Abstract
Background/Objectives: The use of levetiracetam (LEV) in the management of drug-induced seizures has not been systematically investigated. Repetitive and continuous seizures that do not respond to benzodiazepines require second line therapy. Levetiracetam has a unique receptor binding site, rapid absorption, no known cardiac effects at therapeutic doses, and is theoretically a good candidate for use in drug-induced seizures. We evaluate the safety of LEV and its association with seizure cessation in this retrospective chart review of patients who received LEV as a control agent in drug-induced seizures.
Methods: We identified the medical records of patients presenting to an urban, level 1 trauma center between 1 January 2010 and 31 May 2015 by ICD-9 codes based on the following: (1) a poisoning diagnosis, (2) a seizure diagnosis, and (3) administration of LEV. We included patients with a drug-induced seizure based on history, electroencephalogram results, blood alcohol concentrations, urine drug screens, and adequate documentation. We excluded patients with alcohol withdrawal, anoxic brain injury, subtherapeutic concentrations of other antiepileptics, hypoglycemia, and pseudoseizures. Primary outcomes of interest included cessation of active seizures or the prevention of seizure recurrence. We assessed safety by the presence or absence of adverse drug effects (ADE) attributed to the administration of LEV.
Results: Thirty-four patients met inclusion and exclusion criteria. Half of the study cohort (17) presented with generalized tonic-clonic seizures (TCS); half (17) presented in generalized convulsive status epilepticus (GCSE). Six patients in GCSE received LEV during their seizures; 2 also received fosphenytoin. One improved immediately following LEV administration, and the remaining 5 had seizure control. Eleven GCSE patients (65%) remained seizure free after LEV therapy. The patients with TCS (17) received LEV after seizure(s) control. Sixteen (94%) were seizure-free during their hospital course. We found no adverse drug effects. In total, 27 of 34 patients (79%) had a return to baseline neurological and physical health. Six had long-term sequelae; none of which are known LEV side-effects. We identified 46 toxic substances and 22 known seizurogenic agents (48%). The median length of stay was 3.7 days (0.4–96), and the median duration of in-hospital LEV therapy was 1.6 days (0–49).
Conclusions: Levetiracetam used as a second-line agent was associated with control of drug-induced seizures and prevention of seizure recurrence without obvious adverse effects. A prospective study is needed to confirm these results.
Background
Drug toxicity causes two to ten percent of status epilepticus cases [Citation1–5]. Clinicians must recognize and terminate drug-induced status epilepticus early to prevent mortality and poor functional outcomes [Citation6–9]. Because there are no validated guidelines for managing toxic seizures, further guidance – especially for seizures refractory to benzodiazepines – is necessary [Citation10–16].
The choice of second line agents is not clear in this setting. Phenytoin, valproic acid, and carbamazepine have relative contraindications due to ion channel blocking effects and paradoxical seizures in high doses. Phenobarbital and propofol are two recommended second line agents for drug-induced seizures; however, there are no prospective trials for their use in this setting.
Levetiracetam has a unique binding site with a different mechanism of action than that of benzodiazepines, propofol, and barbiturates. Levetiracetam is rapidly absorbed, renally excreted, does not modulate sodium, potassium, or calcium channels, and has no reported cardiotoxic effects at therapeutic doses [Citation17–19]. Because of levetiracetam’s favorable pharmacologic profile and minimal drug interactions, it may be preferred for use in this setting.
Clinicians at our institution utilize levetiracetam for benzodiazepine-resistant seizure cessation and seizure suppression, including toxic seizures. However, data concerning the use of levetiracetam in drug-induced seizures are lacking. We aimed to describe whether levetiracetam controls drug-induced seizures. Our secondary aim was to describe adverse effects associated with levetiracetam when used for toxic seizures.
Methods
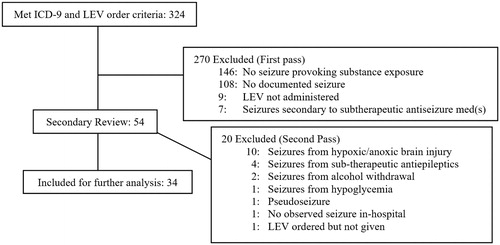
We performed a chart review of patients treated at the University of New Mexico Hospital, between 1 January 2010 and 31 May 2015 with ICD-9 codes for (1) a poisoning diagnosis, and (2) a seizure diagnosis, and (3) administration of levetiracetam intravenously. Of the 324 charts identified by ICD-9 screening, we reviewed cases for those with a documented history of toxic overdose, seizure(s), and levetiracetam administration for seizure termination and/or ongoing seizure control. We excluded cases with no documentation of seizure (e.g. known history of seizure disorder but no seizure on presentation), levetiracetam administration, or if there was a clear non-toxicological etiology for seizure (e.g. alcohol withdrawal, intracranial hemorrhage, hypoglycemia, pseudoseizures, etc.). Of the 54 remaining cases, all six clinical investigators reviewed case summaries, and we obtained a unanimous consensus for inclusion or exclusion (second-pass). illustrates the included and excluded patients. The authors involved in screening included two board certified medical toxicologists, a neuro-intensivist, a clinical toxicologist, a pharmacist and an emergency medicine resident. One toxicologist and the neuro-intensivist are also board certified in emergency medicine.
We reviewed medical records of the 54 included cases for provider notes, laboratory and other testing, medication administration indications, timing and response to treatment. We further divided patients into two groups: (1) those presenting in generalized convulsive status epilepticus (GCSE); and (2) those with generalized tonic-clonic seizures (TCS). We defined generalized convulsive status epilepticus based on two standardized criteria: (1) a seizure lasting greater than five minutes OR (2) more than one seizure occurring within five minutes without return to baseline neurologic function [Citation20,Citation21].
Primary outcomes were: (1) Therapeutic response to levetiracetam, determined by cessation of active seizures in a time-frame consistent with its pharmacologic properties or the absence of seizure recurrence and (2) Safety of levetiracetam in toxic seizures, determined by the presence or absence of adverse drug effects attributed to the administration of levetiracetam, based on known side effects of levetiracetam. Because respiratory depression is not a known side-effect of levetiracetam, we did not include intubation or respiratory depression as an adverse event.
Ethical approval
The University of New Mexico School of Medicine Institutional Review Board reviewed and approved the study.
Results
Thirty-four patient encounters met inclusion and exclusion criteria (). Patient demographics included 11 women and 23 men. The median age was 39 [IQR 24.3–48.3]. Forty-four percent had history of seizures and 56% did not. Further demographic information is listed in .
Table 1. Demographic characteristics of patients treated for toxic seizures.
We identified 46 substances and 22 known seizure-provoking agents (48%) by history and, where available, by laboratory testing (). Toxins most frequently involved in our study were cocaine (6) and amitriptyline (5). We identified more than one seizure provoking substance in 17 patients; the median number of substances was 1.5 [IQR 1–3]. Twenty-nine patients (85%) had serum ethanol measurements, among which four patients tested positive for ethanol. A different subset of 29 patients (85%) underwent immunoassay urine drug screens for drugs of abuse (); we used urine drug screens to confirm history of cocaine and amphetamine use. Seventeen patients (50%) presented with generalized TCS and 17 (50%) presented in GCSE. For a complete list of study patients’ presentations and hospital course please refer to .
Table 2. Substances identified by history, urine, and/or blood.
Table 3. Demographic characteristics of patients treated for toxic seizures.
Table 4. Complete list of patients.
Seizure termination
Among the 17 patients with GCSE, 15 had seizures that did not terminate with benzodiazepines alone. Six patients received levetiracetam during their seizures. The remaining 11 patients received levetiracetam after control of GCSE with other agents. Among the six patients who received levetiracetam during their seizures, one had termination of generalized convulsive status epilepticus immediately following levetiracetam administration; four had seizure control at an unspecified time after administration. One patient received endotracheal intubation, sedation with propofol, and was seizing at the time of levetiracetam administration. An EEG performed two days later was inconclusive.
All the 17 patients with TCS obtained initial seizure cessation spontaneously, either with benzodiazepines, or with other antiseizure medications. Therefore, all the patients with TCS received levetiracetam for seizure suppression, rather than seizure termination.
Seizure suppression
Among the study cohort, 27 of 34 (79%) of the patients with drug-induced seizures remained seizure free after levetiracetam administration. Sixteen of 17 TCS patients remained seizure-free during their hospital course.
All the patients with GCSE received midazolam or lorazepam prior to receiving levetiracetam, with the exception of one patient who only received naloxone. Two patients with GCSE received benzodiazepines and fosphenytoin before levetiracetam. One patient received olanzapine, diazepam, and propofol. Eleven with GCSE remained seizure-free after levetiracetam administration and six had a seizure recurrence. Of the six with seizure recurrence following levetiracetam: (1) Two patients had seizure suppression after titration of levetiracetam dose from 500 to 750 twice daily in one patient and 1000 to 1500 twice daily in the other; (2) One patient who received levetiracetam for clinically apparent seizure (reflected in contemporaneous physician and nursing notes) later had an electroencephalogram (EEG) which showed myoclonic jerking without epileptiform activity. We included this patient based upon the empirical use of levetiracetam for apparent seizure; (3) Three patients had a seizure after discontinuing levetiracetam during their hospital stay; their seizures occurred 1 day, 3 days, and 7 days, respectively, after in-hospital levetiracetam termination.
Of the 15 patients with a seizure history, levetiracetam achieved seizure control in seven of eight prior levetiracetam users and four of seven with no prior levetiracetam use.
The median length of stay was 3.7 days (range of 0.4–96 days), and the median duration of in-hospital levetiracetam therapy was 1.6 days (range of 0–49 days). Twenty-seven of 34 patients (79%) had a return to baseline neurological and physical health.
Adverse events associated with levetiracetam
lists all recorded in-hospital adverse events. The discharge summary for one patient mentioned change of affect. However, confounding factors may include the patient’s hospitalization of 3.7 days and substance abuse. The only death occurred in an 88-year-old woman who succumbed to sepsis from aspiration pneumonia nine days after achieving seizure control with levetiracetam. No contemporaneous record attributed any adverse event to LEV. On secondary review, the investigators did not attribute any adverse event to levetiracetam.
Discussion
Many commonly used second line treatments lack efficacy data in drug-induced seizure or have adverse effects. Propofol is effective but may cause respiratory depression [Citation22,Citation23], and phenobarbital may be associated with respiratory depression and paradoxical seizures [Citation11,Citation24,Citation25,Citation26]. Phenytoin, a sodium channel blocker, may increase the risk of adverse cardiac events in amitriptyline poisoning and causes paradoxical seizures in high doses [Citation16,Citation27,Citation28,Citation29]. Valproic acid, in addition to blocking sodium channels, also blocks calcium channels [Citation30,Citation31,Citation32]. Carbamazepine, like phenytoin, causes paradoxical seizures in supratherapeutic doses and may induce cardiac arrhythmias [Citation33,Citation34].
Levetiracetam is an effective first and second-line control agent for the management of generalized convulsive status epilepticus [Citation35,Citation36,Citation37]. Whether this translates to efficacy in drug-induced seizures is unknown. Interestingly, the overall rate of seizure suppression in our study approximates levetiracetam’s efficacy in non-drug induced seizures (44–94%) [Citation35,Citation36].
Levetiracetam has a distinct binding site at the synaptic vesicle protein 2A (SV2A) by which it may inhibit neurotransmitter release [Citation38]. This unique mechanism of action may modulate seizure suppression by targeting a site different from the primary insult in drug-induced seizures. Furthermore, it may suppress seizures resistant to GABA-agonists.
We identified no evidence of levetiracetam-related adverse effects. Common levetiracetam side effects include somnolence, dizziness, irritation, hostility, nervousness, and aggression; these behavior changes are primarily documented during chronic use [Citation39]. Given the severity of illness in our study sample, clinical documentation may not record minor adverse effects. For instance, treating physicians may have attributed symptoms such as somnolence of dizziness to post-ictal condition or to co-administered medications.
Limitations
This is a retrospective case series and subject to all of the limitations inherent in that study design. We relied upon clinical records of contemporaneous care. Laboratory investigations and management of seizures occurred at the discretion of the treating physicians without regard to research. Seizure control may have resulted from levetiracetam, other agents, or the combination. Seizures may have terminated on their own. Contemporaneous records may not systematically describe response to treatment or capture all adverse drug effects. Some patients’ seizures may have resulted from withdrawal of anti-epileptic medication, alcohol, or other medications. Since this is a retrospective chart review without a control or comparison group, we cannot determine efficacy of levetiracetam, and we are likely underestimating adverse effects.
Conclusions
Levetiracetam used as a second-line agent was associated with control of drug-induced seizures and prevention of seizure recurrence without obvious adverse effects. We propose a prospective clinical trial to more completely assess efficacy and safety.
Disclosure statement
The authors have no conflicts of interest to report.
References
- Alldredge BK, Lowenstein DH, Simon RP. Seizures associated with recreational drug abuse. Neurology. 1989;39:1037–1039.
- Alldredge BK, Lowenstein DH. Status epilepticus related to alcohol abuse. Epilepsia. 1993;34:1033–1037.
- Lowenstein DH, Alldredge BK. Status epilepticus at an urban public hospital in the 1980s. Neurology. 1993;43:483–488.
- Pesola GR, Avasarala J. Bupropion seizure proportion among new-onset generalized seizures and drug related seizures presenting to an emergency department. J Emerg Med. 2002;22:235–239.
- Lowenstein DH. Status epilepticus: an overview of the clinical problem. Epilepsia. 1999;40:S3–S8.
- Cheng JY. Latency to treatment of status epilepticus is associated with mortality and functional status. J Neurol Sci. 2016;370:290–295.
- DeLorenzo RJ, Garnett LK, Towne AR, et al. Comparison of status epilepticus with prolonged seizure episodes lasting from 10 to 29 minutes. Epilepsia. 1999;40:164–169.
- Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5:246–256.
- Jagoda A, Riggio S. Refractory status epilepticus in adults. Ann Emerg Med. 1993;22:1337–1348.
- DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–1035.
- Thundiyil JG, Rowley F, Papa L, et al. Risk factors for complications of drug-induced seizures. J Med Toxicol. 2011;7:16–23.
- Lui HK, Hui KF, Fong WC, et al. De novo status epilepticus is associated with adverse outcome: an 11-year retrospective study in Hong Kong. Seizure. 2016;40:42–45.
- Alvarez V, Drislane FW. Is favorable outcome possible after prolonged refractory status epilepticus? J Clin Neurophysiol. 2016;33:32–41.
- Foreman B, Hirsch LJ. Epilepsy emergencies: diagnosis and management. Neurol Clin. 2012;30:11–41, vii.
- Shah AS, Eddleston M. Should phenytoin or barbiturates be used as second-line anticonvulsant therapy for toxicological seizures? Clin Toxicol. 2010;48:800–805.
- Chen HY, Albertson TE, Olson KR. Treatment of drug-induced seizures. Br J Clin Pharmacol. 2016;81:412–419.
- Lynch BA, Lambeng N, Nocka K, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–9866.
- Rüegg S, Naegelin Y, Hardmeier M, et al. Intravenous levetiracetam: treatment experience with the first 50 critically ill patients. Epilepsy Behav. 2008;12:477–480.
- Patsalos PN. Clinical pharmacokinetics of levetiracetam. Clin Pharmacokinet. 2004;43:707–724.
- Brophy GM, Bell R, Claassen J, Neurocritical Care Society Status Epilepticus Guideline Writing Committee, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23.
- Al-Mufti F, Claassen J. Neurocritical care: status epilepticus review. Crit Care Clin. 2014;30: 751–764.
- Cantrell FL, Wardi G, O'Connell C. Propofol use for toxin-related seizures. Pharmacotherapy. 2016;36:702–704.
- Parviainen I, Kälviäinen R, Ruokonen E. Propofol and barbiturates for the anesthesia of refractory convulsive status epilepticus: pros and cons. Neurol Res. 2007;29:667–671.
- Hassanian-Moghaddam H, Ghadiri F, Shojaei M, et al. Phenobarbital overdose presenting with status epilepticus: a case report. Seizure. 2016;40:57–58.
- Kikuchi K, Hamano S, Oritsu T, et al. Effectiveness and safety of non-intravenous high-dose phenobarbital therapy for intractable epilepsy during childhood. Brain Dev. 2011;33:379–383.
- Lützen L, Poulsen LM, Ulrichsen J. [Respiratory depression in delirium tremens patients treated with phenobarbital. A retrospective study]. Ugeskr Laeger. 2008;170:2018–2022.
- Callaham M, Schumaker H, Pentel P. Phenytoin prophylaxis of cardiotoxicity in experimental amitriptyline poisoning. J Pharmacol Exp Ther. 1988;245:216–220.
- Osorio R, Burnstine B, Remler R, et al. Phenytoin-induced seizures: a paradoxical effect at toxic concentrations in epileptic patients. Epilepsia. 1989;30:230–240.
- Chua HC, Venketasubramanian N, Tan CB, et al. Paradoxical seizures in phenytoin toxicity. Singapore Med J. 1999;40:276–277.
- Tomson T, Battino D, Perucca E. Valproic acid after five decades of use in epilepsy: time to reconsider the indications of a time-honoured drug. Lancet Neurol. 2016;15:210–218.
- Perucca E. Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs. 2002;16:695–714.
- Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46:1323–1338.
- Schmidt S, Schmitz-Buhl M. Signs and symptoms of carbamazepine overdose. J Neurol. 1995;242:169–173.
- Megarbane B, Leprince P, Deye N, et al. Extracorporeal life support in a case of acute carbamazepine poisoning with life-threatening refractory myocardial failure. Intensive Care Med. 2006;32:1409–1413.
- Trinka E, Hofler J, Leitinger M, et al. Pharmacotherapy for Status Epilepticus. Drugs 2015;75:1499–1521.
- Grover EH, Nazzal Y, Hirsch LJ. Treatment of Convulsive Status Epilepticus. Curr Treat Options Neurol. 2016;18:11.
- Misra UK, Kalita J, Maurya PK. Levetiracetam versus lorazepam in status epilepticus: a randomized, open labeled pilot study. J Neurol. 2012;259:645–648.
- Yang XF, Weisenfeld A, Rothman SM. Prolonged exposure to levetiracetam reveals a presynaptic effect on neurotransmission. Epilepsia. 2007;48:1861–1869.
- Verrotti A, Prezioso G, Di Sabatino F, et al. The adverse event profile of levetiracetam: a meta-analysis on children and adults. Seizure. 2015;31:49–55.