Abstract
Background: Ibogaine is an agent that has been evaluated as an unapproved anti-addictive agent for the management of drug dependence. Sudden cardiac death has been described to occur secondary to its use. We describe the clinical effects and toxicokinetics of ibogaine and noribogaine in a single patient. For this purpose, we developed a LC-MS/MS-method to measure ibogaine and noribogaine plasma-concentrations. We used two compartments with first order absorption.
Case details: The maximum concentration of ibogaine was 1.45 mg/L. Our patient developed markedly prolonged QTc interval of 647ms maximum, several multiple cardiac arrhythmias (i.e., atrial tachycardia and ventricular tachycardia and Torsades des Pointes). QTc-prolongation remained present until 12 days after ingestion, several days after ibogaine plasma-levels were low, implicating clinically relevant noribogaine concentrations long after ibogaine had been cleared from the plasma. The ratio k12/k21 for noribogaine was 21.5 and 4.28 for ibogaine, implicating a lower distribution of noribogaine from the peripheral compartment into the central compartment compared to ibogaine.
Conclusions: We demonstrated a linear relationship between the concentration of the metabolite and long duration of action, rather than with parent ibogaine. Therefore, after (prolonged) ibogaine ingestion, clinicians should beware of long-term effects due to its metabolite.
Introduction
Ibogaine is an alkaloid derived from the root of the Tabernanthe iboga-plant that possesses anti-addictive properties for drug dependence through reduction of craving induced by substance withdrawal. The growing use of ibogaine for this purpose is at least controversial since it has been associated with sudden death due to cardiac arrest [Citation1]. Although human studies on ibogaine are relatively scarce, recent literature has detailed the pharmacokinetic and pharmacodynamic profile of ibogaine. In the liver and gut wall, ibogaine is metabolized into biologically active noribogaine by mostly CYP2D6 and to a lesser extent by CYP2C9 and CYP 3A4 [Citation2,Citation3]. As a consequence, blood concentrations of ibogaine and noribogaine can be influenced by polymorphism in the CYP2D6 enzyme and co-ingestion of other medication metabolized by CYP2D6 may have clinical impact [Citation4]. Ibogaine and noribogaine both are an agonist of the opioid receptor and an antagonist of the N-methyl-D-aspartate (NMDA) glutamate and a3b4 nicotinic acetylcholine receptors [Citation5], but they differ in receptor affinity and potency. Noribogaine might be a safer alternative for medical purposes than ibogaine, since in rats, noribogaine induced less adverse effects like tremors and stress-axis activation. Interestingly, noribogaine increased extracellular serotonin levels more than ibogaine [Citation6]. Central serotonin activity is believed to be responsible for QTc prolongation, therefore, whether noribogaine has a safer profile than ibogaine remains to be discussed [Citation7].
Recently, the pharmacokinetic and pharmacodynamic profiles of ibogaine were reviewed in this journal [Citation8]. The half-life of noribogaine is much longer than ibogaine, resulting in relevant plasma-concentrations of noribogaine long after clearance of ibogaine [Citation9,Citation10]. They also described a post-mortem analysis of a patient following ingestion of ibogaine, which revealed high tissue concentrations of ibogaine and noribogaine in liver, spleen, lung and brain, implicating high lipophilicity of both ibogaine and its metabolite [Citation11].
We describe the clinical effects and toxicokinetics of ibogaine and noribogaine in a single patient using internet purchased ibogaine to control heroin addiction.
Case details
A 46-year-old woman presented to our emergency department after being found unconscious by her spouse a few hours after repeated ingestion over a period of 12 h of internet-purchased ibogaine capsules with a total amount of 1400 mg. She purchased the product to curb withdrawal symptoms of her heroin addiction. The ingested dose is in range with the usual off-label dose mentioned in the “Clinical guidelines for ibogaine-assisted detoxification” [Citation12]. The patient's past medical history revealed addiction to alcohol, cocaine and cannabis. No cardiovascular disease or any other chronic outpatient therapy were maintained. On admission, she was conscious and breathing independently with a pulse of 85/min and blood pressure was 126/86 mmHg. Physical examination did not show any abnormalities. A immunofluorescence drug screening assay (Triage TOX Drug Screen) on urine revealed no abuse of co-ingestants of (meth)amphetamines, barbiturates, benzodiazepines, cocaine, methadone, opiates heroin, tetrahydrocannabinol or tricyclic antidepressants. The electrocardiogram showed a markedly prolonged QTc-interval of 647 ms, i.e., atrial tachycardia and ventricular tachycardia. Just before transfer to the coronary care unit, she experienced Torsades des Pointes (TdP), which spontaneously terminated. Isoproterenol was started intravenously to induce tachycardia and suppress initiation of TdP. Despite these measures, she remained in TdP, and a temporary pacemaker lead was inserted with a rate set for pacing at 130/min. One day after admission she developed hypokalaemia of 3.4 mmol/L (ref. 3.5–4.5 mmol/L) and hypomagnesaemia of 0.68 mmol/L (ref. 0.75–1.03 mmol/L), for which intravenous supplementation was started. With these measures, TdP nor any other cardiac arrhythmia did not recur. Pacing-rate was gradually reduced to 100/min over 72 h and the pacemaker-lead was removed after 5 days.
In order to measure plasma-concentrations of ibogaine and its metabolite noribogaine we developed a validated LC-MS/MS method for the desirable range from 10 to 2,000 μg/L. The lower limit of quantification was 3 μg/L for both analytes. Data were analyzed using the pharmacokinetic program MW/Pharm 3.60 (MediWare, Czech). The toxicokinetics of ibogaine and noribogaine were described using two-compartments with first-order absorption without lag-time.
In plasma-concentration versus time curves for ibogaine and noribogaine in this patient are shown with time points for clinically relevant cardiac events. This curve demonstrates the effect of prolonged elevations in plasma-concentrations of noribogaine despite very low ibogaine plasma-levels. The maximum concentrations (Cmax) of ibogaine and noribogaine were 1.45 mg/L (12.7 h) and 0.569 mg/L (98 h), respectively.
Figure 1. Plasma-concentration versus time curve of ibogaine and noribogaine with important clinical interventions and observations.
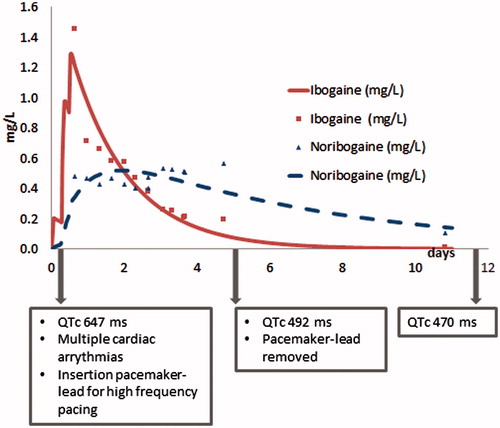
demonstrates toxicokinetics of ibogaine and noribogaine as determined in our patient. The transfer of substance between the central and peripheral compartment is expressed in k12 and k21, while the ratio k12/k21 displaces the net transfer into the peripheral compartment. For noribogaine this ratio was 21.5 and much higher compared to 4.28 for ibogaine. The calculated logP at pH 7.4 were 2.19 for ibogaine and 2.03 for noribogaine.
Table 1. Toxicokinetic parameters of ibogaine and noribogaine using two compartments.
Discussion
We described a patient case in which noribogaine was associated with life-threatening cardiac arrhythmias due to its prolonged presence, while ibogaine plasma-levels were very low. Prolonged noribogaine plasma-levels are consistent with previous research [Citation6,Citation8]. The ratio k12/k21 for noribogaine was higher than for ibogaine, which implicates a lower net distribution of noribogaine into the central compartment compared to ibogaine. The slightly higher logP for ibogaine compared to noribogaine indicates that ibogaine is slightly more lipophilic than noribogaine. We found a much higher half-life of noribogaine in our patient than the authors of a dose-ascending study investigating the PK/PD in healthy volunteers: 2540 h vs. 28–49 h [Citation9]. However, the toxicokinetic parameters in our patient revealed a much higher Cmax, which may account for the difference in these evaluated parameters. In literature, due to fluctuations in individual distribution–phase concentration–time profiles, the presence of enterohepatic recirculation for noribogaine was suggested as a factor for prolonged elimination [Citation9]. In our patient, we measured fluctuating noribogaine plasma-concentrations. This indeed may be the result of an enterohepatic recirculation and may thereby have contributed to long-term noribogaine plasma-concentrations. Polymorphism in the CYP2D6 enzyme can influence blood concentrations of both ibogaine and noribogaine [Citation4]. Unfortunately, we did not perform genotyping in our patient. Finally, both hypokalemia and hypomagnesemia may increase the risk of TdP [Citation3]. Even though the disturbances were minimal in our patient, we cannot be certain it did not have an impact on the manifestation of cardiac arrhythmias.
Conclusions
Although we assessed the toxicokinetic parameters of ibogaine and noribogaine in a single patient, our results are consistent with the sparse literature available on this subject.
We demonstrated a direct relationship between the concentration of the metabolite and long duration of action, rather than ibogaine. Therefore, after (prolonged) intake of ibogaine, clinicians should beware of long-term cardiac effects due to its metabolite.
Disclosure statement
The authors report no declarations of interest.
References
- Alper KR, Stajic M, Gill JR. Fatalities temporally associated with the ingestion of ibogaine. J Forensic Sci. 2012;57:398–412.
- Obach RS, Pablo J, Mash DC. Cytochrome P4502D6 catalyzes the O-demethylation of the psychoactive alkaloid ibogaine to 12-hydroxyibogamine. Drug Metab Dispos. 1998;26:764–768.
- Koenig X, Hilber K. The anti-addiction drug ibogaine and the heart: a delicate relation. Molecules. 2015;20:2208–2228.
- Yip L, Deng JF. On the toxicity of ibogaine. Clin Toxicol. 2016;54:605.
- Hildyard C, Macklin P, Prendergast B, et al. A case of QT prolongation and torsades de pointes caused by ibogaine toxicity. J Emerg Med. 2016;50:83–87.
- Baumann MH, Pablo JP, Ali SF, et al. Noribogaine (12-hydroxyibogamine): a biologically active metabolite of the antiaddictive drug ibogaine. Ann N Y Acad Sci. 2000;914:354–368.
- Asua I. Growing menace of ibogaine toxicity. Br J Anaesth. 2013;111:1029–1030.
- Litjens RPW, Brunt TM. How toxic is ibogaine? Clin Toxicol. 2016;54:297–302.
- Glue P, Lockhart M, Lam F, et al. Ascending-dose study of noribogaine in healthy volunteers: pharmacokinetics, pharmacodynamics, safety, and tolerability. J Clin Pharmacol. 2015;55:189–194.
- Mash DC, Kovera CA, Pablo J, et al. Ibogaine in the treatment of heroin withdrawal. Alkaloids Chem Biol. 2001;56:155–171.
- Kontrimaviciute V, Mathieu O, Mathieu-Daude JC, et al. Distribution of ibogaine and noribogaine in a man following a poisoning involving root bark of the Tabernanthe iboga shrub. J Anal Toxicol. 2006;30:434–440.
- Alliance. GIT. Clinical Guidelines for Ibogaine-Assisted Detoxification [Internet]. 2016 [updated Febuary 2016; cited 2016]. Available from: https://www.ibogainealliance.org/guidelines/
