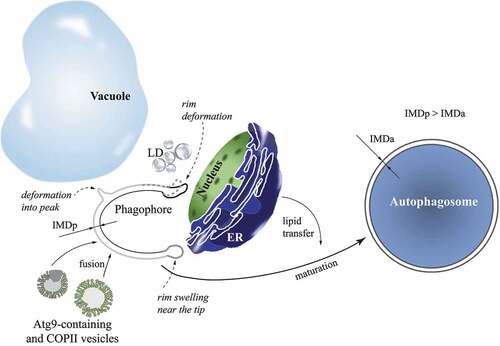
A sphericity index calculated from the best-fitting ellipsoids shows the general geometry of autophagic structures. Although the overall size of the open expanded phagophore and closed autophagosome is similar, the autophagosome is a sphere, whereas the phagophore is rather ellipsoidal. Revealing data comes from the application of a special algorithm that yields measurements of intermembrane distances in double-membrane organelles. The intermembrane distance in the phagophore (IMDp) and autophagosome (IMDa) are significantly smaller than the distances in the nuclear envelope, ER, or mitochondria. The intermembrane spacing of 9–11 nm in the autophagic structures is also very homogeneous. The mean intermembrane distance evaluated throughout the process of phagophore expansion shows a decrease that occurs as there is an increase in degree of maturation, meaning that the phagophore is thinning as it grows.
The shape of the autophagosome, resembling a nearly perfect double-membrane sphere, makes it possible to calculate a total membrane area to intermembrane lumen volume ratio. Closed autophagosomes exhibit an average ratio of 0.53 ± 0.10 nm−1, a value that is significantly higher than the ratio of 0.35–0.13 nm−1 calculated for Atg9-containing and COPII vesicles, based on their previously reported diameters of 30–60 nm and >60 nm, respectively. These diameters are in agreement with measurements from the tomograms that show on average a diameter of 40 nm for vesicles observed within the distance of 100 nm from autophagic structures. Because two process are thought to build autophagosomes, vesicle fusion and direct lipid transfer, Bieber et al. reason that a high area:volume ratio for autophagosomes can be obtained only by a significant membrane contribution from direct lipid transfer or synthesis. Vesicle fusion alone cannot yield a high area:volume ratio because vesicles add both membrane area and lumen volume to the growing phagophore.
Analysis of the tomograms visualizing phagophores shows that the cup-shaped structure of the phagophore is highly curved at the opening and appears dilated. The authors inspected in detail 26 well-resolved phagophore rims, and analyzed the intermembrane distances near the tip and further in the back of the phagophore. The data clearly show rim swelling (, dark gray line) at the average position of 17 ± 7 nm from the tip. A ratio of the intermembrane distance at the maximal dilation to the intermembrane distance toward the back of the phagophore yields a dilation factor for each phagophore rim. Dilation factors of all analyzed rims have a mean value of 1.35 ± 0.15. Bieber et al. speculate that a purpose of the rim swelling might be a reduction in the local mean curvature and ultimately in the bending energy, relative to a rim that is not dilated near the tip. To test this idea, an artificial nondilated rim was constructed and compared to the experimental dilated rim with the same overall shape and membrane area. Calculation of the bending energy shows that 7 out of 8 experimental rims with dilation factor >1.3 have a lower bending energy than the artificial nondilated rim. Furthermore, energy calculations reveal that the rim undergoes dynamic changes as the phagophore expands. The degree of curvature at the rim tip and the bending energy per nanometer of rim increase with the extent of phagophore maturation. These results suggest that a decrease in bending energy by swelling near the rim tip can be a compensating measure in order to stabilize the open phagophore state.
In addition to capturing transient intermediates in the process of autophagosome formation, the study by Bieber et al. employs numerous computational algorithms that provide revealing measurements and conclusions about autophagic structures. For example, the decreasing intermembrane distance observed during phagophore expansion indicates an increasing membrane area:lumen volume ratio with the progression from the early to late phagophore. This finding implies that membrane sources shift from vesicle fusion to a direct lipid transfer when the phagophore expands and transitions into the autophagosome (). Triggering signals and regulating factors for such a shift remain to be elucidated. Another interesting output from the tomograms is the observation that there is no correlation between the size and intermembrane distance in autophagosomes. This result shows that a bigger autophagosome is not thinner than a smaller autophagosome and that lipid transfer, which promotes phagophore thinning during expansion, does not control the differential size of autophagosomes. Thereby, the authors propose that the final size of the autophagosome might be restrained by the number and fusion rate of vesicles joining the growing phagophore because only fusing vesicles presumably control the lumenal volume of the autophagosome.
Initial vesicular contribution of the lipid membrane and lumen to the phagophore is in accord with numerous biochemical studies. These studies together suggest that Atg9-containing lipid vesicles are involved in protein-protein and protein-lipid interactions with the multimeric self-assembly of the Atg1 complex, where intrinsically disordered domains promote phase separation of the complex to form liquid droplets of the phagophore assembly site/PAS [Citation2–8]. The phagophore assembly site is tethered to the vacuolar membrane via the Atg13-Vac8 interaction, where the C terminus of Atg13 binds Vac8 that is anchored in the vacuolar membrane by its N terminus [Citation9–11], and is concentrated in membrane subdomains from which the vacuolar protein Vph1 is excluded [Citation12]. This intricate protein interplay somehow specifically generates the preferential contact of the vacuole at the back and side of the phagophore, which is seen in the tomograms. How this orientation of contact sites is mechanistically achieved remains an intriguing question for future investigations. One would expect that mechanisms involved in guiding the phagophore-vacuole contacts and positions are elegantly efficient because the tomograms show different phagophore sizes near the vacuole and the presence of open phagophores directly beside (but on the opposite side of the vacuolar membrane) autophagic bodies in the vacuole, which indicates the usage of the same vacuolar site in fast succession.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bieber A, Capitanio C, Erdmann PS, et al. In situ structural analysis reveals membrane shape transitions during autophagosome formation. Proc Natl Acad Sci U S A. 2022 Sep 27;119(39):e2209823119.
- Sekito T, Kawamata T, Ichikawa R, et al. Atg17 recruits Atg9 to organize the pre-autophagosomal structure. Genes Cells. 2009 May;14(5):525–538.
- Suzuki SW, Yamamoto H, Oikawa Y, et al. Atg13 HORMA domain recruits Atg9 vesicles during autophagosome formation. Proc Natl Acad Sci USA. 2015 Mar 17;112(11):3350–3355.
- Rao Y, Perna MG, Hofmann B, et al. The Atg1-kinase complex tethers Atg9-vesicles to initiate autophagy. Nat Commun. 2016 Jan 12;7:10338.
- Fujioka Y, Alam JM, Noshiro D, et al. Phase separation organizes the site of autophagosome formation. Nature. 2020 Feb;578(7794):301–305.
- Gatica D, Wen X, Cheong H, et al. Vac8 determines phagophore assembly site vacuolar localization during nitrogen starvation-induced autophagy. Autophagy. 2021 Jul;17(7):1636–1648.
- Uversky VN. Recent developments in the field of intrinsically disordered proteins: intrinsic disorder-based emergence in cellular biology in light of the physiological and pathological liquid-liquid phase transitions. Annu Rev Biophys. 2021 May;6(50):135–156.
- Yamamoto H, Fujioka Y, Suzuki SW, et al. The intrinsically disordered protein Atg13 mediates supramolecular assembly of autophagy initiation complexes. Dev Cell. 2016 Jul 11;38(1):86–99.
- Gatica D, Damasio A, Pascual C, et al. The carboxy terminus of yeast Atg13 binds phospholipid membrane via motifs that overlap with the Vac8-interacting domain. Autophagy. 2020 Jun;16(6):1007–1020.
- Hollenstein DM, Gomez-Sanchez R, Ciftci A, et al. Vac8 spatially confines autophagosome formation at the vacuole in Scerevisiae. J Cell Sci. 2019 Nov 14;132:22.
- Park J, Kim HI, Jeong H, et al. Quaternary structures of Vac8 differentially regulate the Cvt and PMN pathways. Autophagy. 2020 Jun;16(6):991–1006.
- Munzel L, Neumann P, Otto FB, et al. Atg21 organizes Atg8 lipidation at the contact of the vacuole with the phagophore. Autophagy. 2021 Jun;17(6):1458–1478.
