Abstract
Importance: Acetaminophen toxicity is common and is characterized by hepatic failure. In cases that are not improving with standard medical therapy with N-acetylcysteine, some patients may require hepatic transplant. While there are various criteria to predict patients who might benefit from transplant, the King’s College criteria remain one of the most widely used. However, the King’s College criteria have several limitations and do not incorporate glucose, an important marker of hepatic function.
Objective: The primary objective of this study is to compare the presence of hypoglycemia, coagulopathy, and metabolic acidosis with the King’s College criteria for predicting a composite endpoint of death or transplant.
Design: This study is a retrospective cohort study of adult patients admitted with a discharge diagnosis of acetaminophen-induced liver failure.
Setting: The patients were admitted at one of six university-affiliated teaching hospitals in the United States.
Results: A total of 334 subjects were identified who met inclusion criteria. Fifty-one subjects (15.3%) met the composite endpoint of death or transplant. Ninety-six (28.7%) subjects met the King’s College criteria for transplant. The presence of hypoglycemia increased the odds of reaching the composite endpoint by 3.39-fold. This model performed better than the King’s College criteria (pseudo R2 for the area under the curve of 0.93 vs. 0.20 for the King’s College criteria).
Conclusions: The combination of hypoglycemia, coagulopathy, and lactic acidosis performed better than the King’s College criteria for predicting death or transplant.
Introduction
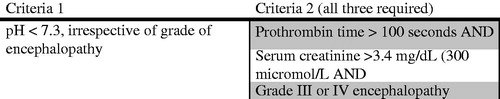
Acetaminophen is one of the most widely consumed over the counter analgesic medications and is one of the most common medications toxicologists encounter in overdose [Citation1]. Toxicity can result in severe liver damage and standard therapy includes N-acetylcysteine (NAC). In patients with acetaminophen-induced acute liver failure who fail medical management with NAC, orthotopic liver transplant is considered as a last resort. One of the most commonly used criteria to predict patients who would benefit from a transplant in acetaminophen-induced hepatic failure are the King’s College criteria [Citation2]. Published in 1989, these criteria incorporate several factors, including pH, renal function, encephalopathy, and coagulopathy () [Citation3]. However, these criteria have limitations [Citation2]. Because the liver serves a critical role in glycolysis and gluconeogenesis, hypoglycemia may develop in patients with hepatic failure. In patients with acutely decompensated cirrhosis, hypoglycemia has been associated with increased mortality [Citation4]. In addition, patients with acetaminophen-induced hepatic failure can develop a lactic acidosis mostly due to impaired lactate clearance, but also due to tissue hypoperfusion and anaerobic respiration [Citation5]. Acutely, patients with massive acetaminophen toxicity may have an acidosis due to accumulation of 5-oxoproline. The development of post-resuscitation acidosis, which is often driven by lactic acid accumulation has been shown to be a strong predictor of mortality in acetaminophen overdose [Citation5]. The implication of hypoglycemia in acetaminophen-induced hepatic failure is not known. This study attempted to compare the presence of hypoglycemia, metabolic acidosis, and coagulopathy with the King’s College criteria for predicting a composite endpoint of death or transplant in patients with acetaminophen-induced hepatic failure.
Methods
This is a retrospective cohort study of all patients with the age of 14 years or older, who were admitted to one of six university-affiliated teaching hospitals throughout the United States, with a discharge diagnosis of acetaminophen-induced hepatic failure. The study was approved by the institutional review board at each of the participating medical centers.
Patient selection
Patients with the age of 14 years or older, who were admitted between 1 January 2008 and 30 June 2013, and had a discharge diagnosis of acetaminophen-induced hepatic failure were included. Patients with ambiguity regarding the etiology of the hepatic dysfunction were excluded. Patients either had a documented toxic serum acetaminophen concentration or a non-detectable acetaminophen concentration in the context of a history of acetaminophen ingestion reported with a time course consistent with hepatic dysfunction. Patients without a clear history of acetaminophen ingestion, including those with an overdose of an unknown agent were excluded. Patients were identified via either with a search of ICD-9 codes (965.0 and 965.4) or pharmacy records for NAC administration. Because many patients received NAC without hepatic dysfunction or for other reasons (e.g., prevention of contrast nephropathy), each record was manually reviewed to confirm eligibility. Similarly, patients identified as having received NAC from pharmacy billing records were not included, unless there is a clear diagnosis of hepatic injury in the record. If an individual had multiple admissions during the study period, only the index hospitalization was included.
Definitions
Hepatic injury was defined as an aspartate transaminase (AST) or an alanine transaminase (ALT) exceeding 1000 IU/L at any point during the index hospitalization. Length of the stay was defined in calendar days, not 24 h periods. This study utilized a composite endpoint of liver transplant or death. The King’s College criteria include a creatinine of 3.4 mg/dL as one of the endpoints. Patients who were started on continuous renal replacement therapy (CRRT) or hemodialysis prior to reach a creatinine of 3.4 mg/dL were considered to have met these criteria, as we assumed that creatinine would likely rise to that level without intervention in that context of acute renal insufficiency. The King’s College “endpoint” for coagulopathy and acidosis were defined as a prothrombin time (PT) > 100 s and a pH of <7.30, respectively. An “elevated” PT or lactic acid was defined as any laboratory value above the hospital’s reference range. Metabolic acidosis was defined as a pH ≤7.30. Hypoglycemia was defined as a venous or capillary glucose <50 mg/dL. In cases of exogenous insulin administration, the hypoglycemic episode must not have been within 3 h of the administration of regular insulin to be considered. For purposes of calculations, any glucose reading of “low” or “less than 5 mg/dL” was interpreted as 0. Similarly, any laboratory parameter that was recorded as “greater than XX” was interpreted as that result (e.g., an AST >60,000 IU/L was interpreted as 60,000 for purposes of calculations).
Data abstraction
Data abstracted from the medical records included age, sex, reason for the ingestion, and initial acetaminophen concentration (number of hours after ingestion that the serum concentration was obtained), along with the presence or absence of baseline medical conditions, including diabetes, hepatitis, alcohol abuse, and pre-existing renal failure. Laboratory parameters recorded included initial and peak AST and ALT, initial and nadir glucose, lactic acid, and the initial and peak PT. Collected treatment interventions included the administration of pharmaceuticals and/or blood products (vitamin K, fresh frozen plasma, and recombinant factor VIIa), and exogenous dextrose administration. The need for transplant, length of stay, and disposition was also recorded.
The data was collected using a standardized abstraction form and entered into a spreadsheet Excell 2000 version 9.0.2720 (Microsoft, Redmond, WA) by one member of the study staff at each center. Ten percent of the charts from different centers were subsequently abstracted and a weighted kappa was performed to assess inter-rater reliability. The abstractors were blinded to the original study hypothesis.
Data analysis
Data analysis was performed using Stata version 14 MP (StataCorp, College Station, TX). Significance was set at p < .05, with confidence intervals reported at the 95% level. For categorical variables, independent associations were assessed via a Chi-squared test and Fisher’s exact test (as appropriate) whereas a Student’s t-test was used to assess continuous variables.
Univariate analysis identified candidates for exploration in logistic regression, using a standard cutoff of p < .20 [Citation6]. Logistic regression with dichotomous endpoints of death or transplant was executed on the cases for which data were available for all covariates. The final logistic regression model included three independent variables. No other covariates were significant, and there was no effect modification or confounding identified when non-significant covariates were reintroduced into the model [Citation6].
Results
A total of 334 unique cases were identified. The median (IQR) age was 38 (27–50), with an overall age range of 14–80. Males accounted for 33%. The median (IQR) acetaminophen concentration was 29 mg/dL (0–100), with the median (IQR) acetaminophen level being obtained at 13.5 (8–34) h. Single acute ingestions accounted for 151 (45.2%) subjects, whereas 61 (18.3%) has multiple, staggered ingestions throughout a 24 h period. Chronic supratherapeutic acetaminophen abuse was noted in 80 (24%) subjects, whereas the pattern of ingestion was unable to be ascertained in 42 (12.6%) subjects.
Medical comorbidities are presented in , and a summary of pertinent laboratory results are presented in . Nineteen patients were listed for transplant; 13 (3.9%) of patients listed received a transplant. In addition, 40 (12%) of subjects died including the two who died after transplant. Fifty-one subjects (15.3%) met the composite endpoint of death or transplant. The median (IQR) length of stay was 7 (4–11) d. Ninety-six (28.7%) of subjects met the King’s College criteria for transplant.
Table 1. Patient characteristics.
Table 2. Laboratory results.
The median (IQR) initial and nadir blood glucose was 113 (89–145) and 79 (68–89) mg/dL, respectively. Hypoglycemia occurred in 29 (8.6%) of subjects. On logistic regression, the presence of hypoglycemia increased the odds of reaching the composite endpoint 3.39-fold (95% CI 1.20–9.6). For every one-point increase in the lactic acid, the odds of reaching the composite endpoint increased 1.23-fold (95% CI 1.14–1.32). For each 1-point increase in the PT, the chance of reaching the composite endpoint increased 3.8% (OR 1.03; 95% CI 1.02–1.06). This three-variable model was associated with an area under the curve on ROC analysis of 0.9277. In contrast, the AUC for the King’s College criteria for predicting the composite endpoint was 0.77.
Interpretation of the odds ratios (ORs) is exampled by the OR for the first covariate for lactate: for every 1-point increase in lactate, the risk of the composite endpoint increased by 23%. Hypoglycemia increased the odds of the endpoint’s occurrence by over three-fold and for every 1-point increase in PT, the chance of the endpoint increased by 4% ().
Table 3. Multivariate logistic regression: factors associated with composite endpoint (death or transplant).
Diagnostic testing demonstrated acceptability of the regression model. The area-under-curve (AUC) assessment for the model (0.93) indicated good model discrimination. The Hosmer–Lemeshow goodness-of-fit test p value (.79) indicated good model fit (also known as calibration).
Not only with the results from evaluation of logistic regression model has become positive, but they were also better than the results of a similar model that was fit using the King’s College criteria. The pseudo-R2 was higher for this model, as was the AUC (0.93 vs. 0.20). The kappa statistic was >0.8 for all variables abstracted.
Discussion
For patients with severe acetaminophen-induced hepatotoxicity, liver transplantation is considered as a standard treatment for those not expected to survive [Citation2]. In this study, the combination of hypoglycemia, lactic acidosis, and elevation of the PT were better predictors of the composite endpoint of death or transplant than the King’s College criteria, which has traditionally been widely regarded as the primary criteria to guide transplant. The use of the King’s College criteria, however, poses several significant challenges. First, the ideal time to institute transplant evaluation is prior to the development of encephalopathy. However, one of the entry criteria of the King’s College criteria is the presence of a grade III or IV hepatic encephalopathy. Waiting for the patients to have this degree of encephalopathy prior to instituting transplant evaluation is suboptimal, as the patient is not able to participate in the psychosocial assessment once encephalopathic. Furthermore, determining the degree of encephalopathy is somewhat subjective. In one study, by the time patients fulfilled the King’s College Criteria, many were felt to be medically unfit for surgery [Citation7]. In addition, external interventions (e.g., hemodialysis) may artificially prevent patients from reaching all of the required end-points in the King’s College criteria. Thus, the use of the King’s College criteria may be suboptimal for many reasons. The modified King’s College criteria do incorporate arterial lactate. This modified criteria has slightly increased sensitivity but decreased specificity compared with the original King’s College criteria [Citation8]. Furthermore, many of the same limitations with the original criteria still are applicable to the modified criteria.
Because of these limitations in both the original and modified King’s College criteria, there has been an interest in determining alternative transplant guidelines. Recently, the sequential organ failure assessment (SOFA) score has been shown to perform better than the APACHE II score or the King’s College criteria as a prognostic score [Citation9]. However, similar to the King’s College criteria, neither the APACHE II score nor the SOFA score includes glucose in their scoring. Hypoglycemia may represent not only impaired storage of glycogen, but impaired ability of the liver to synthesize glucose via gluconeogenesis. Thus, when assessing prognostic implications of the liver following acetaminophen overdose, incorporating glucose concentration is physiologically sound. Furthermore, unlike the King’s College criteria which rely on a subjective determination of the degree of encephalopathy, this model relies on data subject to little interpretation.
In this study, we opted to include a dichotomous cut-off of death or need for this transplant. While some patients that were listed for transplant may have ultimately survived without transplant, we had to assume that all patients that were transplanted would not have survived without the intervention.
This manuscript did not use a dichotomous “cutoff” point for elevation of the PT or lactate. The use of such dichotomous “cutoffs” incurs the risk of significant information loss. This study was not designed to develop a specific clinical application to replace the King’s College Criteria; rather it was to identify potentially useful information to modify the King’s College or create an alternative scoring system. The use of specific cut offs or specific covariates would need to be incorporated on subsequent analysis.
This study was limited by its retrospective design. As a result, the conclusions are limited by the quality and completeness of the data recorded in the medical record. While some data in the medical record (e.g., electrocardiogram interpretation) may be subject to considerable inter-rater reliability [Citation10], this study uses continuous variables (e.g., glucose) and dichotomous outcomes (e.g., transplant or no transplant). These choices likely reduced if not eliminated abstractor bias, thus minimizing some of the limitations of a retrospective review. In addition, patients in whom the etiology of the liver failure was ambiguous were excluded. It is possible that there was variation in excluding patients, which may have resulted in a potential bias study population. The results of this study provide the framework for the design of a prospective study.
Conclusion
In this study, incorporating hypoglycemia, along with coagulopathy and acidosis, more accurately predicts the composite endpoint of death or transplant than does the King’s College criteria. A prospective validation using an external dataset is required.
Disclosure statement
There are no financial, litigational, or other conflicts of interest to disclose.
References
- Farrugia LA, Rhyee SH, Campleman SL, et al. The toxicology investigators consortium case registry – the 2015 experience. J Med Toxicol. 2016;12:224–247.
- Ding GK, Buckley NA. Evidence and consequences of spectrum bias in studies of criteria for liver transplant in paracetamol hepatotoxicity. QJM. 2008;101:723–729.
- O’Grady JG, Alexander GJ, Haylar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445.
- Pfortmueller CA, Wiemann C, Funk GC, et al. Hypoglycemia is associated with increased mortality in patients with acute decompensated liver cirrhosis. J Crit Care. 2014;29:e7–12.
- Shah AD, Wood DM, Dargan PI. Understanding lactic acidosis in paracetamol (acetaminophen) poisoning. Br J Clin Pharmacol. 2011;71:20–28.
- Hosmer D, Lemeshow S, Sturdivant R, Applied logistic regression. 3rd ed. Hoboken (NJ): John Wiley & Sons; 2013.
- Simpson KJ, Bates CM, Henderson NC. The utilization of liver transplantation in the management of acute liver failure: comparison between acetaminophen and non-acetaminophen etiologies. Liver Transpl. 2009;15:600–609.
- Schmidt LE, Larsen FS. Prognostic implications of hyperlactatemia, multiple organ failure, and systemic inflammatory response syndrome in patients with acetaminophen-induced acute liver failure. Crit Care Med. 2006;34:337–423.
- Cholongitas E, Theocharidou E, Vasianopoulou P, et al. Comparison of the sequential organ failure assessment score with the King’s College Hospital criteria and the model for end-stage liver disease score for the prognosis of acetaminophen-induced acute liver failure. Liver Transpl. 2012;18:405–412.
- Gilbert EH, Lowenstein SR, Koziol-McLain J, et al. Chart reviews in emergency medicine research: where are the methods? Ann Emerg Med. 1996;27:305–308.