Abstract
Background: Physostigmine has long been recognized as an antidote to reverse anticholinergic delirium. However, its effectiveness, safety profile, and dosing have been disputed.
Objectives: To describe effectiveness, adverse events, and dosing associated with the use of physostigmine to reverse anticholinergic delirium.
Methods: A retrospective cohort study of hospitalized patients reported to a regional poison center system between 2003 and 2012 who received physostigmine to reverse an anticholinergic toxidrome. Data extraction of a priori defined variables were recorded with concurrence of investigators. The cases were stratified by the primary ingestant as the presumed causative agent and associations for response were performed using odds ratios (ORs), 95% confidence intervals (CI’s), and p values.
Results: Of the 1422 cases identified, 191 met the inclusion criteria. Patients exposed to non-diphenhydramine antihistamines (n = 14), antipsychotics (n = 4), and tricyclic antidepressants (n = 3) had 100% response to physostigmine, whereas anticholinergic plants (n = 46/67; 68.7%, OR: 0.70; CI: 0.36–1.35), diphenhydramine (n = 43/56; 64.2%, OR: 1.30; CI: 0.63–2.68), and combination products (n = 8/10; 80%, OR: 1.48; CI: 0.30–7.24) had partial response rates. Of the included patients, 142 (74.3%) were treated with physostigmine alone, and 16 (8.4%) of these patients were discharged directly from the emergency department (ED).
Discussion: Most patients, 182 (95.3%), had no documented adverse effects. Four patients (2.1%) experienced emesis, two experienced QTc prolongation (1.0%), and two experienced seizures (1.0%). There was a single fatality 6 h after physostigmine administration. Average initial total doses of physostigmine ranged from 1.0 to 1.75 mg. Most patients were admitted to the ICU (n = 110; 57.6%), however, 36 (18.8%) patients were discharged directly from the ED.
Conclusions: In this retrospective cohort study, physostigmine administration to reverse anticholinergic delirium had a good safety profile, and often improved or resolved anticholinergic delirium when administered in doses less than 2 mg.
Introduction
Physostigmine is a tertiary carbamate and reversible acetylcholinesterase inhibitor that can provide rapid reversal of anticholinergic toxicity [Citation1]. Its rapid reversal anticholinergic delirium makes physostigmine a useful diagnostic and therapeutic tool [Citation2–6]. Reversal of agitated delirium secondary to anticholinergic toxicity may prevent complications such as agitation, rhabdomyolysis, and hyperthermia [Citation1,Citation7]. Concern for cholinergic excess including seizure, bradycardia, emesis, and asystole in the setting of tricyclic overdose have been reported and often limits use [Citation2–6,Citation8–10], however, previous studies have shown that adverse reactions are infrequent [Citation3,Citation6].
Dosing regimens utilized for physostigmine vary. Higher dosage regimens have been associated more frequent adverse effects of cholinergic excess [Citation9]. More conservative dosage regimens using an initial dose of 0.5–1 mg, then additional doses every 5–15 min up to a maximum of 2 mg over the first hour have been recommended to reduce the likelihood of adverse events [Citation9,Citation11].
We completed a large retrospective review of all patients treated with physostigmine reported to the California Poison Control Systems (CPCS) from 1 January 2003 to 31 December 2012 to evaluate the safety and effectiveness of physostigmine to reverse anticholinergic delirium as the result of any ingestion or exposure. It was our objective to describe patient response to physostigmine, adverse events associated with physostigmine administration, and determine dosing regimens associated with effective treatment of anticholinergic delirium stratified by the causative agent.
Methods
This retrospective review of CPCS records included patients reported to CPCS from 2003 to 2012 treated with physostigmine. CPCS is comprised of four poison control centers, and provides a toll-free 24-h hotline service for the public and health care professionals seeking guidance for the care of accidental or intentional poisonings, adverse drug reactions, envenomations, or environmental exposures. All calls received by CPCS are entered into an electronic database VDL (Visual Dotlab Enterprises), and are reviewed and coded by symptom, treatment, and outcome as outlined by American Association of Poison Control Centers (AAPCC). Patients are followed remotely by poison control staff until clinical resolution of symptoms attributed to the toxicologic emergency, and the CPCS record for each patient is then available for review for research and quality control purposes. For this study, the database was queried for patients of all ages and sex reported to CPCS between the years of 2003 and 2012. The search criteria included all cases that included “physostigmine” in the treatment field, or if the term “physostigmine” was included in the text of the CPCS record.
Patients were included if he or she received physostigmine regardless if it was or was not recommended by CPCS. Patients were excluded if the patient did not receive physostigmine, it was unclear if the patient received physostigmine, or if the case was a duplicate case. Three trained abstractors reviewed cases and abstracted data, while a fourth abstractor reviewed and resolved discrepancies. This study was reviewed and approved by the University of California San Francisco Committee on Human Research.
CPCS records included in the study were reviewed for demographic and clinical data utilizing an a priori defined set of variables and definitions including: patient demographics, presenting symptoms, substances ingested, dosage of physostigmine, response to therapy and need for repeat dosing, adverse effects, disposition, and final outcomes.
Of note, no specific protocol for physostigmine dosing was utilized, however, CPCS considers the handbook, Poisoning and Drug Overdose [Citation11] as the guideline for the management of poisonings. The handbook recommends a conservative dosage regimen using an initial dose of 0.5–1 mg and then additional doses every 5–10 min up to a maximum of 2 mg over the first hour.
Patient demographic data included patient age and sex. Clinical information included primary ingestion (the substance deemed as the primary causative agent for anticholinergic delirium), presenting signs and symptoms, dose of physostigmine received, response to and repeat dose of physostigmine, adverse events, disposition, and outcome.
Presenting signs and symptoms were either defined as described, or inferred by the abstractor on review of the CPCS record. Signs and symptoms included: tachycardia defined as a heart rate (HR) greater than 100 beats per minute (bpm), bradycardia defined as a HR less than 60 bpm, mydriasis, evidence of anticholinergic delirium (defined as altered mental status, agitation, hallucinations (audio, visual, or tactile), drowsiness, and/or obtundation following ingestion of a suspected anticholinergic substance), dry skin, hyperthermia defined as a temperature greater than 38.0 °C or if specifically noted in the CPCS record, urinary retention, seizure, QTc prolongation (as stated in the text of the CPCS record or QTc >450 ms), QRS prolongation (as stated in the text of the CPCS record or a documented QRS >100 ms), or both QTc and QRS prolongation.
Clinical response to physostigmine was defined as improvement in anticholinergic delirium as noted explicitly by the primary team after the initial dose of physostigmine, or if the patient was noted to have improved symptoms of anticholinergic delirium as determined by the reviewer.
Adverse events reported include: emesis, QTc or QRS prolongation, seizure, or death. QTc and QRS prolongation were defined as any noted QTc or QRS prolongation (as defined above) compared with an EKG prior to receiving physostigmine, or as noted after receiving physostigmine whether or not a prior QTc or QRS was recorded.
The dose of physostigmine given, if recorded in the CPCS record, was reported as the mean, median, and range of physostigmine doses, stratified by substance category. Repeat dose was defined as an additional dose of physostigmine given after the initial dose. The milligram dosage of repeat doses was not recorded. For patients who did not receive repeat doses of physostigmine, CPCS records were reviewed to determine why additional doses of physostigmine were not given, and if additional agents were administered.
Disposition was defined as admission to the intensive care unit (ICU), admission to a non-critical care unit (with or without telemetry monitoring), treated and released from the ED, death, or unknown.
Data analysis
Associations between response rates by primary causative substance were analyze by univariate analysis to determine odds ratios (ORs), 95% confidence intervals (CIs), and associated p values. The significance threshold was ≤.05 in all tests. Data were analyzed using Microsoft™ Excel™ 2010 (version 14.0.6123.5001) (Microsoft Corp., Redmond, WA). All other variables were analyzed with descriptive statistics of the aggregated group (presenting signs and symptoms, adverse effects, reason for not repeating the dose of physostigmine, disposition, outcomes) or stratified by substance category of primary ingestant (response to physostigmine) with reported frequencies.
Results
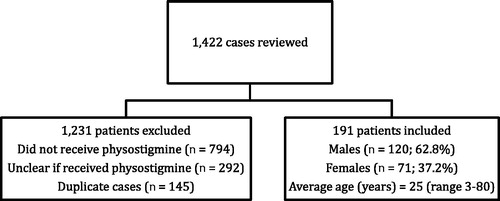
On review of CPCS records, 1422 total patients were identified, and 1231 were excluded. Of the excluded patients, 794 did not receive physostigmine, it was unclear whether physostigmine was given to 292 patients, and 145 cases were duplicates. After patients were excluded, 191 patients were included in the final analysis. Of the 191 included patients, 120 patients were male (62.8%), 71 patients were female (37.1%). The average age of patients was 25 years old (range: 3–80 years old). is an algorithm of records reviewed and provides a summary of reasons for exclusion and the sex of included patients.
Figure 1. Flow diagram of poison center cases identified with mention of physostigmine and demographics of included patients.
The presenting signs and symptoms are shown on . All patients (n = 191) were noted to have one or more symptoms of anticholinergic delirium (as defined above), most commonly agitation (n = 113, 59.1%), and altered mental status (n = 99, 51.8%). Additional presenting signs and symptoms include tachycardia (n = 163, 85.3%), mydriasis (n = 105, 55.0%), dry skin (n = 49, 25.7%), hyperthermia (n = 31, 16.2%), seizures (n = 13, 6.8%), urinary retention (n = 8, 4.2%), QTc prolongation (n = 8, 4.2%), QRS prolongation (n = 3, 1.6%), and 1 (0.5%) patient demonstrated both QTc and QRS prolongation.
Table 1. Presenting signs and symptoms.
Effectiveness
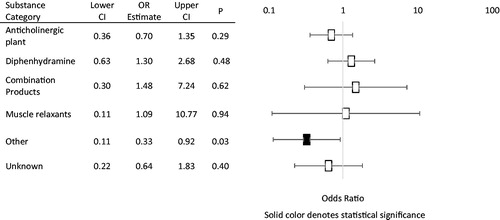
The frequency of cases by primary causative substance and their associated response rates, and repeat dosing for physostigmine are detailed in and , respectively. Odds ratios (OR) for response rates categorized by primary causative substance are also included in . The most common ingestions reported were anticholinergic plants (n = 67, 35.1%), and diphenhydramine (n = 56, 29.3%). Ingestion was unknown in 17 patients (8.9%). Of the 191 included patients, 140 (73.3%) patients had improvement or resolution of delirium following an initial dose of physostigmine, and 69 (36.1%) patients received additional doses of physostigmine. Of the 191 included patients, only 69 (36.1%) of patients received additional doses of physostigmine.
Table 2. Patients with response to initial dose of physostigmine, defined as an improvement or normalization of mental status with associated odds ratio (or), 95% confidence intervals (95% CI), and p values.
Table 3. Patients requiring repeat doses of physostigmine.
The reasons why patients did not receive additional doses of physostigmine are outlined in . Of these patients, 23/113 (20.4%) were noted to have resolution of anticholinergic delirium not requiring additional doses of physostigmine or any additional sedating agents. Fifty (50/113, 44.2%) patients were noted to have improved symptoms of delirium without requiring additional benzodiazepines or neuroleptics, while 11/113 (9.7%) patients were given additional benzodiazepines or neuroleptics to treat symptoms of delirium. Ten patients (10/113, 8.8%) were intubated either prior to or after receiving physostigmine. Finally, 3/113 (2.7%) patients who were not given repeat doses of physostigmine were noted to have adverse drug reactions (seizure in two patients, emesis in one).
Table 4. Reasons physostigmine dose was not repeated during hospitalization based upon review of CPCS records.
The odds ratios of the response to physostigmine by substance category are shown in . Patients with 100% response rates were excluded from calculations as an odds ratio cannot be calculated for these patients. The only odds ratio to reach statistical significance was that of patients who ingested other anticholinergic substances 8/16 (50%, OR: 0.33; CI: 0.11–0.92; p = .03). Of the patients who ingested anticholinergic plants, 46/67 (68.7%, OR: 0.70; CI: 0.36–1.35) had improvement or reversal of delirium after the initial dose of physostigmine. Of the patients who ingested diphenhydramine, 43/56 (64.2%, OR: 1.30; CI: 0.63–2.68) patients responded with improvement or resolution of anticholinergic delirium following the initial dose of physostigmine. Of the 10 patients who ingested combination products, 8/10 (80%, OR: 1.48; CI: 0.30–7.24) responded to physostigmine. All of the patients who ingested non-diphenhydramine antihistamines (n = 14, 100%) and tricyclic antidepressants (n = 3, 100%) responded to physostigmine.
Adverse events
Adverse events following physostigmine administration are detailed in . Of the 191 patients, the majority of patients, 182 (95.3%), had no adverse reactions after receiving physostigmine. Four patients (2.1%) experienced emesis, though one of these patients was noted to have emesis prior to and after administration of physostigmine. Two patients (1.0%) developed QTc prolongation. One of these two patients was noted to have a QTc of 530 ms documented after receiving physostigmine, however, the QTc prior to administration of physostigmine was not reported. The second patient had a documented of QTc 494 ms prior to administration of physostigmine, and 516 ms after receiving physostigmine. Two patients (1.0%) were noted to have seizures followings physostigmine administration. One of these two patients developed seizure in the setting of a combined ingestion of quetiapine and bupropion, and the second patient experienced seizures prior to and after receiving physostigmine. Adverse events were unknown in one patient (0.5%).
Table 5. Reported adverse events following physostigmine administration.
Regarding the single fatality, this patient was a 21-year-old male who presented to the ED with agitation after ingesting 950 mg of diphenhydramine. His vital signs on arrival were: blood pressure (BP) 110/70 mm Hg, HR 154 bpm, and respiratory rate (RR) 20 bpm. He was given 0.5 mg of physostigmine, without response. Approximately 6 h after physostigmine administration, the patient developed a wide complex tachycardia and a subsequent cardiac arrest and ultimately expired despite resuscitative measures. His urine drug screen was positive for cocaine, amphetamines, and benzodiazepines.
Dosing
Doses of physostigmine were known in 121 (63.4%) patients, and the median, mean, and range of total initial doses administered to patients, arranged by substance category are detailed in . Of the patients with known initial physostigmine doses, 89/121 (73.4%) were treated with a total of less than 2 mg, with 36/121 (29.8%) of patients receiving a total of less than 1 mg of physostigmine, and only 3/121 (2.5%) patients received greater than a total of 4 mg. Median total physostigmine doses ranged from 1.0 to 1.38 mg and mean doses ranging from 1.0 to 1.75 mg. Antipsychotics were not included in this table as dose was only available for one patient (2 mg total).
Table 6. Doses of physostigmine, by substance category of primary ingestant.
Disposition
Regarding disposition, the majority of patients were admitted to the ICU (n = 110; 57.6%), 36 (18.8%) patients were discharged directly from the ED, 35 (18.3%) patients were admitted to a non-critical care floor, 10 (5.2%) patients had an unknown disposition, and one fatality (0.5%) was reported. Of the patients who were discharged from the ED, 16 (16/36, 44.4%) were treated with physostigmine only.
Discussion
The reversal of anticholinergic delirium with physostigmine provides diagnostic certainty, avoiding unnecessary and potentially invasive testing [Citation6]. The most feared complication of physostigmine administration is asystole, described in two patients with significant tricyclic antidepressant toxicity treated with physostigmine [Citation8]. Review of these cases, however, suggests the fatalities were more likely the result of severe tricyclic antidepressant toxicity rather than a consequence of physostigmine administration [Citation12,Citation13]. As expected, this case series resulted in hesitancy to use physostigmine as an antidote despite its demonstrated effectiveness [Citation2–6,Citation9,Citation10]. This retrospective review investigates the safety and effectiveness of physostigmine to reverse anticholinergic delirium.
Few adverse events were reported in our cohort. Only two patients were noted to have seizure following physostigmine administration. In one instance, the patient was described to have seizure following ingestion of multiple medications with a high incidence of seizure in overdose, and the second patient was noted to have seizure activity preceding physostigmine administration. No patients were noted to have QRS prolongation, and QTc prolongation was rare, and not associated with arrhythmia. A single fatality was noted in this case series, however, the patient developed a wide complex tachycardia greater than 6 h from the administration of a small dose (0.5 g) of physostigmine. Given the serum half-life of physostigmine is short (22 min [Citation9]), it is very unlikely physostigmine contributed to the patient’s arrhythmia.
Physostigmine was effective in reversing or improving anticholinergic delirium in a majority (73.8%) of the patients after an initial dose of physostigmine, similar to previous reviews noting reversal of delirium in 73% [Citation4] and 80% of patients [Citation2]. Physostigmine alone was safely used to treat 74.3% (n = 142) of patients in our cohort, and 16 (8.4%) of these patients were discharged from the ED after its use, suggesting physostigmine may be considered as a safe first-line therapy to treat anticholinergic delirium. Furthermore, doses less than 2 mg of physostigmine were often effective in reversing anticholinergic delirium, decreasing the potential for adverse events.
The varied duration of response following physostigmine administration is best explained by the pharmacodynamics of cholinesterase inhibition [Citation9,Citation14]. The onset of action of physostigmine is within 3–8 min and the duration of effect is usually 30–60 min [Citation15] following parenteral administration. While the average elimination half-life of physostigmine has been observed at 22 min [Citation9,Citation15,Citation16], the half-life of cholinesterase inhibition is notably longer ranging from 83.7 ± 5.2 min, and the effects on cholinesterase inhibition approximately five times longer than the elimination half-life of physostigmine [Citation17].
A difference in the response rate of physostigmine to reverse anticholinergic delirium was observed, and varied by substance. The explanation for this variable response rate is likely multi-factorial. The difference in the number of patients comprising these groups may account for the differences in physostigmine response. A larger sample of poisoning cases is likely to have a wider range of ingestion histories (e.g., doses, time to presentation, co-morbidities, and co-ingestants). The severity of presenting signs and symptoms may also account for some of the variation in response rate. For example, 73.1% (n = 49) of patients with anticholinergic plant ingestions were described as agitated, compared with 42.9% (n = 6), of patients with non-diphenhydramine antihistamine ingestions. Other possibilities for partial response to physostigmine may include the relative low dose of physostigmine administered, mixed pharmacological actions (e.g., non-anticholinergic), variable mechanisms of action (antagonism of post-synaptic muscarinic receptor, decrease of acetylcholine release), receptor affinity, and target organ concentration (CNS) for substances causing an anticholinergic delirium.
Limitations
There are several limitations to this study. First, this study is a retrospective review with inherent biases, and also makes temporal relationships difficult to assess. The data source used (CPCS records) is limited in the completeness of the data recorded, and CPCS personnel documenting cases were not collecting information specific to this study and therefore data was missing from this data set. Patients are followed remotely by CPCS staff until clinical resolution of symptoms of a toxicologic emergency, however, patients may be lost to follow-up for various reasons not under the control of CPCS staff (e.g., patients may have been discharged prior to completion of follow-up or treatment teams could not be reached). The patient information provided is limited to information provided by the care team caring for patients and the information recorded in the CPCS database. In addition, the ingestion history is reliant on patient report rather than laboratory confirmation, and thus ingestion histories may be inaccurate. The QRS and QTc values included in this case series were as reported on the EKG, measured by the EKG machine and thus may limit accuracy. The use of the physostigmine dosing guidelines as outlined in Poisoning and Drug Overdose [Citation11] used by CPCS staff may have influenced dosing recommendations in this cohort. Finally, this sample is relatively small, including only 191 patients.
Conclusions
Physostigmine improved anticholinergic delirium in a majority of patients in this cohort with few adverse events. Physostigmine administration to reverse anticholinergic delirium was often used as a single agent in doses less than 2 mg, with few adverse events and no fatalities as a result of its use. However, intoxications from some substance categories may be less responsive to physostigmine therapy.
Acknowledgements
The authors would like to acknowledge the contribution of Brian Kearney, Ph.D., who assisted with the statistical analysis and constructed the forest plots for .
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Betten DP, Vohra RB, Cook MD, et al. Antidote use in the critically ill poisoned patient. J Intensive Care Med. 2006;21:255–277.
- Walker WE, Levy RC, Hanenson IB. Physostigmine-its use and abuse. JACEP. 1976;5:436–439.
- Schneir AB, Offerman SR, Ly BT, et al. Complications of diagnostic physostigmine administration to emergency department patients. Ann Emerg Med. 2003;42:14–19.
- Greene LT. Physostigmine treatment of anticholinergic-drug depression in postoperative patients. Anesth Analg. 1971;50:222–226.
- Holzgrafe RE, Vondrell JJ, Mintz SM. Reversal of postoperative reactions to scopolamine with physostigmine. Anesth Analg. 1973;52:921–925.
- Burns MJ, Linden CH, Graudins A, et al. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35:374–381.
- Krenzelok EP. Aspects of Datura poisoning and treatment. Clin Toxicol. 2010;48:104–110.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9:588–590.
- Dawson AH, Buckley NA. Pharmacological management of anticholinergic delirium – theory, evidence and practice. Br J Clin Pharmacol. 2016;81:516–524.
- Watkins JW, Schwarz ES, Arroyo-Plasencia AM, Toxicology Investigators Consortium investigators, et al. The use of physostigmine by toxicologists in anticholinergic toxicity. J Med Toxicol. 2015;11:179–184.
- Olson KR, editor. Poisoning & drug overdose. 6th ed. New York, NY: McGraw-Hill Companies Inc; 2007.
- Suchard JR. Assessing physostigmine's contraindication in cyclic antidepressant ingestions. J Emerg Med. 2003;25:185–191.
- Kulig K, Rumack BH. Physostigmine and asystole. Ann Emerg Med. 1981;10:228–220.
- Knapp S, Wardlow ML, Albert K, et al. Correlation between plasma physostigmine concentrations and percentage of acetylcholinesterase inhibition over time after controlled release of physostigmine in volunteer subjects. Drug Metab Dispos. 1991;19:400–404.
- Hartvig P, Wiklund L, Lindström B. Pharmacokinetics of physostigmine after intravenous, intramuscular and subcutaneous administration in surgical patients. Acta Anaesthesiol Scand. 1986;30:177–182.
- Triggle DJ, Mitchell JM, Filler R. The pharmacology of physostigmine. CNS Drug Rev. 1998;4:87–136.
- Asthana S, Greig NH, Hegedus L, et al. Clinical pharmacokinetics of physostigmine in patients with Alzheimer's disease. Clin Pharmacol Ther. 1995;58:299–309.