ABSTRACT
Reticulophagy, which directs the endoplasmic reticulum (ER) to the phagophore for sequestration within an autophagosome and subsequent lysosomal degradation via specific receptors, is essential for ER quality control and is implicated in various diseases. This study utilizes Drosophila to establish an in vivo model for reticulophagy. Starvation-induced reticulophagy is detected across multiple tissues in Drosophila. Whole-body upregulation or downregulation of the expression of reticulophagy receptors, atl and Rtnl1, negatively affects fly health. Notably, moderate upregulation of reticulophagy in neuronal tissues by overexpressing these receptors reduces age-related degeneration. In a Drosophila Alzheimer model expressing human APP (amyloid beta precursor protein), reticulophagy is compromised. Correcting reticulophagy by enhancing atl and Rtnl1 expression in the neurons promotes APP degradation, significantly reducing neurodegenerative symptoms. However, overexpression of mutated atl and Rtnl1, which disrupts the interaction of the corresponding proteins with Atg8, does not alleviate these symptoms, emphasizing the importance of receptor functionality. These findings support modulating reticulophagy as a therapeutic strategy for aging and neurodegenerative diseases associated with ER protein accumulation.
Macroautophagy/autophagy refers to the formation of a double-membrane structure, a phagophore, that encloses cytosolic substrates; upon closure, the resulting autophagosome fuses with the lysosome for degradation. Autophagy can be categorized into nonselective and selective types. Selective autophagy involves the direct binding of cargo receptors with the autophagy protein Atg8. Reticulophagy/ER-phagy specifically degrades the endoplasmic reticulum (ER) and is required for ER quality control. Dysfunctions in reticulophagy are associated with various diseases, such as neurodegeneration. Previous studies on reticulophagy have predominantly utilized yeast or in vitro cultured cells, lacking in vivo animal models. Consequently, the physiological and pathological functions of reticulophagy remain unclear.
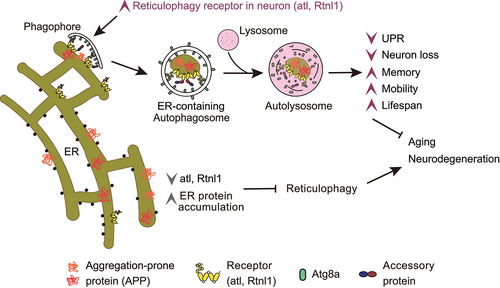
To study the function of reticulophagy, we first established an animal model in Drosophila. Reticulophagy is successfully induced in various organs of Drosophila, including the fat body and brain, through starvation or treatment with the mTor inhibitor rapamycin. Homology analysis identified Drosophila atl (CG6668) and Rtnl1 (CG33113) as homologs of human reticulophagy receptors ATL3 and RTN3L, respectively. Knockdown of atl or mutation of Rtnl1 in Drosophila blocks reticulophagy without affecting nonselective autophagy. These manipulations result in an increased unfolded protein response/UPR, decreased climbing ability, extensive brain neuron death, and shortened lifespan. These findings suggest that disrupting the expression of reticulophagy receptors atl and Rtnl1 impairs reticulophagy and triggers neurodegenerative phenotypes in Drosophila.
The finding that impaired reticulophagy compromises nervous system health led us to investigate whether enhancing reticulophagy could benefit the organism. Surprisingly, we discovered that globally upregulating reticulophagy by overexpressing atl or Rtnl1 harms the health of Drosophila, suggesting the adverse effects of overactivating reticulophagy. To overcome this, we employed the GeneSwitch system in Drosophila, which allows for controlled timing, targeted tissue expression, and adjustable gene expression levels. Using this system, we found that moderate upregulation of atl or Rtnl1 in Drosophila neurons reduces the age-related increase in the unfolded protein response, improves climbing ability, and extends lifespan. To determine whether these beneficial effects are due to the function of Rtnl1 or atl as reticulophagy receptors, we constructed transgenic flies expressing Rtnl1 with LC3-interacting region (LIR) mutations (Rtnl1-LIRm) and atl with a GABARAP-interacting motif (GIM) mutation (atl-GIMm), which lose their ability to bind Atg8. Overexpression of atl-GIMm or Rtnl1-LIRm neither promotes reticulophagy nor delays aging. Taken together, these results suggest that moderate overexpression of reticulophagy receptors atl or Rtnl1 in Drosophila neurons promotes reticulophagy and delays aging.
Recognizing the importance of reticulophagy in maintaining neuronal health, we explored its ability to clear neurodegeneration-related proteins such as APP, associated with familial Alzheimer disease (AD). After synthesis, APP is processed in the ER and then localized to the cell membrane through the secretory pathway. Given its potential accumulation in the ER, we utilized Drosophila models expressing human APP to see if reticulophagy could eliminate it. We found that a substantial amount of APP colocalizes with the ER. Flies expressing APP exhibit inhibited reticulophagy but normal nonselective macroautophagy. These flies display neurodegenerative phenotypes, including decreased climbing ability, impaired learning and memory, increased neuronal cell death, and shortened lifespan. Transcriptional analysis in APP flies shows specific downregulation of the reticulophagy genes atl and Rtnl1, with no significant changes in nonselective autophagy genes. This may be attributed to the specific disruption of reticulophagy and the accumulation of APP in the ER.
To correct the reticulophagy defect in APP flies, we upregulated the expression of atl and Rtnl1 in the brain of these flies. We found that increasing reticulophagy effectively promotes the degradation of ER-localized APP, thus significantly alleviating the neurodegenerative pathology. However, overexpression of atl-GIMm or Rtnl1-LIRm in APP flies does not promote APP degradation nor alleviate neurodegenerative pathology. These results suggest that upregulation of the reticulophagy receptors atl and Rtnl1 in neurons facilitates APP degradation via reticulophagy and alleviates its neurotoxicity.
In summary, our study demonstrates for the first time at an individual level the function of reticulophagy in delaying aging and its effectiveness in clearing APP accumulated in the ER () [Citation1]. Our finding that enhancing reticulophagy to degrade pathogenic proteins like APP in Alzheimer disease alleviates symptoms suggests a broader therapeutic strategy for conditions characterized by ER protein aggregation. Future work will be needed to uncover: 1) how reticulophagy is specifically blocked in APP-expressing flies; 2) whether the induction of reticulophagy varies in its protective effectiveness across different brain regions or neuronal cell types; 3) how activating reticulophagy in the brain contributes to overall organismal health; 4) whether the findings from this study in flies are conserved in higher organisms. Ultimately, finding ways to moderately activate neuronal reticulophagy to avoid overactivation and treat aging and protein aggregation diseases is a crucial future step.
Figure 1. Activating reticulophagy in the brain protects Drosophila from aging and ER protein accumulation toxicity. Impaired reticulophagy in aged or atl or Rtnl1 receptor-downregulated flies leads to neurodegeneration. Enhancing reticulophagy via increased atl or Rtnl1 delays aging and enhances fitness in normal flies. This enhancement in an Alzheimer Drosophila model reduces neurotoxicity by clearing APP.
Disclosure statement
No potential conflict of interest was reported by the author(s).
