Abstract
Context: Intentional self-poisoning with the herbicide paraquat has a very high case-fatality and is a major problem in rural Asia and Pacific.
Objectives: We aimed to determine whether the addition of immunosuppression to supportive care offers benefit in resource poor Asian district hospitals.
Materials and methods: We performed a randomised placebo-controlled trial comparing immunosuppression (intravenous cyclophosphamide up to 1 g/day for two days and methylprednisolone 1 g/day for three days, and then oral dexamethasone 8 mg three-times-a-day for 14 days) with saline and placebo tablets, in addition to standard care, in patients with acute paraquat self-poisoning admitted to six Sri Lankan hospitals between 1st March 2007 and 15th November 2010. The primary outcome was in-hospital mortality.
Results: 299 patients were randomised to receive immunosuppression (147) or saline/placebo (152). There was no significant difference in in-hospital mortality rates between the groups (immunosuppression 78 [53%] vs. placebo 94 [62%] (Chi squared test 2.4, p = .12). There was no difference in mortality at three months between the immunosuppression (101/147 [69%]) and placebo groups (108/152 [71%]); (mortality reduction 2%, 95% CI: −8 to +12%). A Cox model did not support benefit from high-dose immunosuppression but suggested potential benefit from the subsequent two weeks of dexamethasone.
Conclusions: We found no evidence that high dose immunosuppression improves survival in paraquat-poisoned patients. The continuing high mortality means further research on the use of dexamethasone and other potential treatments is urgently needed.
Introduction
Deaths from pesticide self-poisoning is a major clinical and public health problem in rural Asia [Citation1–3]. The herbicide paraquat is a leading cause of death [Citation4] from pesticide self-poisoning [Citation3–8]. In a prospective observational cohort of 9300 patients with pesticide self-poisoning, paraquat had a case fatality of 42% and accounted for 25% of pesticide poisoning deaths [Citation4].
Ingestion of paraquat results in rapid multi-organ failure or more drawn out lethal pulmonary fibrosis. Paraquat’s very high case-fatality is due both to its inherent toxicity and the lack of any effective treatment. There are no widely accepted guidelines on treatment of patients with paraquat self-poisoning; treatment regimens vary from supportive care alone to combinations of immunomodulation, anti-oxidant therapy, haemoperfusion and haemodialysis [Citation9].
Since paraquat leads to an acute inflammatory response [Citation9], immunosuppression with cyclophosphamide, methylprednisolone and dexamethasone has become a frequent method of treatment for paraquat self-poisoning. The regimen was first suggested in 1986 in a report of an uncontrolled study [Citation10]. A series of small clinical studies using immunosuppression reported a marked improvement in survival [Citation11–13]. However, a systematic review found insufficient evidence from high quality randomised controlled trials (RCT) to recommend its use [Citation14]. We established an RCT in 2007 to determine the effectiveness of immunosuppression versus placebo in preventing deaths from paraquat self-poisoning.
Materials and methods
The RCT (ISRCTN85372848) was conducted in six Sri Lankan district hospital sites. Ethics approval was received from the Ethics committees of Peradeniya, Colombo and Ruhuna Faculties of Medicine, Sri Lanka, and the Australian National University. Written informed consent was taken from each patient in their own language.
Participants
We approached all patients aged 14 years and over with paraquat pesticide self-poisoning admitted to adult wards with systemic exposure to paraquat as indicated by a positive urine dithionite test, a marker of poor prognosis [Citation15]. Patients were initially approached if they presented within 24 h of ingestion; following consultation with the DMEC in May 2009, all patients who presented within 48 h of ingestion were approached to improve recruitment. None of the patients received other specific treatments such as haemodialysis or haemoperfusion.
Exclusion criteria included: age < 14 years, known pregnancy, and known allergy to, or prior therapy with, trial medication. In addition, patients with a systolic blood pressure less than 70 mmHg that did not respond to 1 L of intravenous fluid or a Glasgow Coma score less than 8/15 were excluded; these criteria were intended to exclude severely poisoned patients who were expected to die imminently without any chance of responding to therapy.
Outcomes, objectives and hypotheses
The primary aim was to determine whether, in addition to standard care (intravenous fluid, activated charcoal and pain relief), high dose immunosuppression with cyclophosphamide, methylprednisolone and dexamethasone reduced in-hospital death from all causes in paraquat self-poisoning compared with no immunosuppression. Secondary outcomes were all-cause mortality at three months post-ingestion and lung function in survivors at three months.
At trial registration, we specified in-hospital deaths as the primary outcome. As people may die over one to two months after discharge [Citation16] we planned a secondary outcome of three-month survival. The other pre-specified secondary outcome of lung function [formal lung functions and a high resolution CT scan (HRCT) to be performed in tertiary referral hospitals at long-term follow-up] did not prove possible to obtain from a meaningful number of patients.
Randomisation
Randomisation was done using purpose-designed computer software. The random sequence and allocation were concealed prior to randomisation. The program randomised eligible patients in a 1:1 ratio to the placebo and immunosuppression arms (without block-allocation). The allocation sequences were generated and encrypted independently by an IT consultant who had no role in patient recruitment, treatment and assessment.
The randomisation was performed by study pharmacists at a central location in each hospital. Upon recruiting a patient, the pharmacist was provided with the name, hospital number and weight of the patient. The pharmacist randomised the patient and subsequently prepared treatment packs. The allocation was only known to the pharmacists who had no other role in patient management and data collection. The other members of the team could neither predict allocation nor alter randomisation.
Intervention
Patients randomised to the intervention arm were treated as described in Box S1 (Treatment Protocol). We visited patients three months after discharge to record if they were still alive or dead. Deaths were confirmed by observation of death certificates issued by the registrar of deaths. Survivors were interviewed to see if they were engaged in their daily routines and basic cardiovascular and respiratory clinical examinations were performed.
Blood and urine sampling
A urine sample and a 10 mL blood sample were obtained on admission after clinical stabilisation. A dithionite test was immediately performed on the admission urine sample [Citation17]. Five ml of blood was sampled at 8 h intervals up to three days and daily thereafter until death or discharge from hospital. These blood samples were used to measure plasma paraquat (as described elsewhere [Citation18]), creatinine, liver enzymes and full blood count. The treating physicians performed daily blood sugars using venous blood.
Sample size
In the largest previous study, overall survival in the control group was 18% (12/65) compared with 32% (18/56) in the treatment arm [Citation11]. An absolute increase in survival of 10–28%, would be clinically important. In order to be able to detect whether either regimen increases survival from 18% to 28%, with a significance level (alpha) of 5% and a power of 80%, a minimum of 295 patients must be recruited to each arm of the trial (i.e., 590 patients in total) (see Table S1 in the online data supplement).
Independent data monitoring & ethics committee
An independent data monitoring and ethics committee (DMEC) was established. The DMEC met each year and reviewed data supplied by the trial statistician applying the O’Brien-Fleming stopping rule (which has minimal effect on the power of the final analysis). In the light of interim data, and emerging evidence from other studies, the DMEC was tasked with advising the trial investigators to terminate the study if a treatment effect was clearly demonstrated, or if continuation would be detrimental to future enrolled patients’ health, or it was evident that no clear outcome would be obtained. The DMEC was also consulted when a paraquat import ban resulted in a collapse in recruitment, endorsed the decision to terminate the trial and reviewed the final manuscript.
Statistical analysis
All analyses were conducted using Stata Version 13. We compared demographic factors and clinical characteristics between treatment groups to assess randomisation.
The primary analysis was conducted using an unadjusted chi-squared test to compare in-hospital death between placebo and immunosuppression groups. We also did an intention to treat analysis of three-month survival (for this analysis we assumed that the six patients discharged alive and lost to follow up survived.) We use and present this superior outcome in all subsequent analyses; but conducted a sensitivity analysis restricted to in-hospital deaths whenever statistical tests were applied.
The best estimates of prognosis come from a timed plasma paraquat concentration on admission plotted on the Proudfoot nomogram [Citation19] or converted to the Severity Index of Paraquat Poisoning (SIPP = plasma paraquat concentration in mg/L multiplied by time in hours since ingestion) score [Citation20], the dose ingested and the serum creatinine [Citation9]. Eight values of zero for SIPP were replaced with 0.05 (half the lowest measured value). The two prediction methods are equally accurate but SIPP score has an advantage over the Proudfoot (and other) nomograms as it can be applied to any timed sample [Citation21]. A SIPP could not be calculated for 47 patients mostly due to a sample not having been analysed for paraquat. We carried out adjusted regression analyses to assess whether controlling for age, gender, volume of ingestion, or SIPP score altered the estimate of treatment effect. We used Cox proportional hazards for survival time.
Post hoc analysis
Lin et al. in a post hoc analysis of patients who survived for seven days and reported a significantly lower case fatality of 18.2% (2/22) in the immunosuppression group compared with 57% (16/28) case fatality in the control group [Citation11]. We performed the same analysis of case fatality of patients who survive seven days or longer.
Results
Patients were enrolled from 1st March 2007 until 15th November 2010. As a result of regulatory decisions, the use of paraquat was phased out from Sri Lankan agricultural practice through 2008 and 2009 and finally banned in August 2010. The trial was stopped in November 2010 after consultation with the DMEC due to a collapse in recruitment.
Participants
About 604 patients with paraquat poisoning were assessed on admission; 305 patients were excluded from the trial [negative urine test (149), refused consent (42), died before randomisation (46), late admission (>48 h post ingestion) (39), GCS < 8 (2), pregnancy [Citation3], age less than 14 (3), other reasons [Citation21]. 299 urine positive patients were eligible, consented, and randomised into the trial: 152 received placebo and 147 received immunosuppression ().
Figure 1. CONSORT statement flow diagram of patient progress through the RCT. **Three patients died and one withdrew consent after randomisation but before allocated treatment was given. One patient randomised to placebo was given immunosuppressive treatment. ##Three patients died and one left against medical advice after randomisation but before allocated treatment was given. ***Eighteen patients left hospital against medical advice after starting allocated treatment (median of 1.9 days [IQR 0.7–3.7, range 0.16–5.1]). ###Twenty-six patients left hospital against medical advice after starting allocated treatment (median of 2.1 days [IQR 1.1–3.0, range 0.20–9.7]).
![Figure 1. CONSORT statement flow diagram of patient progress through the RCT. **Three patients died and one withdrew consent after randomisation but before allocated treatment was given. One patient randomised to placebo was given immunosuppressive treatment. ##Three patients died and one left against medical advice after randomisation but before allocated treatment was given. ***Eighteen patients left hospital against medical advice after starting allocated treatment (median of 1.9 days [IQR 0.7–3.7, range 0.16–5.1]). ###Twenty-six patients left hospital against medical advice after starting allocated treatment (median of 2.1 days [IQR 1.1–3.0, range 0.20–9.7]).](/cms/asset/681486d5-4f26-46ea-aa08-7fb282d7dca6/ictx_a_1394465_f0001_b.jpg)
Baseline characteristics
Baseline demographic and clinical characteristics are presented in , and dose ingested in Figure S1. Admission plasma paraquat concentrations were available in 127 (84%) of the placebo group and 125 (85%) of the immunosuppression group. The immunosuppression group had a slightly higher median paraquat concentration and SIPP score but there were no other substantial differences.
Table 1. Baseline demographics, clinical parameters, and prognostic markers.
Primary outcome – mortality
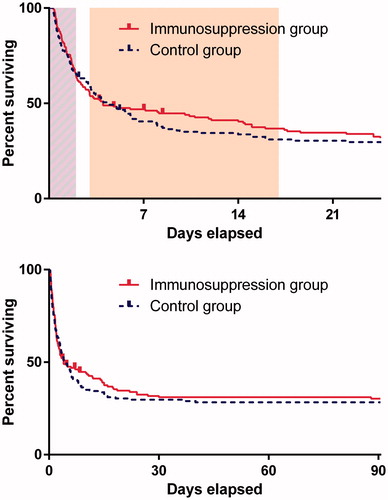
In-hospital mortality was 172/299 (58%). There was no significant difference in in-hospital mortality between the placebo (61.8%) and immunosuppression (53.1%) groups (mortality reduction 8.8%, 95% CI −2.4% to 20.0%, p = .1). There was also no evidence of a difference in case fatality at three months post ingestion between patients in placebo (71.1%) and immunosuppression (68.7%) groups (mortality reduction 2.3%, 95% CI: −8.1% to 12.7%, p = .7). The paraquat concentrations of survivors and non-survivors from both groups plotted against two risk prediction nomograms in , indicate that death was predictable from exposure in most individuals, with few individuals below the lines dying and few above surviving, and no obvious difference with treatment. On an intention to treat basis, there was no evidence of a difference in survival between the two groups (, log rank p = .6).
Figure 2. Admission plasma paraquat concentrations plotted against Proudfoot (with Schermann’s extension) and SIPP score =10 lines [concentrations above these shown to be highly predictive of a fatal outcome]. Patients lost to follow up before three months but assumed to have survived shown with green highlight. Lines on Proudfoot nomogram appear curved due to log transformed y-axis.
![Figure 2. Admission plasma paraquat concentrations plotted against Proudfoot (with Schermann’s extension) and SIPP score =10 lines [concentrations above these shown to be highly predictive of a fatal outcome]. Patients lost to follow up before three months but assumed to have survived shown with green highlight. Lines on Proudfoot nomogram appear curved due to log transformed y-axis.](/cms/asset/18ca22ba-4c86-4397-b1bf-a83217845a3b/ictx_a_1394465_f0002_c.jpg)
Figure 3. Timing of deaths in the two study arms. Percentage of patients still alive shown. The clock has been started at randomisation and stops either at death or last follow-up or three months. The days on which cyclophosphamide were given and dexamethasone are shown in light purple and orange, respectively in the top panel. Censored patients (lost to follow-up) are tagged.
We were able to follow up all but six patients post discharge. HRCT and lung function became available during the second year in one centre and the third year in another centre. However, patients were unwilling to travel long distances for HRCT and formal lung functions, mostly because for many this opportunity came one to two years after their poisoning. These outcomes were only obtained on 17 patients (<20%), were mostly normal, and provide no insight into treatment effectiveness.
Pre-specified multivariable analysis
The Cox proportional hazards model suggests a small, albeit non-statistically-significant, beneficial effect from immunosuppression (adjusted HR for death 0.74 (95%CI: 0.54–1.01) – ). Looking at this more closely, introducing a time dependent treatment effect into the model showed that the favourable effect was not observed during the high-dose immunosuppression but was restricted to the time of the dexamethasone administration (), (see Table S2 in the online data supplement).
Table 2. Cox model of hazard of death (n = 252) without including time dependent treatment effects (†) and incorporating an analysis of treatment effect based on the time in which high-dose immunosuppression (days 0–3), dexamethasone (days 3–17) and no treatment (>day 17) were given (††).
Post hoc analysis of patients surviving seven days or longer
We compared the case fatality of the 124 patients who survived seven days or longer. Death occurred in 17/59 (28%) patients in the placebo group and 22/65 (34%) in the immunosuppression group (mortality increase 5%, 95% CI: −11% to +21%).
Adverse events
Each patient was assessed twice daily for two specific adverse effects (haematuria, bladder pain) attributable to cyclophosphamide, but these were not observed.
Discussion
This trial, the largest RCT in paraquat poisoning to date, showed no benefit of high dose immunosuppression with cyclophosphamide, methylprednisolone and dexamethasone in acute self-poisoning with paraquat. Previous reports have claimed large benefits from immunosuppression but not provided convincing evidence [Citation22]. The original report claimed improved survival on the basis that 6 of the 18 patients who had plasma paraquat concentrations over 2 mg/L survived and that the mortality in this group should be 100% [Citation10]. This was followed by a negative observational study [Citation23], and more recently by a series of very small RCTs [Citation11–13,Citation24]. The trials have all been unregistered and no power calculations would have supported trials this small. Hence, it seems likely that most or all of these studies were prematurely stopped based on unplanned interim analyses with no adjustment to the statistical plan to avoid inflated type I error. A forest plot of the various RCTs shows a very strong inverse relationship between study and effect size (Figure S3), suggesting there may also be publication bias and that any pooled analysis suggesting a larger overall benefit should be interpreted cautiously. We believe our trial (which has more than half the total randomised patients) provides a more accurate estimate of the likely treatment effect. In the next largest RCT, on intention to treat analysis 12/65 control patients and 18/56 patients receiving immunosuppression survived (p = .09). The authors presented a post-hoc analysis in which only patients who survived the first week after randomisation were compared and claimed they had demonstrated benefit from immunosuppression in this sub-group. Their study reported deaths (in those surviving more than a week) in 4/28 patients with immunosuppression compared with 16/28 controls. Our post hoc analysis of more than twice this number does not provide any evidence that supports survival benefit from immunosuppression in this sub group. This illustrates (if any further illustrations are needed) the pitfalls of conclusions based on post-hoc sub-group comparisons in clinical trials.
Despite that caveat, our own secondary analysis does suggest a potentially important favourable effect of the two week dexamethasone course that followed the high dose immunosuppression (; Figure S2). There is also a separation of the survival curves between the 5th and the 14th day. From around that time onwards, the survival curves come back together (). Dexamethasone was the only active treatment during this time and was stopped on the 17th day. While this effect was non-significant on the intention to treat analysis, was not sustained after treatment stopped, and is also quite modest, any future studies using steroids should consider continuing this treatment for the five- to six-week period in which deaths occur. It is possible, although less likely that this time course reflected a delayed response to immunosuppression. However, in contrast to high-dose immunosuppression dexamethasone is inexpensive, of low toxicity and easily administered. A large nation-wide study of 1811 patients from Taiwan concluded that patients receiving immunosuppression had modestly better outcomes (29% vs 24% survival). Intriguingly, the only immunosuppressive regimens demonstrating improved outcomes were the 4 out of 7 which contained dexamethasone [Citation25]. Further, animal studies have shown dexamethasone may be effective, and also that it has two mechanisms of action. As well as an anti-inflammatory effect, it induces P-glycoprotein which may increase efflux of paraquat from pulmonary tissue [Citation26].
In contrast, a recent review of pathophysiological mechanisms in paraquat poisoning found neither animal models nor plausible pathophysiological explanation for why high dose immunosuppression would be effective in paraquat poisoning in humans [Citation9]. Paraquat rapidly generates reactive oxygen species which causes cellular damage via lipid peroxidation, activation of nuclear factor kappa B, mitochondrial damage and apoptosis in many organs. This leads to rapid deterioration of renal and liver function and development of acute alveolitis. Cyclophosphamide and methylprednisolone have not been shown to have beneficial effects in scavenging free radicals during the acute stage. Nor were these treatments alone (without dexamethasone) associated with better outcomes in the large nationwide study from Taiwan [Citation25].
Limitations
The most important limitation of our study was that, while it is much larger than the preceding trials, we were unable to recruit the planned number of patients into the study as the sale of paraquat was banned in Sri Lanka. However, as the difference in case fatality with 298 patients is minimal, it seems unlikely that continuation of the trial would have led to a trial result that supported immunosuppression improving overall survival (the primary outcome). To achieve a 9% difference in survival with statistical significance, the survival in the immunosuppression arm would need to increase to 50% in the remaining 150 patients with the placebo group continuing at the current survival of 29%. This is a 19% increase in survival from the current level of 31%. This is extremely unlikely to occur. Other limitations largely relate to the resource poor setting of rural Asian hospitals; typical of paraquat poisoning but not clinical trials. In a Western country such severely ill patients would be in intensive care; in rural Asia they are on crowded open wards with few staff. There was very limited laboratory testing and infrequent clinical monitoring of many patients. Thus we cannot report on any disease modifying effects of treatment (e.g., less or more kidney failure) that did not affect mortality, although such information would not alter our overall conclusions. Further, we did not exclude patients based on pre-existing medical conditions, however, there were very few in this predominately young healthy population and none that were regarded as having directly contributed to death.
Conclusions
Intentional self-poisoning with paraquat continues to kill many people throughout rural Asia. We have little evidence that supports any active medical treatment. Clinical trials to identify more effective therapy are urgently needed in places where paraquat has not been banned. Our study and observational studies suggest any possible benefit is due to the dexamethasone component rather than high-dose immunosuppression. We believe clinical research efforts would be best spent on exploring the optimal dose of dexamethasone and other inexpensive and low toxicity antidotes with favourable effects in animal studies (for example acetylcysteine) [Citation9,Citation27].
ICTX_Mohamed_et-al-Supplemental_Content.docx
Download MS Word (109.2 KB)Disclosure statement
MW was an employee of Syngenta at the time of trial inception and until 2009. AHD received funding to attend DMEC meeting from Syngenta. ME have received travel expenses from Syngenta to attend meetings of a scientific advisory group in relation to studies of new paraquat formulations. No other conflicts declared.
Funding
This study has been funded by Syngenta Crop Protection AG; Wellcome Trust/National Health and Medical Research Council International Collaborative Research Grant [ICRG 071669]; Australian Leadership Award [ALA00379].
References
- Jeyaratnam J. Acute pesticide poisoning: a major global health problem. World Health Stat Q. 1990;43:139–144.
- Eddleston M, Phillips MR. Self poisoning with pesticides. BMJ. 2004;328:42–44.
- Gunnell D, Eddleston M, Phillips MR, et al. The global distribution of fatal pesticide self-poisoning: systematic review. BMC Public Health. 2007;7:357.
- Dawson AH, Eddleston M, Senarathna L, et al. Acute human lethal toxicity of agricultural pesticides: a prospective cohort study. PLoS Med. 2010;7:e1000357.
- Patel V, Ramasundarahettige C, Vijayakumar L, et al. Suicide mortality in India: a nationally representative survey. Lancet. 2012;379:2343–2351.
- Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. QJM. 2000;93:715–731.
- Yin Y, Guo X, Zhang SL, et al. Analysis of paraquat intoxication epidemic (2002–2011) within China. Biomed Environ Sci. 2013;26:509–512.
- Chang SS, Lu TH, Eddleston M, et al. Factors associated with the decline in suicide by pesticide poisoning in Taiwan: a time trend analysis, 1987-2010. Clin Toxicol. 2012;50:471–480.
- Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72:745–757.
- Addo E, Poon-King T. Leucocyte suppression in treatment of 72 patients with paraquat poisoning. Lancet. 1986;1:1117–1120.
- Lin JL, Leu ML, Liu YC, et al. A prospective clinical trial of pulse therapy with glucocorticoid and cyclophosphamide in moderate to severe paraquat-poisoned patients. Am J Respir Crit Care Med. 1999;159:357–360.
- Lin JL, Wei MC, Liu YC. Pulse therapy with cyclophosphamide and methylprednisolone in patients with moderate to severe paraquat poisoning: a preliminary report. Thorax. 1996;51:661–663.
- Lin JL, Lin-Tan DT, Chen KH, et al. Repeated pulse of methylprednisolone and cyclophosphamide with continuous dexamethasone therapy for patients with severe paraquat poisoning. Crit Care Med. 2006;34:368–373.
- Eddleston M, Wilks MF, Buckley NA. Prospects for treatment of paraquat-induced lung fibrosis with immunosuppressive drugs and the need for better prediction of outcome: a systematic review. QJM. 2003;96:809–824.
- Scherrmann JM, Houze P, Bismuth C, et al. Prognostic value of plasma and urine paraquat concentration. Hum Toxicol. 1987;6:91–93.
- Wilks MF, Fernando R, Ariyananda PL, et al. Improvement in survival after paraquat ingestion following introduction of a new formulation in Sri Lanka. PLoS Med. 2008;5:e49.
- Knepil J. Measurement of plasma-paraquat concentration. Lancet. 1979;2:699.
- Wunnapuk K, Medley GA, Liu X, et al. Simple and sensitive liquid chromatography-tandem mass spectrometry methods for quantification of paraquat in plasma and urine: application to experimental and clinical toxicological studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:3047–3052.
- Proudfoot AT, Stewart MS, Levitt T, et al. Paraquat poisoning: significance of plasma-paraquat concentrations. Lancet. 1979;314: 330–332.
- Sawada Y, Yamamoto I, Hirokane T, et al. Severity index of paraquat poisoning. Lancet. 1988;1:1333.
- Senarathna L, Eddleston M, Wilks MF, et al. Prediction of outcome after paraquat poisoning by measurement of the plasma paraquat concentration. QJM. 2009;102:251–259.
- Li LR, Sydenham E, Chaudhary B, et al. Glucocorticoid with cyclophosphamide for paraquat-induced lung fibrosis. Cochrane Database Syst Rev. 2014;8:CD008084.
- Perriens JH, Benimadho S, Kiauw IL, et al. High-dose cyclophosphamide and dexamethasone in paraquat poisoning: a prospective study. Hum Exp Toxicol. 1992;11:129–134.
- Ghorbani A, Masoumi K, Forouzan A, etet al. Effect of pulse therapy with glucocorticoids and cyclophosphamide in patients with paraquat poisoning. Hong Kong J Emerg Med. 2015;22:235–240.
- Wu WP, Lai MN, Lin CH, et al. Addition of immunosuppressive treatment to hemoperfusion is associated with improved survival after paraquat poisoning: a nationwide study. PLoS One. 2014;9:e87568.
- Dinis-Oliveira RJ, Duarte JA, Remiao F, et al. Single high dose dexamethasone treatment decreases the pathological score and increases the survival rate of paraquat-intoxicated rats. Toxicology. 2006;227:73–85.
- Yeh ST, Guo HR, Su YS, et al. Protective effects of N-acetylcysteine treatment post-acute paraquat intoxication in rats and in human lung epithelial cells. Toxicology. 2006;223:181–190.
