Abstract
Context: Poison centers (PCs) frequently manage patients with antimuscarinic delirium. However, controversy surrounds the antidotal use of physostigmine for its treatment. The aim of this study was to prospectively investigate physostigmine versus non-antidote therapy for the management of antimuscarinic delirium in a single regional PC.
Methods: This was a prospective observational analysis of patients diagnosed with antimuscarinic delirium and treated in consultation with a regional PC. Certified Specialists in Poison Information (CSPIs) use a clinical guideline to recommend the use of physostigmine. Using a previously derived altered mental status score, we quantified the rate of delirium improvement with physostigmine compared to non-antidote therapy two hours after initial patient identification. We also recorded adverse events (defined a priori as bradycardia, vomiting, seizures) and resource utilization (intubation and physical restraint).
Results: We identified 245 patients and included 154 in the analysis. The most common exposure classes were antihistamines (68%), analgesics (19%), and antipsychotics (19%). CSPIs recommended physostigmine in 81% (125) of cases and the treatment team administered it in 37% (57) of these. We observed delirium control in 79% of patients who received physostigmine versus 36% of those who did not. The odds of delirium control were six times greater for patients receiving physostigmine than for patients treated with non-antidote therapy (OR 6.6). Adverse events were rare and did not differ significantly between the groups. Physostigmine was not associated with changes in the incidence of intubation or restraint.
Conclusions: This study provides further evidence of both the safety and efficacy of physostigmine in the treatment of antimuscarinic delirium.
Introduction
Central antimuscarinic delirium is a commonly encountered constellation of symptoms seen in patients poisoned with medications that antagonize muscarinic receptors in the central nervous system. It has recently been reported that 15–20% of admissions for acute poisoning are due to antimuscarinic delirium [Citation1]. This unique delirium is characterized by carphologia (picking or grasping at inanimate objects), mumbled speech, confusion, and inability to follow commands [Citation1]. Peripheral symptoms of the antimuscarinic toxidrome include tachycardia, dry skin and mucous membranes, skin flushing, mydriasis, and urinary retention. A variety of medication classes (e.g., cyclic antidepressants, many antipsychotics and antihistamines) and plants containing atropine, hyoscyamine, or scopolamine (e.g. Datura stramonium or “jimsonweed”) are also known to cause the antimuscarinic toxidrome [Citation2]. As with aggressive behaviors more broadly, restraints – both physical and chemical – are frequently required [Citation3].
Physostigmine has been known to effectively reverse central antimuscarinic toxicity for over 150 years [Citation4]. The earliest published reported use of physostigmine for antidotal purposes was by Kleinwachter in 1864 [Citation5] to reverse the mydriasis caused by the ingestion of atropine [Citation6]. Almost 100 years later, Forrer and Miller [Citation7] reported the use of physostigmine to reverse antimuscarinic delirium in humans in the setting of atropine-induced comas for the treatment of psychosis. Derived from the Calabar bean (Physostigma venenosum), the drug reached its peak use during the 1960s and 1970s [Citation8], when it was considered not only to be a part of the “coma cocktail” for the treatment of undifferentiated delirium [Citation9], but was also used to treat the cardiovascular and central nervous system effects of tricyclic antidepressants (TCAs) [Citation10–12]. The use of physostigmine declined [Citation5] following a two patient case series published in 1980 by Pentel and Peterson [Citation13] that reported two episodes of asystole temporally associated with physostigmine administration. Despite this, several researchers have continued to investigate the safety and efficacy of the antidote [Citation8,Citation14–17]. Multiple observational studies have demonstrated the safety of physostigmine in a variety of contexts [Citation15–18], but few have compared physostigmine use to non-antidote therapy in a prospective fashion, and none have done so via telephone recommendations from a regional poison center (PC). Therefore, we prospectively compared the use of physostigmine to non-antidotal therapy in the treatment of antimuscarinic delirium. Furthermore, we set out to characterize the impact of physostigmine use on resource utilization (intubation and restraint use) and adverse event rates (vomiting, bradycardia, seizures).
Methods
Study design
This was a prospective observational cohort study completed between 1 June 2016 and 31 May 2017 at a single regional PC. The governing Human Subjects Research Committee approved the study and waived the requirement for informed consent due to the non-interventional nature of the study.
Study setting
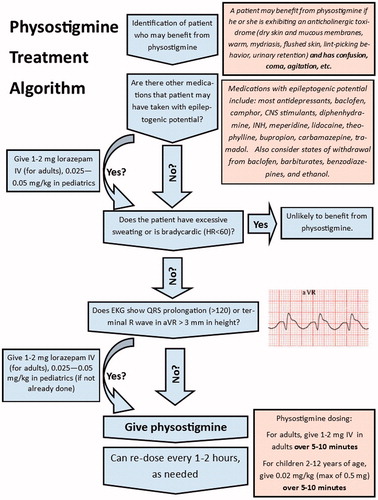
We carried out the study at an American Association of Poison Control Centers (AAPCC) accredited regional PC covering three US states. In 2016, the study PC handled 64,468 calls; 58,838 of these were exposure calls. Pharmacists, who are AAPCC-certified specialists in poison information (CSPIs), almost exclusively manage all PC calls. Calls are also occasionally answered by medical toxicology fellows. Board-certified medical toxicologists are available for consultation 24 hours a day and fully support medical toxicology fellows and CSPIs. The PC routinely recommends physostigmine for central antimuscarinic delirium based upon the clinical assessment of the managing CSPI, and prior to the involvement of on-call medical toxicologists. Recommendations to administer or withhold physostigmine are based on clinical guidelines developed by the PC and available to the CSPI and receiving hospital for reference. displays a portion of these guidelines.
Selection of participants
We considered all calls received by the PC during the study period potentially eligible for study inclusion. All patients that the CSPI or the treating (calling) provider determined were exhibiting symptoms and objective signs of antimuscarinic delirium that would benefit from medication for symptom control were included in the study. We excluded patients who had already received physostigmine for this exposure, suffered a seizure, or were intubated prior to the first call to the poison center. We applied no other a priori exclusion criteria.
Study protocol and variables
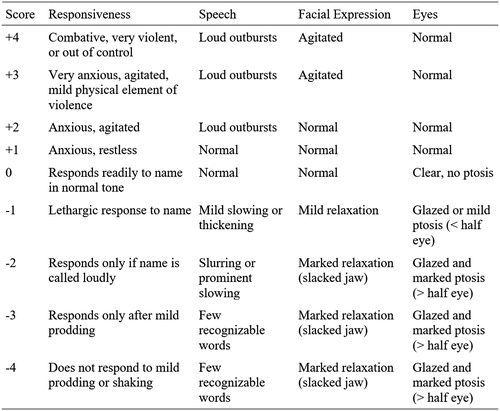
During the initial call, CSPIs collected baseline clinical data including age, gender, current vital signs, whether the patient had a history of a seizure disorder, and what therapies the treating team administered prior to the call. The CSPI then ascertained if the patient had a seizure, had been restrained, or had been intubated prior to the call. The CSPI recorded reported toxic exposure, dose, route, and time of ingestion, when available. All CSPIs were trained by the primary investigator in the altered mental status score (AMSS), a previously derived score used to characterize the severity of altered mental status in the emergency department that ranges from –4 (coma) to +4 (severe agitation) [Citation19]. The CSPI determined an initial AMSS at the time of the call based on the patient’s symptoms at that time (). The score is determined based on the patient’s speech, responsiveness, facial expression, and eyes. CSPIs determined the AMSS using a standardized flowsheet based on the patient details that would be most amenable to obtain over the phone. If patients responded readily to name and had normal speech, the CSPI assigned an AMSS of zero. If they were somnolent, the flowsheet directed the CSPI to the negative numbers on the scale. If they were not, they were directed to the positive numbers. From there, a best fit among the remaining levels was determined. We excluded patients if the AMSS was not calculated at the time of the initial phone call or if the score was not quantifiable retrospectively based on detailed CSPI case notes. CSPIs ultimately recommended delirium treatment based on the PC guidelines. The CSPI recorded whether they recommended the administration of physostigmine. The AMSS was analyzed as in previous research using this score [Citation19].
Allowing for the unpredictable nature of the PC call volume as well as availability of the bedside provider, a CSPI made a follow-up call as close to two hours after the initial call as feasible. If the CSPI did not complete a follow-up call within three hours of the initial call, the patient was excluded from the analysis. Updated vital signs, AMSS, and details of physostigmine administration (yes/no, when given, and dose) were collected, as was information characterizing adverse events (an episode of seizure activity, bradycardia, or vomiting) and resource intensive interventions (intubation or restraint). We defined bradycardia as a heart rate of less than 60. The CSPI did not recommend any PC protocol deviations regarding the treatment of anticholinergic delirium as a part of this study.
The CSPI recorded the data directly into the free text area of the PC case management software (Toxicall®, version 4.7.37, 1999-2013, Computer Automation Systems, Inc., Aurora, CO), and abstracted by the principal investigator (SPB) into an Excel spreadsheet (Microsoft Excel® 2011, Microsoft Excel, Redmond, WA).
Outcomes
The primary outcome was delirium control at the two-hour call-back. We defined delirium control as an absolute change in the AMSS of one point or greater toward a score of zero. For example, if a patient’s AMSS improved from –3 to –1, the absolute change in AMSS was 2, and delirium was considered controlled. Secondary outcomes included bedside provider’s subjective reported delirium control at the two-hour call back (“Did the physostigmine improve the delirium?”), the rate of resource utilization (restraint and intubation), and adverse events (bradycardia, vomiting, and seizures).
Statistical analysis
We used descriptive statistics to characterize the cohort including median age and heart rate for each of the two treatment groups, differentiated by the administration of physostigmine. Welch’s t-test was used to determine if age and heart rate differed significantly between the groups.
We then investigated the association of delirium control at two hours (presence/absence) with administration of physostigmine (presence/absence) by calculating an odds ratio using a 2x2 contingency table and determining statistical significance with a Fisher’s exact test. Then, we investigated the association between administration of physostigmine and adverse events (presence/absence of any episode of bradycardia, vomiting, or seizure), and the association between administration of physostigmine and intubation or restraint (presence/absence) in the same way. We completed the statistical analysis using R statistical software (R Core Team (2017), version 3.4.1. Vienna, Austria) [Citation20].
Results
Characteristics of study patients
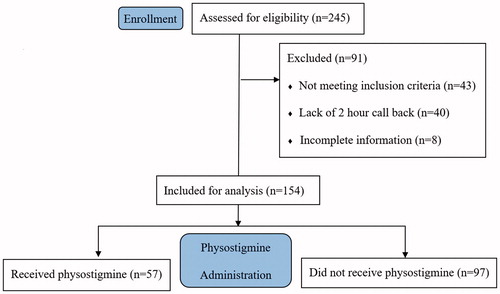
We identified a total of 245 cases during the study period and 154 patients were included in the final analysis (). Of the 91 cases excluded, 43 did not require medication per the CSPI, 40 lacked a two-hour call back, and eight had incomplete information. CSPIs recommended physostigmine in 125 cases (81%); 57 (37%) patients were given physostigmine, while 97 (63%) were not. There was no statistically significant difference in the median ages of the groups, which were 27 (IQR 18–36) and 25 (IQR 17–38) for the physostigmine and non-antidote groups, respectively. We collected the dose of physostigmine in a small minority of cases; where doses were recorded, we found a median administered dose of 2 mg. The treating providers gave benzodiazepines alone for 55 patients (56%) in the non-antidote group, with 35 (36%) of patients receiving no chemical sedation. The remaining sedative medications documented in the non-antidote group included propofol (n = 10), haloperidol (n = 8), olanzapine (n = 4), dexmedetomidine (n = 3), diphenhydramine (n = 2), ketamine (n = 1), ziprasidone (n = 1), fentanyl (n = 1), and pentobarbital (n = 1). The median heart rate across both groups was 120 (IQR 110–135), with no statistically significant differences across groups (). The most common substances involved were antihistamines (68%), analgesics (19%), antipsychotics (19%), and ethanol (13%) (). Most of ingestions involved multiple substances (55%). There were no statistically significant differences observed between the two groups with respect to the distribution of exposure substances.
Table 1. Baseline age, gender, and median heart rate.
Table 2. Substances involved.
Main results
The CSPI observed delirium control in 45 of the 57 patients given physostigmine (79%, 95% CI 68%-–90%) versus 35 of 97 patients not given physostigmine (36%, 95% CI 26%–46%). Results were similar when considering bedside providers’ reports of qualitative delirium control, with 79% (95% CI 68%–90%) versus 25% (95% CI 16%–33%) reporting delirium control for the physostigmine and the non-antidote groups, respectively (). We determined that the odds of attaining delirium control were 6.6 times higher for patients given physostigmine compared to those who were not.
Table 3. Delirium control at two-hour call back.
We found few adverse events in both groups (). No seizures occurred in either group. Vomiting occurred three times in the physostigmine group and once in the non-antidote group. We observed bradycardia a single time, in the non-antidote group. Among those given physostigmine, three patients were physically restrained, and two patients were intubated. In the non-antidote group, seven patients were restrained and five were intubated. None of these differences reached statistical significance. Using Fisher’s exact test, physostigmine was not associated with increased incidence of adverse events (p = .36). Additionally, physostigmine administration was not associated with a decreased rate of intubation or physical restraint (slope: z = −0.50, p = .78). When we analyzed bradycardia, vomiting, intubation, and physical restraint in patients that the CSPI recommended physostigmine but did not receive it, there similarly was no difference between groups.
Table 4. Adverse events and intubation/restraint at two-hour call back.
Discussion
In this prospective observational study, we confirmed previously reported findings [Citation8,Citation14–17] that physostigmine is effective in treating antimuscarinic delirium. Patients with antimuscarinic delirium that the CSPI recognized as one who would benefit from intervention and who were given physostigmine had improved delirium more often both by AMSS as well as by subjective report at two hours than those who were not, with an odds ratio of greater than 6. The delirium control of 79% among patients treated with physostigmine reported, in this study, is similar to the reported delirium control in several previous studies done in different clinical settings. In an inpatient toxicology service, Burns et al. [Citation14] retrospectively compared physostigmine versus benzodiazepine use for the treatment of antimuscarinic delirium in 52 patients and found delirium was controlled in 87% of patients. Rasimas et al. [Citation16] also assessed delirium control in antimuscarinic patients in an ambispective manner. This large study analyzed 1197 patients administered physostigmine by a large toxicology inpatient service. A total of 868 patients, analyzed retrospectively, received physostigmine over a period of 6 years; 81% had improvement in delirium. Prospectively, they documented another 329 patients who received physostigmine over a single year and saw clinical improvement in 74% of these. In a retrospective chart review at a single hospital, Nguyen et al. [Citation18] reported CNS improvement following physostigmine in 87% of 57 antimuscarinic patients who were presented to a single large tertiary center. Finally, in a retrospective study of patients with clinical antimuscarinic delirium given physostigmine at a single poison center, Arens et al. [Citation17] reported improvement or resolution in delirium in 73% of 191 patients after a single dose of physostigmine.
Previously reported frequencies of adverse events related to physostigmine [Citation8,Citation14,Citation16–18] are similar to the present study. Despite including patients without regard to QRS interval or presence of co-ingested sodium channel antagonists in our study, there were no episodes of wide complex tachycardia or bradycardia in the physostigmine group. Rather, the only recorded study episode of bradycardia was in the non-antidote group. These findings resemble those of Rasimas et al. [Citation16], who found one arrhythmia each in the prospective and retrospective arms of their study, both thought not to be directly related to the administration of physostigmine. Similarly, Burns [Citation14] reported one episode of bradycardia, while Arens et al. [Citation17] and Nguyen et al. [Citation18] reported no arrhythmias related to physostigmine administration in their cohorts.
Further, there were relatively few episodes of other adverse events (vomiting and seizures) in the physostigmine group of this study. Vomiting occurred three times in the physostigmine group, and a single time in the non-antidote group; we documented no seizures in either group. This finding of relatively few adverse events is similar to the previously mentioned studies that looked at similar outcomes, which reported adverse event rates of 1.1% in Nyugen’s study [Citation18], 0.9% in Rasimas’ retrospective observational study [Citation16], and 1% in the Burns’ study. In the Burns’ study, the adverse event rate for the benzodiazepine group was significantly higher at 15% [Citation14]. We did not find a significant difference in adverse events between the two groups in our study.
We attempted to determine whether delirium control translated to a decrease in resource utilization – namely, were fewer patients intubated or physically restrained. Our study was not powered to determine a difference between these outcomes as the treating providers intubated or restrained relatively few patients in either group. Previously, it had been suggested that the use of physostigmine may reduce resource utilization [Citation8,Citation14,Citation16,Citation21,Citation22]. Burns found that physostigmine was associated with a lower rate of intubation (1%) compared to benzodiazepine treatment (27%). Watkins found a significant decrease in intubation between patients who received physostigmine only and those who did not, of 1.9% vs. 8.4%, respectively. To our knowledge, no study has directly evaluated the effect of physostigmine on physical restraint use. Although we evaluated this parameter, we found no statistically significant difference between the groups. Although a larger sample size may result in a statistically significant difference between the groups, our findings suggest that decreases in resource utilization due to physostigmine use are minimal.
Despite similarities to previous studies, our study adds to the literature in several ways. To our knowledge, this is the first study to prospectively observe delirium control and adverse events related to the treatment of antimuscarinic delirium, regardless of physostigmine use. Further, we prospectively used the PC to evaluate the treatment of antimuscarinic delirium. Although Arens and Nguyen used a poison center sample, both were retrospective chart reviews that did not attempt to quantify delirium using an altered mental status score and systematic CSPI documentation.
Perhaps the most important and novel finding of our study is that CSPIs following a poison center-specific physostigmine guideline can safely recommend the antidote without the direct approval from a medical or clinical toxicologist. We demonstrated that while physostigmine is not without risks, it has a similar risk profile to other interventions that CSPIs routinely recommend that are reported to have high complication rates (such as n-acetylcysteine, with a reported 14–18% risk of anaphylactoid reactions, and 7% risk of vomiting [Citation23]) but in regular clinical practice, likely have much lower rates of complications.
Limitations
The application of this study is limited by its uncontrolled and observational nature. We included all patients who met the inclusion and exclusion criteria, and no subsequent randomization to physostigmine or standard care was undertaken. Randomization might otherwise address important differences in the study groups identified in our observational study. Of note, baseline AMSS scoring was higher in the group that received physostigmine, indicating that a significant difference in terms of severity was evident between the groups. This may have biased the results to favor the delirium control in the physostigmine group (given the definition of delirium control as an absolute improvement in the score towards zero). Similarly, the size of the cohort studied is an additional limitation. With adverse event rates in both groups relatively low, a larger study will be needed to identify a true difference, if one exists.
The determination of whether a patient had antimuscarinic delirium is also a limitation of the study. Although CSPIs are trained in recognizing symptoms associated with central antimuscarinic delirium, the literature contains no specific tests or other validated criteria to determine whether the toxidrome exists. This may bias the results, as physostigmine can improve other delirium in the context of other toxidromes given its analeptic property.
Our reliance on the AMSS to determine delirium severity is an additional limitation. Although the AMSS has been validated for the use of quantifying altered mental status in the emergency department, it primarily focuses on agitation due to decompensated mental illness, alcohol intoxication, or stimulant-class drug intoxication, rather than symptoms specific to antimuscarinic delirium. For example, a severely antimuscarinic patient may be relatively calm and quiet in their bed, but may exhibit severe carphologia, remove lines and tubes, and try to get out of bed – all of which require significant, resource-intensive interventions. To address this, we asked the bedside providers for a subjective measure of delirium improvement. Using this subjective measure, we saw even better effectiveness with physostigmine compared to non-antidote care than we did using the AMSS-derived score. In our study, there were five patients who had no change in their AMSS, but improved by the completely subjective determination (four in the physostigmine group, and one in the non-antidote group). Using the definition of delirium control that used AMSS alone would therefore bias our results toward favoring the non-antidote arm. This is because these patients were most frequently given benzodiazepines alone, a medication class likely to reduce the AMSS but leave the underlying cause of delirium untreated. Further, we relied on provider-reported information for the purpose of our study, which is prone to biases as discussed in prior publications [Citation24].
Given the setting of our study, a PC staffed by mostly pharmacists acting relatively autonomously, applying our findings to other PC settings may be imprudent at this time. Our findings result largely from exposure to antimuscarinic medications; they may not be generalizable to settings in which most antimuscarinic exposures are from plants, rather than medications.
Finally, many patients did not receive physostigmine despite the CSPI recommendation. An additional limitation of the study is that we did not ask or formally determine why the treating physician did not administer physostigmine. Presumably, it was related to unfamiliarity or discomfort with the medication. Alternatively, it could have been related to preexisting patient factors that we did not assess in the study such as QRS prolongation, history of reaction to physostigmine, or unavailability of the drug at the treating institution.
Conclusions
Our results suggest that physostigmine is superior to non-antidote care in the control of antimuscarinic delirium when recognized by CSPIs and recommended for use. Adverse effects related to physostigmine (including bradycardia, vomiting, seizures) were few and similar to those seen in patients treated by other means. We found no difference in resource utilization (intubation or physical restraint) between the two groups in our sample. Finally, we showed that CSPIs may safely and independently recommend physostigmine for the treatment of antimuscarinic delirium.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Dawson AH, Buckley NA. Pharmacological management of anticholinergic delirium – theory, evidence and practice. Br J Clin Pharmacol. 2016;81:516–524.
- Curry S, O’Connor A, Graeme K, et al. Neurotransmitters and neuromodulators. In: Hoffman R, Howland MA, Lewin N, Nelson L, Goldfrank L, editors. Goldfrank’s toxicologic emergencies. 10th ed. New York: McGraw-Hill; 2015. p. 176.
- Zerbo E, Kondracke A, Psychiatric principles. In: Hoffman R, Howland MA, Lewin N, Nelson L, Goldfrank L, editors. Goldfrank’s toxicologic emergencies. 10th ed. New York: McGraw-Hill; 2015. p. 350–351.
- Dale H. Chemical transmission of the effects of nerve impulses. Br Med J. 1934;1:835–841.
- Kleinwachter. Beobachtung uber die Wirkung des Calabar-Extracts gegen Atropin-vergiftung. Berliner Klin Wochenschrift. 1864;1:369–371.
- Nickalls RWD, Nickalls EA. The first use of physostigmine in the treatment of atropine poisoning: a translation of Kleinwachter’s paper entitled “Observations on the effect of Calabar bean extract as an antidote to atropine poisoning.” Anaesthesia. 1988;43:776–777.
- Forrer GR, Miller JJ. Atropine coma: a somatic therapy in psychiatry. Am J Psychiatry. 1958;115:455–458.
- Watkins JW, Schwarz ES, Arroyo-Plasencia AM, et al. The use of physostigmine by toxicologists in anticholinergic toxicity. J Med Toxicol. 2015;11:179–184.
- Hoffman RS, Goldfrank LR. The poisoned patient with altered consciousness. Controversies in the use of a “coma cocktail.” J Am Med Assoc. 1995;274:562–569.
- Munoz RA. Treatment of tricyclic intoxication. Am J Psychiatry. 1976;133:1085–1087.
- Walker WE, Levy RC, Hanenson IB. Physostigmine – its use and abuse. JACEP. 1976;5:436–439.
- Rumack BH. Anticholinergic poisoning: treatment with physostigmine. Pediatrics 1973;52:449–451.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9:588–590.
- Burns MJ, Linden CH, Graudins A, et al. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35:374–381.
- Schneir AB, Offerman SR, Ly BT, et al. Complications of diagnostic physostigmine administration to emergency department patients. Ann Emerg Med. 2003;42:14–19.
- Rasimas JJ, Ph D, Sachdeva KK, et al. Revival of an antidote: bedside experience with physostigmine. J Am Assoc Emerg Psychiatry. 2014;12:5–24.
- Arens AM, Shah K, Al-Abri S, et al. Safety and effectiveness of physostigmine: a 10-year retrospective review. Clin Toxicol. 2017;56:1–7.
- Nguyen TT, Armengol C, Wilhoite G, et al. Adverse events from physostigmine: an observational study. Am J Emerg Med. 2018;36:141–142.
- Martel M, Sterzinger A, Miner J, et al. Management of acute undifferentiated agitation in the emergency department: a randomized double-blind trial of droperidol, ziprasidone, and midazolam. Acad Emerg Med. 2005;12:1167–1172.
- R Development Core Team R. R software. Environ Stat Comput. 2017. Available from: https://www.R-project.org/
- Stellpflug S, Cole J, Isaacson B, et al. Massive atropine eye drop ingestion treated with high-dose physostigmine to avoid intubation. West J Emerg Med. 2012;13:77–79.
- Cole JB, Stellpflug SJ, Ellsworth H, et al. Reversal of quetiapine-induced altered mental status with physostigmine: a case series. Am J Emerg Med. 2012;30:950–953.
- Hendrickson R, Howland MA, n-Acetylcysteine. In: Hoffman R, Howland MA, Lewin N, Nelson L, Goldfrank L, editors. Goldfrank’s toxicologic emergencies. 10th ed. New York: McGraw-Hill; 2015. p. 465–72.
- Hoffman RS. Understanding the limitations of retrospective analyses of poison center data. Clin Toxicol (Phila). 2007;45:943–945.