Abstract
Context: Acidemia is a marker of prognosis in methanol poisoning, as well as compounding formate-induced cytotoxicity. Prompt correction of acidemia is a key treatment of methanol toxicity and methods to optimize this are poorly defined.
Objective: We studied the efficiency of acidemia correction by intermittent hemodialysis (IHD) and continuous renal replacement therapy (CRRT) in a mass outbreak of methanol poisoning.
Methods: The study was designed as observational cohort study. The mean time for an increase of 1 mmol/L HCO3–, 0.01 unit arterial blood pH, and the total time for correction of HCO3– were determined in IHD- and CRRT-treated patients.
Results: Data were obtained from 18 patients treated with IHD and 13 patients treated with CRRT. At baseline, CRRT group was more acidemic than IHD group (mean arterial pH 6.79 ± 0.10 versus 7.05 ± 0.10; p = 0.001). No association was found between the rate of acidemia correction and age, weight, serum methanol, lactate, formate, and glucose on admission. The time to HCO3– correction correlated with arterial blood pH (r= −0.511; p = 0.003) and creatinine (r = 0.415; p = 0.020). There was association between the time to HCO3– correction and dialysate/effluent and blood flow rates (r= −0.738; p < 0.001 and r= −0.602; p < 0.001, correspondingly).
The mean time for HCO3– to increase by 1 mmol/L was 12 ± 2 min for IHD versus 34 ± 8 min for CRRT (p < 0.001), and the mean time for arterial blood pH to increase 0.01 was 7 ± 1 mins for IHD versus 11 ± 4 min for CRRT (p = 0.024). The mean increase in HCO3– was 5.67 ± 0.90 mmol/L/h for IHD versus 2.17 ± 0.74 mmol/L/h for CRRT (p < 0.001).
Conclusions: Our study supports the superiority of IHD over CRRT in terms of the rate of acidemia correction.
Introduction
Background
Severity of metabolic acidosis on admission is an important prognostic parameter in acute methanol poisoning.[Citation1–3] There is a strong correlation between admission arterial blood pH and mortality, as well as the probability of long-term visual and central nervous system (CNS) sequelae of poisoning in survivors.[Citation4–7] In methanol poisoning, acidosis is caused primarily by metabolism of methanol to formate, and saturation of folate-dependent detoxification of formate results in accumulation of formic acid in blood serum.[Citation8,Citation9]
Patients who die generally have serum formate concentration higher than 12 mmol/L and severe lactacidemia.[Citation10,Citation11] Therefore, given that acidemia has a central role in propagating methanol toxicity, timely and adequate correction of acidemia is crucial for the successful treatment of acute methanol poisoning.
Importance
The role of extracorporeal treatments (ECTRs) in the management of methanol poisoning is well established,[Citation12–15] although there is ongoing discussion regarding indications.[Citation16,Citation17] ECTRs effectively eliminate both methanol and formate.[Citation13–15] According to the current recommendations for ECTR, intermittent hemodialysis (IHD) is the modality of choice in the management of acute methanol poisoning based on a much higher methanol clearance, a greater formate clearance, a lower cost and a higher availability of IHD over continuous renal replacement therapy (CRRT).[Citation15]
There are limited data in the literature regarding the relative rates of correction of acidemia following inhibition of alcohol dehydrogenase (ADH) with ethanol or formepizole. Studies comparing the efficiency of different ECTRs for the correction of acidemia in acute methanol poisoning are non-existent, but case reports note that it may be delayed with CRRT.[Citation15]
More data regarding the efficiency of ECTRs is likely to inform decisions regarding which patients should receive which ECTR. This is particularly important when resources are limited, for example approximately 80% of all dialysis sessions in 2006 were performed in the developed world,[Citation18] whereas the majority of methanol poisoning outbreaks occur in underdeveloped countries where resources are scarce. Therefore, a thorough evaluation of the efficiency and limitations of the various modalities of ECTR on correction of acidemia in acute methanol poisoning is warranted.
Aim of the study
During an outbreak of methanol poisoning in the Czech Republic in 2012,[Citation19–29] we compared the efficiency of ECTRs for correction of acidemia in relation to the severity of poisoning and burden of formate toxicity. The epidemiological description of the outbreak has been presented elsewhere.[Citation3]
Materials and methods
Patients and procedures
The study was designed as an observational cohort study. The hemodialysis modality, type of antidote, and other treatments were selected by clinicians on the basis of clinical status and availability. A total of 137 cases of methanol poisoning occurred between the 3rd of September 2012 and 31st of August 2014, most of the poisonings (121) occurred in 2012; 106 patients were treated in hospitals. A detailed history of methanol exposure, onset, and dynamics of ocular and systemic toxicity was obtained in a prospective manner directly from the patients or from relatives of critically ill patients upon admission. Detailed neurological and ophthalmological examinations were performed during hospitalization and on discharge.
Laboratory analyses were performed on admission to hospital and the patients with an established diagnosis of methanol poisoning were identified. Diagnosis was established when (i) known history of recent ingestion of illicit spirits and serum methanol was higher than 6.2 mmol/L (20 mg/dL), or (ii) history/clinical suspicion of methanol poisoning, and serum methanol was above the limit of detection (1.9 mmol/L, or 6 mg/dL) with at least two of the following: pH <7.3, serum bicarbonate <20 mmol/L, or anion gap (AG) ≥ 20 mmol/L.
Treatment
All patients were treated in accordance with AACT/EAPCCT practice guidelines on the treatment of methanol poisoning.[Citation12] NaHCO3 8.4% solution was given intravenously as a buffer to the patients with metabolic acidosis. Ethanol was administered intravenously as 10% solution according to the following scheme: the loading dose of approximately 7.5–8.0 ml/kg during 1 h, followed by the maintenance dose 1.0–2.0 ml/kg/h or 2.5–3.0 ml/kg/h during hemodialysis. If ethanol was administered per os, 0.7–1.0 ml/kg/h of 20% solution was generally applied in boluses every 3 h.[Citation24,Citation30] The dose of oral ethanol was calculated based on average pharmacokinetic values presented in AACT/EAPCCT practice guidelines on the treatment of methanol poisoning. Standard maintenance dose for ethanol abusers is 0.46 ml/kg/h of 43% oral solution.[Citation12] To ensure better tolerance of oral solution, we diluted it to 20% solution with appropriate increase of the volume: 0.46 ml/kg/h of 43% solution are 0.99 ml/kg/h of 20% solution. As long as in conscious patients oral ethanol solution cannot be administered continuously, it was applied in boluses. For 70 kg patient, one-hour bolus made 69.3 ml of 20% solution. From practical point of view based on the frequency of serum ethanol measurements and optimal workload distribution of medical personnel, 3-h boluses were applied with approximately 200 ml of 20% solution. In these cases, the intravenous administration of ethanol was followed by oral administration after the patients had been dialyzed, until the serum methanol level was below 6.2 mmol/L (20 mg/dL) and the metabolic acidosis was corrected. The solutions of ethanol for intravenous and oral administration were diluted and sterilized by inpatient pharmacy solutions department from the manufactured 96% solution of medicinal ethanol. Fomepizole (Fomepizole EUSA, EUSA Pharma, France) was given as a bolus dose of 15 mg/kg i.v. diluted in isotonic saline, followed by 10 mg/kg every 12 h (every 4 h during hemodialysis).
ECTR was performed if the patients met any of the following criteria: serum methanol higher than 15.6 mmol/L (50 mg/dL), metabolic acidosis with a pH <7.30, or visual disturbances.[Citation12] The mode of ECTR–IHD, extended daily dialysis (EDD), or CRRT – was based on several factors, such as the hemodynamic stability of the patient on admission, the severity of poisoning, and the availability of dialysis equipment. Taking into account the relatively small number of patients with EDD, we combined the patients treated with EDD and IHD in one group for further analysis, given the closer resemblance between EDD and IHD, compared to EDD and CRRT. Excluding the EDD from the analysis did not change the results.
In all 31 cases, a dialysate solution with bicarbonate was applied (BIC 315, BIC 322, and Braun SW139A with 32.6 mmol/L of bicarbonate concentration in the IHD group, and MEDISOL Bi0, Bi4, and ACCUSOL with 40.0 and 35.0 mmol/L of bicarbonate concentration, correspondingly, in the CRRT group). Folate (folinic acid or folic acid) was administered to substitute the endogenous pool of folate.
Laboratory investigations
Arterial blood gases were analyzed on admission, before the start of dialysis, during the dialysis sessions, and until full correction of acidemia, at the end of dialysis sessions, and each 2–4 h during the next eight hours after the dialysis session.
Venous blood for methanol, ethanol, and formate analysis were obtained on admission, at the start of hemodialysis, each 2–4 h during the dialysis session, and at the end of dialysis. The stated parameters of CRRT were maintained during the observation time. Blood samples were spun, serum separated and frozen until analyses.
Calculations and data analysis
The laboratory and clinical data were compared using two-sample assuming unequal variances (equal means), two-sample f-test for variances, bias test, and two-sample Kolmogorov–Smirnov test. The normality of data distribution was characterized using skewness and kurtosis tests. Data are expressed as arithmetic means with confidence interval (significance level α = 0.05). Pearson’s and Spearman’s rank correlation, exploratory factor analysis, and Chi-square tests were used to analyze the associations between the following variables: demographic parameters of the patients (age, gender, and weight), laboratory parameters on admission (serum methanol, ethanol, formate, lactate, glucose, creatinine, potassium, and osmolal gap), parameters of acidemia (arterial blood pH, bicarbonate, pCO2, and anion gap), clinical signs on presentation (Glasgow coma scale, and mean arterial pressure), treatments (dialysis modality, bicarbonate substitution, antidote, folate substitution, inotropes, and noradrenaline administration), technical parameters of ECTRs (blood flow rate, dialysate/effluent flow rate, and dialyzer surface), outcomes (death, survival with and without sequelae), and parameters of acidemia correction (time needed for 0.01-unit increase of arterial blood pH, time to standard bicarbonate increase of 1 mmol/L). Statistically significant parameters were subsequently used in multivariate linear regression models of ordinal multinomic logistic regression based on likelihood ratio estimation. Statistical analysis was performed using Excel (Microsoft, Redmond, WA), and the formal calculations were produced in QC Expert software 3.1 (Trilobyte, Pardubice, Czech Republic) and in IBM SPSS ver. 17.0 (IBM, Armonk, NY) and Statistica SW ver. 10.0 (Dell Inc., Round Rock, TX).
Ethics
The study was approved by the General University Hospital Ethics Committee in Prague, Czech Republic.
Results
Dialysis was carried out in 81 cases in 30 hospitals located in 11 different regions of the Czech Republic: intermittent modalities were applied in 35 cases, continuous modalities in 41 cases, and EDD in 5 cases. Among these, there were sufficient data in 31 cases to be included in the present study: 13 patients were treated with IHD, 5 patients with EDD, whereas the other 13 patients were treated with CRRT.
Admission laboratory data, clinical features, and outcomes
The two groups of patients were comparable by age, gender, circumstances of poisoning, and time from ingestion to hospital treatment. The patients in both groups were “late-presenters” admitted to hospitals more than 12 h after methanol ingestion, with a mean time to treatment of 42 ± 11 hours in the CRRT group and 48 ± 8 h in the IHD group (p > 0.05). The laboratory data, clinical features on admission, and treatment outcomes are presented in .
Table 1. Laboratory and clinical data on admission prior to administration of antidotes, bicarbonate or renal replacement therapy in 18 patients treated with intermittent and extended daily hemodialysis (IHD 1–13)/(EDD 14–18), and 13 patients treated with continuous renal replacement therapy (CRRT 19–31).
The patients treated with CRRT were more acidemic on admission with lower arterial blood pH and standard bicarbonate, and higher serum lactate compared to the IHD group. They had higher serum glucose, suggesting more severe poisoning on admission. They also had slightly higher serum creatinine and potassium, although not statistically significant.
More patients in the group treated with CRRT were admitted in coma (11/13 versus 5/18 for IHD; p = 0.002). The groups did not differ in the number of cases with fomepizole administration or folate substitution (6/13 versus 7/18; p = 0.686, and 11/18 versus 10/13; p = 0.353, correspondingly). The number of patients with poor outcome (death or visual and/or CNS sequelae) was higher in the group treated with continuous modalities of hemodialysis (13/13 versus 12/18; p = 0.020).
Technical parameters of hemodialysis and dynamics of acidemia correction
Data on the technical parameters of ECTR and time to correction of acidemia are presented in . The time needed for 0.01-unit increase of arterial blood pH was shorter in the patients with more severe acidemia on admission, which is likely to have reflected the administration of intravenous NaHCO3 before or during the first hours of dialysis (). The time to increase arterial blood pH was significantly shorter for the IHD group in patients with acidemia of the same severity before the start of dialysis, despite the fact that the patients in the CRRT group received more NaHCO3 intravenously ().
Figure 1. Time to increase arterial blood pH by 0.01 pH unit depending on arterial blood pH before the start of extracorporeal treatment. IHD: intermittent hemodialysis; EDD: extended daily hemodialysis; CRRT: continuous renal replacement therapy.
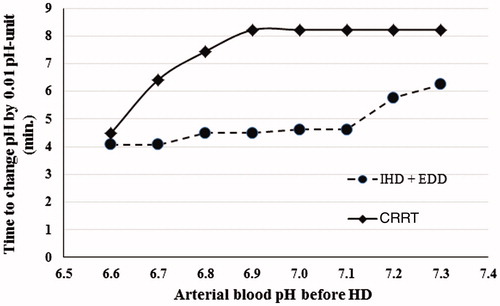
Table 2(A). Correction of acidemia and technical parameters of hemodialysis in the patients treated with intermittent (IHD 1–13) and extended daily hemodialysis (EDD 14–18).
Table 3. Time for arterial blood pH to increase by 0.01-point depending on arterial blood pH on admission in the patients on intermittent and extended daily hemodialysis (IHD/EDD) versus continuous renal replacement therapy (CRRT).
The time to standard bicarbonate increase of 1 mmol/L in the patients from IHD group was significantly shorter for IHD group compared to the patients with the same range of standard bicarbonate on admission in the CRRT group (, ).
Figure 2. Time to increase standard HCO3– by 1.0 mmol/L depending on standard HCO3– before the start of extracorporeal treatment. IHD: intermittent hemodialysis; EDD: extended daily hemodialysis; CRRT: continuous renal replacement therapy.
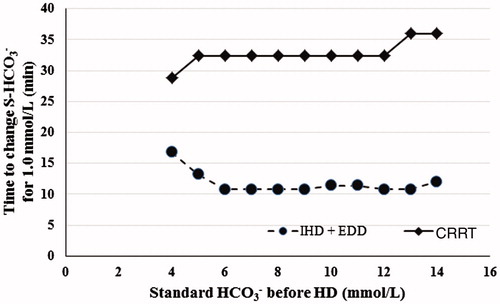
Table 4. Time for arterial blood standard HCO3– to increase by 1 mmol/L depending on standard HCO3– concentration on admission in the patients on intermittent and extended daily hemodialysis (IHD/EDD) versus continuous renal replacement therapy (CRRT).
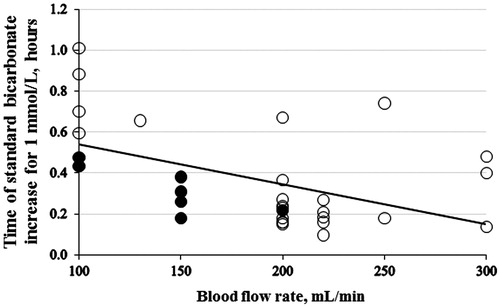
The rate of increase of standard bicarbonate by 1 mmol/L was higher when the dialysate/effluent flow rate was higher () and the blood flow rate was higher (). In these graphs it is also noted that the rate of correction was accelerated by administration of intravenous NaHCO3.
Figure 3. The average rate of standard HCO3– correction for 1 mmol/L versus dialysate flow rate (r = 0.677; p < 0.001, n = 31). The full black points indicate the patients administered more than 500 mmol of NaHCO3. Dialysate flow rate includes effluent flow rate in the cases of hemofiltration and hemodiafiltration.
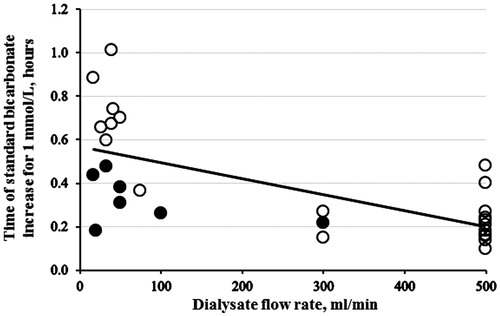
Figure 4. The average rate of standard HCO3– correction for 1 mmol/L versus blood flow rate (r = 0.485; p = 0.006, n = 31). The full black circles indicate the patients administered more than 500 mmol of NaHCO3.
Based on the results of the univariate regression analysis, the time needed for standard bicarbonate increase for 1 mmol/L correlated with the following parameters (n = 31):
The modality of dialysis (r = 0.724, p < 0.001);
The amount of NaHCO3 substitution (r = 0.873; p < 0.001);
The dialysate/effluent flow rate (r= −0.677; p < 0.001);
The blood flow rate (r= −0.485; p = 0.006);
The serum creatinine (r = 0.415; p = 0.020);
The dialyzer surface area (r = 0.397; p = 0.027).
No association was found between the rate of standard bicarbonate correction and age, gender, weight of a patient, serum methanol, formate, lactate, and glucose on admission, type of antidote administered, and folate substitution.
The results of multivariate regression analysis of variables influencing the rate of correction of acidemia based on all 31 cases are shown in . For the multivariate analysis, the following variables were included: time needed for 0.01-unit increase of arterial blood pH, arterial blood pH before dialysis session, Δ pH during dialysis session, NaHCO3 substitution, serum creatinine, dialyzate/effluent flow rate, blood flow rate, dialyzer surface area. Three independent variables included in the model explained 78.1% of dispersion in the rate of acidemia correction. These variables were the difference between the initial and the corrected arterial blood pH, bicarbonate substituted by infusions, and the dialysate/effluent flow rate.
Table 5. The multivariate regression analysis of time needed to 0.01-increase of arterial blood pH (R2 = 0.781).
Complications during dialysis sessions were seen in one case of CRRT (bleeding during ECTR and rebound of acidosis after it was stopped), in one case of IHD (filter clotting), and in three cases of EDD (one case of filter clotting and rebound of acidosis, one case of dialyzer failure and further IHD application, and one case of EDD discontinuation and further CVVHD application due to hemodynamic instability). In both cases of rebound of acidosis due to subtherapeutical serum ethanol level after ECTR was prematurily ended, ethanol was administered as antidote ().
Discussion
Methanol poisoning is a condition in clinical toxicology where methods of enhanced elimination play an essential role.[Citation12,Citation15] Extensive toxicokinetic and clinical data exist on its capacity to remove methanol [Citation31–35] and formate.[Citation14,Citation36,Citation37] Nevertheless, there are no studies evaluating the effectiveness of different ECTRs for correction of acidemia in the patients with acute methanol poisoning.
Our results prove the superiority of IHD over the CRRT as regards to the rate of correction of acidemia. Both time to 0.01-unit increase of arterial blood pH and time to 1 mmol/L increase of standard bicarbonate were significantly shorter with IHD. The risk of hemodynamic instability from IHD is a consideration when choosing an ECTR modality because this may complicate management including exacerbation of lactic acidosis. In our study, patients treated with CRRT had lower mean arterial blood pressure and needed more pressors/inotropes as compared to those treated with IHD.
Admission laboratory data, clinical features, and outcomes
We did not find any correlation between demographic parameters as age, gender, or weight of the patients and the rate of acidemia correction. Further, no association with serum methanol, lactate, formate, glucose, and time for 0.01-unit increase of arterial blood pH or time to 1 mmol/L-increase of standard bicarbonate level was present. Finally, no association between the rate of acidemia correction and the type of antidote (fomepizole or ethanol) and folate substitution was found.
The CRRT group had a poorer outcome as such, but they were more severely poisoned on admission, and our data does not suggest increased survival based on the choice of dialysis modality. As long as severity of acidemia is a prognostic parameter of outcome, higher rate of acidemia correction on IHD provides certain theoretical rationale for the superiority of IHD in terms of clinical effectiveness. But further clinical studies with more cases are necessary to demonstrate whether the modality of hemodialysis also affects the mortality and long-term visual and/or CNS sequelae in methanol-poisoned patients.
Technical parameters of hemodialysis and dynamics of acidemia correction
The mean time to 0.01-unit increase of arterial blood pH was 40% shorter (typically 4.5 min versus 8 min) ( and ), and the mean time to 1 mmol/L-increase in standard bicarbonate was 60% shorter (typically 10–15 min versus 30–35 min) on IHD compared to CRRT ( and ). All 13 patients in the CRRT group received intravenous NaHCO3 at the start or during the first hours of dialysis, whereas only 12/18 (67%) of the patients on IHD received it. Further, the amount of NaHCO3 administered intravenously was significantly lower in the IHD group (see ). Therefore, the actual efficiency of acidemia correction by IHD compared to CRRT was even higher than it appears in our data.
Table 2(B). Correction of acidemia and technical parameters in the patients treated with continuous renal replacement therapy (CRRT).
The variability in the rate of acidemia correction in patients receiving CRRT (lower dialysate/effluent flow rate, represented on the left handside of ) reflected in part the administration of varying amounts of intravenous NaHCO3 administered before the start of dialysis. The higher NaHCO3 infusion in the most acidemic CRRT-patients may also explain the shorter time for CRRT to correct pH (see ), and bicarbonate (see ). If the patients were not given additional NaHCO3 substitution, one would expect the time to correction to be longer for the more acidotic patients, because of the need to compensate for the other buffers (proteins, etc.).[Citation38] A lack of hyperventilation in the most acidotic IHD patients could be part of the explanation for them to need longer ECTR and bicarbonate correction, but this can only be hypothezised as the pCO2 on admission were not reported in five of the patients on IHD.
Our results support that the time needed for correction of acidemia depends on modality and technical parameters of dialysis: The higher the flows (blood and dialysate/effluent), the shorter time to correction of acidosis. Based on these findings, the blood flow and the dialysate/effluent flow when using continuous dialysis should be increased as much as possible, taking the technical facilities of the dialyser (including clotting of filters, catheter problems, etc.) and the patient’s condition into account. Depending on the local dialysis equipment (citrate or heparin anticoagulation), this should be done under a close evaluation of the acid-base and electrolyte status of the patient.
Regarding the complications of both modalities, filter clotting was the most common one. Two cases of rebound of acidemia after stopping dialysis occurred after premature stopping dialysis due to technical problems (filter clotting, bleeding) were reported. In both cases, ethanol was being used to inhibit ADH, suggesting that ethanol concentration decreased to subtherapeutical level during dialysis despite an increase in dosage to account for this. Finally, in three of five cases of EDD it had to be stopped and, in two of these cases, followed by other modalities (IHD and CVVHD) due to hemodynamic instability and technical complications during the ECTR session.
Strength and limitations
The limitations of this study can be attributed to certain confounders: possible variations in the time, amount and patterns of toxic liquor intake, individual differences in the methanol and formate metabolism, and the available modalities for treatment (other than the modality of enhanced elimination). However, the two groups of patients treated with different modes of enhanced elimination were comparable by age, time to presentation, and size; most of the collected data exhibited normal distribution.
This study was not designed as a randomized controlled trial, because the choice of the method of enhanced elimination in each case was based on clinical practice at individual sites, and conditioned by different factors (hemodynamic stability, severity of poisoning, availability of dialysis facilities, and so on). This raises the possibility of allocation bias, and differences in baseline acidemia suggests that this did occur. Nevertheless, this study was not conceived as a study looking at defining the best modality in terms of clinical outcomes such as death and morbidity, but rather to describe the difference in the rate of correction of acidemia which are important and relevant surrogate clinical markers for comparing the effect of different ECTRs.
Acknowledgements
Supported with the Project 16-27075A of AZV VES 2016, the Project 43/16/RPZP of the Ministry of Health of the Czech Republic, and the Projects P25/1LF/2 and P28/1LF/6 of Charles University in Prague.
Disclosure statement
The authors of the manuscript state no conflict of interest.
References
- Paasma R, Hovda KE, Hassanian-Moghaddam H, et al. Risk factors related to poor outcome after methanol poisoning and the relation between outcome and antidotes – a multicenter study. Clin Toxicol. 2012;50:823–831.
- Hovda KE, Hunderi OH, Tafjord AB, et al. Methanol outbreak in Norway 2002-2004: epidemiology, clinical features and prognostic signs. J Intern Med. 2005;258:181–190.
- Zakharov S, Pelclova D, Urban P, et al. Czech mass methanol outbreak 2012: epidemiology, challenges and clinical features. Clin Toxicol. 2014;52:1013–1024.
- Zakharov S, Nurieva O, Navratil T, et al. Acute methanol poisonings: folates administration and visual sequelae. J Appl Biomed. 2014;12:309–316.
- Vaneckova M, Zakharov S, Klempir J, et al. Methanol intoxication on magnetic resonance imaging – case reports. Cesk Slov Neurol N. 2014;77:235–239.
- Hubacek JA, Pelclova D, Seidl Z, et al. Rare alleles within the CYP2E1 (MEOS system) could be associated with better short-term health outcome after acute methanol poisoning. Basic Clin Pharmacol Toxicol. 2015;116:168–172.
- Vaneckova M, Zakharov S, Klempir J, et al. Imaging findings after methanol intoxication (cohort of 46 patients). Neuro Endocrinol Lett. 2015;36:737–744.
- Liesivuori J, Savolainen H. Methanol and formic acid toxicity – biochemical mechanisms. Pharmacol Toxicol. 1991;69:157–163.
- Jacobsen D, Hovda KE. Methanol. In: Shannon MW, Borron SW, Burns MJ, editors. Haddad and Winchester's clinical management of poisoning and drug overdose. Philadelphia (PA): WB Saunders; 2007. p. 605–611.
- Zakharov S, Kurcova I, Navratil T, et al. Is the measurement of serum formate concentration useful in the diagnostics of acute methanol poisoning? A prospective study of 38 patients. Basic Clin Pharmacol Toxicol. 2015;116:445–451.
- Jacobsen D, Ovrebo S, Sejersted OM. Toxicokinetics of formate during hemodialysis. Acta Med Scand. 1983;214:409–412.
- Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA. American academy of clinical toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol-Clin Toxicol. 2002;40:415–446.
- Chow MT, Di Silvestro VA, Yung CY, et al. Treatment of acute methanol intoxication with hemodialysis using an ethanol-enriched, bicarbonate-based dialysate. Am J Kidney Dis. 1997;30:568–570.
- Zakharov S, Pelclova D, Navratil T, et al. Intermittent hemodialysis is superior to continuous veno-venous hemodialysis/hemodiafiltration to eliminate methanol and formate during treatment for methanol poisoning. Kidney Int. 2014;86:199–207.
- Roberts DM, Yates C, Megarbane B, et al. Recommendations for the role of extracorporeal treatments in the management of acute methanol poisoning: a systematic review and consensus statement. Crit Care Med. 2015;43:461–472.
- Hassanian-Moghaddam H. Prioritizing the intoxicated patients for extracorporeal treatments in methanol poisoning. Crit Care Med. 2015;43:e210–e211.
- Roberts DM, Hoffman RS, Gosselin S, et al. The authors reply. Crit Care Med. 2015;43:e211–e212.
- Aviles-Gomez R, Luquin-Arellano VH, Garcia-Garcia G, et al. Is renal replacement therapy for all possible in developing countries? Ethn Dis. 2006;16:S2-70-2.
- Nurieva O, Kotikova K, Urban P, et al. Prevalence, dynamics, and biochemical predictors of optic nerve remyelination after methanol-induced acute optic neuropathy: a 2-year prospective study in 54 patients. Monatsh Chem. 2016;147:239–249.
- Zakharov S, Navratil T, Pelclova D. Analysis of serum anion gap and osmolal gap in diagnosis and prognosis of acute methanol poisoning: clinical study in 86 patients. Monatsh Chem. 2015;146:787–794.
- Zakharov S, Nurieva O, Kotikova K, et al. Factors predicting optic nerve axonal degeneration after methanol-induced acute optic neuropathy: a 2-year prospective study in 54 patients. Monatsh Chem. 2016;147:251–261.
- Bezdicek O, Klempir J, Liskova I, et al. Sequelae of methanol poisoning for cognition. Cesk Slov Neurol N. 2014;77/110:320–325.
- Zakharov S, Navratil T, Pelclova D, et al. Fomepizole versus ethanol in the treatment of acute methanol poisoning: comparison of clinical effectiveness in a mass poisoning outbreak. Clin Toxicol. 2015;53:797–806.
- Zakharov S, Navratil T, Pelclova D. Fomepizole in the treatment of acute methanol poisonings: experience from the Czech mass methanol outbreak 2012–2013. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:641–649.
- Zakharov S, Pelclova D, Diblik P, et al. Long-term visual damage after acute methanol poisonings: longitudinal cross-sectional study in 50 patients. Clin Toxicol. 2015;53:884–892.
- Urban P, Zakharov S, Diblík P, et al. Visual evoked potentials in patients after methanol poisoning. Int J Occup Med Environ Health. 2016;29:471–478.
- Zakharov S, Pelclova D, Urban P, et al. Use of out-of-hospital ethanol administration to improve outcome in mass methanol outbreaks. Ann Emerg Med. 2016;68:52–61.
- Zakharov S, Nurieva O, Kotikova K, et al. Positive serum ethanol concentration on admission to hospital as the factor predictive of treatment outcome in acute methanol poisoning. Monatsh Chem. 2016; [Epub ahead of print]. doi 10.1007/s00706-016-1846-z.
- Zakharov S, Kotikova K, Vaneckova M, et al. Acute methanol poisoning: prevalence and predisposing factors of haemorrhagic and non-haemorrhagic brain lesions. Basic Clin Pharmacol Toxicol. 2016;119:228–238.
- Zakharov S, Navratil T, Salek T, et al. Fluctuations in serum ethanol concentration in the treatment of acute methanol poisoning: a prospective study of 21 patients. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:666–676.
- Garella S. Extracorporeal techniques in the treatment of exogenous intoxications. Kidney Int. 1988;33:735–754.
- Dorval M, Pichette V, Cardinal J, et al. The use of an ethanol- and phosphate-enriched dialysate to maintain stable serum ethanol levels during haemodialysis for methanol intoxication. Nephrol Dial Transplant. 1999;14:1774–1777.
- Chebrolu SB, Hariman A, Eggert CH, et al. Phosphorus-enriched hemodialysis for the treatment of patients with severe methanol intoxication. Int J Artif Organs. 2005;28:270–274.
- Peces R, Fernandez R, Peces C, et al. Effectiveness of pre-emptive hemodialysis with high-flux membranes for the treatment of life-threatening alcohol poisoning. Nefrologia. 2008;28:413–418.
- Bayliss G. Dialysis in the poisoned patient. Hemodial Int. 2010;14:158–167.
- Hantson P, Haufroid V, Wallemacq P. Formate kinetics in methanol poisoning. Hum Exp Toxicol. 2005;24:55–59.
- Kerns W, II, Tomaszewski C, McMartin K, et al. Formate kinetics in methanol poisoning. J Toxicol Clin Toxicol. 2002;40:137–143.
- Fernandez PC, Cohen RM, Feldman GM. The concept of bicarbonate distribution space: the crucial role of body buffers. Kidney Int. 1989;36:747–752.
