ABSTRACT
Conjugation of ATG8 to single membranes (CASM) at endolysosomal compartments has attracted attention as the non-autophagic function of the Atg8-family protein conjugation system, and the V-ATPase-ATG16L1 axis has emerged as a core mechanism. Our recent research has revealed that this mechanism contributes to the lysosomal recruitment and activation of LRRK2, a Parkinson disease-associated kinase that phosphorylates a subset of RAB GTPases. The activated LRRK2 under CASM-causing lysosomal stress acts to regulate lysosomal morphology and stimulate extracellular secretion of lysosomal contents, thereby promoting the lysosomal stress response.
The Atg8-family protein conjugation system, consisting mainly of the ATG12–ATG5-ATG16L1 complex as well as ATG7 and ATG3, is well known to conjugate Atg8-family proteins (MAP1LC3/LC3, GABARAP, etc.) to phagophores – precursors to double-membrane autophagosomes – during macroautophagy (hereafter autophagy). However, it is now widely accepted that the Atg8-family protein conjugation system also functions to conjugate Atg8-family proteins to single membranes of the stressed endolysosomes, which occurs independent of autophagy. This phenomenon has long been referred to as “noncanonical autophagy”, but because it does not necessarily accompany intracellular degradation and is potentially confusing with other autophagy-related phenomena, another term, “CASM” (conjugation of ATG8 to single membranes), has recently become established. CASM is induced by a variety of stimuli, including lysosomotropic and proton ionophore drug treatment, activation of STING1 or MCOLN1/TRMPL1, influenza A virus infection, and phagocytosis of pathogens and dead cells. Specifically, LC3 recruitment onto pathogen-containing phagosomes has also been referred to as LC3-associated phagocytosis/LAP. Mechanistically, a major type of CASM does not require autophagy upstream machinery (e.g., the ULK complex and the class III phosphatidylinositol 3-kinase complex) but critically requires the interaction between the lysosomal vacuolar-type H+-translocating ATPase (V-ATPase) and the WD40 domain of ATG16L1, a domain inessential for canonical autophagy; this machinery is now specifically called “the V-ATPase-ATG16L1 axis”. However, the roles and significance of this machinery in cells have not been well understood.
We found a clue about the significance of CASM from our research on familial Parkinson Disease (PD). LRRK2 (leucine rich repeat kinase 2) is the most common causative gene for autosomal-dominant PD and encodes a large serine/threonine kinase that phosphorylates a subset of RAB GTPases. Previous studies by us and others have revealed that lysosomal stress loading such as that due to treatment with chloroquine (a lysosomotropic agent) or zymosan (a yeast cell wall preparation, mimicking pathogen infection) induces LRRK2 recruitment to the stressed lysosomal membranes as well as its activation. Under such conditions, we found that LRRK2 is mostly colocalized with LC3 on enlarged lysosomes. This colocalization with LC3 is classically suggestive of the localization of LRRK2 to double-membrane autophagosomes, but electron microscopy by CLEM confirmed that LRRK2 and LC3 are indeed found on single-membrane lysosomes.
Then, we hypothesized that LRRK2 recruitment onto lysosomes is regulated by the mechanism of CASM. Lysosomal/phagolysosomal recruitment of LRRK2 upon treatment with chloroquine or zymosan is suppressed by knockdown of ATG16L1 or ATG5, but not by that of RB1CC1/FIP200 or ATG13 that constitute part of the autophagy initiation complex, suggesting the involvement of CASM but not classical autophagy [Citation1]. To examine the involvement of the V-ATPase-ATG16L1 axis in CASM, we analyzed cells harboring mutant ATG16L1 that endogenously lacks its WD40 domain only (thus preserving canonical autophagic activity), or those overexpressing bacterial SopF protein that interferes with the interaction between lysosomal V-ATPase and ATG16L1; both of these cells show reduced recruitment of LRRK2 to lysosomes following chloroquine treatment. Furthermore, this mechanism is responsible for the activation of lysosome-localized LRRK2 as well as the resultant exocytic secretion of lysosomal contents and morphological regulation of lysosomes ().
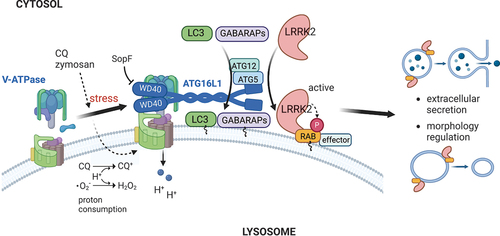
Figure 1. A schematic model of LRRK2 regulation by CASM through the V-ATPase-ATG16L1 axis. CASM-inducing stimuli, such as chloroquine (CQ) or zymosan treatment, cause pH-increasing stress to lysosomes and upregulate the V-ATPase-ATG16L1 axis, which then recruits LRRK2 as well as Atg8-family proteins onto lysosomes. LRRK2 is activated on membranes and phosphorylates RAB GTPases there, leading to the extracellular secretion of lysosomal contents and morphological regulation of lysosomes. This figure was created with BioRender.com.
Together, these findings suggest a new function of CASM that utilizes LRRK2 to facilitate lysosomal stress responses. This CASM-LRRK2 pathway is considered to act as one of the cellular defense systems that cope with lysosomal stress and maintain their homeostasis. The biggest unresolved question here would be how CASM regulates LRRK2. Although LRRK2 has several putative LC3-interacting region/LIR motifs, we have not succeeded in the detection of an interaction between LRRK2 and Atg8-family proteins. We have additionally observed that, when LC3 lipidation status is changed by manipulating ATG4, RAB10 phosphorylation does not correlate with the amount of LC3-II, suggesting independency of LRRK2 activity from Atg8-family protein lipidation (the data are available in the published review history of this paper). Conversely, another recent study in a preprint has shown the direct regulation of LRRK2 by GABARAP, and, given additional molecules assumed to play together in CASM, LRRK2 recruitment via such molecules would also be possible. It is also unclear how LRRK2 activity regulates lysosomal secretion and morphology under lysosomal stress. As LRRK2 phosphorylates a subset of RAB GTPases (e.g., RAB8A and RAB10) that are well-known regulators of membrane trafficking, it would be worth further investigation as to how lysosomal membrane dynamics are controlled downstream of LRRK2.
In relevance to PD, aberrant activation of LRRK2 has been demonstrated in disease tissues, and it is possible that abnormal CASM leading to LRRK2 activation may contribute to the pathogenesis in such a way that lysosomal or intracellular/extracellular dynamics of aggregated proteins are affected. To date, aberrant autophagic activity in PD has been repeatedly reported, but some of the observations such as the elevated LC3 lipidation or immunostaining might be detecting CASM, and therefore it will be important to distinguish CASM from autophagy in future disease research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
