Abstract
Introduction: γ-Hydroxybutyric acid is an endogenous substance, a therapeutic agent, and a recreational drug of abuse. This psychoactive substance acts as a depressant of the central nervous system and is commonly encountered in clinical and forensic practice, including impaired drivers, poisoned patients, and drug-related intoxication deaths.
Objective: The aim of this review is to assist clinical and forensic practitioners with the interpretation of γ-hydroxybutyric acid concentrations in blood, urine, and alternative biological specimens from living and deceased persons.
Methods: The information sources used to prepare this review were PubMed, Scopus, and Web-of-Science. These databases were searched using keywords γ-hydroxybutyrate (GHB), blood, urine, alternative specimens, non-conventional biological matrices, saliva, oral fluid, sweat, hair, vitreous humor (VH), brain, cerebrospinal fluid (CSF), dried blood spots (DBS), breast milk, and various combinations thereof. The resulting 4228 references were screened to exclude duplicates, which left 1980 articles for further consideration. These publications were carefully evaluated by taking into account the main aims of the review and 143 scientific papers were considered relevant.
Analytical methods: The analytical methods used to determine γ-hydroxybutyric acid in blood and other biological specimens make use of gas- or liquid-chromatography coupled to mass spectrometry. These hyphenated techniques are accurate, precise, and specific for their intended purposes and the lower limit of quantitation in blood and other specimens is 0.5 mg/L or less.
Human pharmacokinetics: GHB is rapidly absorbed from the gut and distributes into the total body water compartment. Only a small fraction of the dose (1–2%) is excreted unchanged in the urine. The plasma elimination half-life of γ-hydroxybutyric acid is short, being only about 0.5–0.9 h, which requires timely sampling of blood and other biological specimens for clinical and forensic analysis.
Endogenous concentrations of GHB in blood: GHB is both an endogenous metabolite and a drug of abuse, which complicates interpretation of the laboratory results of analysis. Moreover, the concentrations of GHB in blood and other specimens tend to increase after sampling, especially in autopsy cases. This requires the use of practical “cut-off” concentrations to avoid reporting false positive results. These cut-offs are different for different biological specimen types.
Concentrations of GHB in clinical and forensic practice: As a recreational drug GHB is predominantly used by young males (94%) with a mean age of 27.1 years. The mean (median) and range of concentrations in blood from apprehended drivers was 90 mg/L (82 mg/L) and 8–600 mg/L, respectively. The concentration distributions in blood taken from living and deceased persons overlapped, although the mean (median) and range of concentrations were higher in intoxication deaths; 640 mg/L (280 mg/L) and 30–9200 mg/L, respectively.
Analysis of GHB in alternative specimens: All biological fluids and tissue containing water are suitable for the analysis of GHB. Examples of alternative specimens discussed in this review are CSF, saliva, hair strands, breast milk, DBS, VH, and brain tissue.
Conclusions: Body fluids for the analysis of GHB must be obtained as quickly as possible after a poisoned patient is admitted to hospital or after a person is arrested for a drug-related crime to enhance chances of detecting the drug. The sampling of urine lengthens the window of detection by 3–4 h compared with blood samples, but with longer delays between last intake of GHB and obtaining specimens, hair strands, and/or nails might be the only option. In postmortem toxicology, the concentrations of drugs tend to be more stable in bladder urine, VH, and CSF compared with blood, because these sampling sites are protected from the spread of bacteria from the gut. Accordingly, the relationship between blood and urine concentrations of GHB furnishes useful information when drug intoxication deaths are investigated.
Introduction
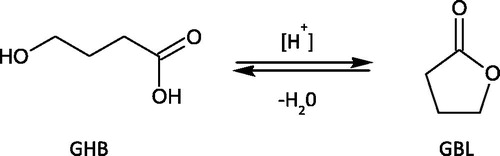
γ-Hydroxybutyric acid (4-hydroxybutyric acid, GHB) is a straight chain carboxylic acid comprised of four carbon atoms and a single hydroxyl (–OH) group bonded to C-4 (). Its molecular weight is 104.1 g/mol. The acid (–COOH) and alcohol (–OH) functional groups in close proximity mean that GHB can undergo intramolecular esterification. In aqueous solution, depending on time, temperature, and pH, GHB is converted to the corresponding cyclic ester (γ-butyrlolactone, GBL), as depicted in . Accordingly, at low pH (acid catalyzed) the cyclization is favored and GHB is dehydrogenated to yield GBL. In basic media (high pH) the reverse reaction occurs and GBL is hydrolyzed back to GHB [Citation1].
GHB and its analogs hit the headlines in the early 1980s when they were first used as recreational drugs of abuse and were culpable in a number of drug overdose deaths [Citation2]. Earlier GHB was investigated as a possible general anesthetic [Citation3,Citation4], but was rejected for clinical purposes owing to its lack of an analgesic effect and the fact that some patients vomited after treatment [Citation5]. Shortly afterward, GHB could be purchased from health food stores and was touted as a dietary supplement for use by body builders to stimulate the growth hormone and increase muscle mass (anabolic effect) [Citation6,Citation7].
According to the UK Misuse of Drugs Act, GHB is classified as a controlled substance (Class C), making it a crime to possess, manufacture or supply the drug. The precursor chemical GBL is also a scheduled substance belonging to Class C, whereas 1,4-butanediol (1,4-BD) is not prohibited. Both GBL and 1,4-BD are rapidly and effectively converted to GHB in the body and are therefore subject to abuse [Citation8–11]. There is an ongoing diversion of these organic solvents from the legal to the illegal drug market [Citation12–14].
Small recreational doses of GHB produce feelings of euphoria with loss of inhibitions, whereas large acute doses have more dramatic effects on a person’s performance and behavior. People become dizzy and drowsy, they experience nausea and vomiting, and often become unconscious and require emergency hospital treatment [Citation10]. Furthermore, people intoxicated by GHB suffer from anterograde amnesia, which is one of the reasons this drug is used by perpetrators in sexual assault cases [Citation15–19]. The difference between a recreational dose of GHB and a toxic or lethal dose is fairly narrow, which probably accounts for many accidental overdose deaths involving this drug [Citation20–22].
GHB has been implicated in many types of forensic cases including sexual assaults (date rape) [Citation23,Citation24], in the context of rave-parties and “chemsex” [Citation25], driving under the influence of drugs [Citation26,Citation27], and in drug overdose deaths and murder [Citation28,Citation29].
The sodium salt of GHB (sodium 4-hydroxybutyrate), generic name sodium oxybate, has been developed and registered as a pharmaceutical product. This medication is sold under the trade name Xyrem® when prescribed for treating people with a sleep disorder, such as narcolepsy [Citation30,Citation31] and the proprietary name is Alcover® when used to alleviate alcohol withdrawal syndrome and treat alcohol dependence [Citation32,Citation33].
Objective
The aim of this review is to assist clinical and forensic practitioners in interpreting GHB concentrations in blood, urine, and alternative biological specimens. Relevant aspects of the chemistry and pharmacology of GHB are covered, along with information about absorption, distribution, metabolism, and excretion patterns in humans.
Methods
Relevant scientific articles were identified by searching PubMed, Scopus, and Web-of-Science up to May 2018 using the keywords: γ-hydroxybutyrate, GHB, blood, urine, alternative specimens, non-conventional biological matrices, oral fluid, saliva, hair, vitreous humor (VH), brain, cerebrospinal fluid (CSF), breast milk, and dried blood spots (DBS).
The main keywords γ-hydroxybutyrate and GHB were searched individually and also in association with the other keywords listed. In addition, various institutional and government websites dealing with abused drugs were scrutinized. The resulting 4228 references were screened and duplicates were excluded, which left 1980 articles for further consideration.
All publications were carefully evaluated by taking into account the main aims of the review, namely interpreting GHB concentrations in biological specimens for clinical and forensic purposes. Articles in languages other than English were not considered. We were mainly interested in (1) human studies; (2) original research articles, single case reports, and case series; (3) papers classified as reviews, mini-reviews, letters-to-the-editor, and commentaries; and (4) documents and guidelines promulgated by scientific societies and international organizations. This left 143 scientific papers, distributed as 85 original research articles, 32 single case reports and case studies, 20 reviews and/or mini-reviews, 4 letters-to-the-editor, and 2 documents published on institutional websites.
Analytical methods
The analytical methods available for the determination of GHB in biological specimens are sufficiently reliable for their intended purpose and are not considered further in this review [Citation34]. Most laboratories utilize chromatographic procedures based on gas chromatography (GC) with flame ionization detector (FID) or GC coupled to mass spectrometry (MS) or liquid chromatography (LC) procedures. These hyphenated techniques of GC-FID, GC-MS, GC-MS/MS, or LC-MS/MS are highly sensitive and specific for quantification analysis of GHB in a wide range of biological specimens including blood, saliva, urine, hair strands, etc [Citation34–36].
The GHB concentrations observed in peripheral blood after recreational use of the drug are often in the range 80–200 mg/L in living subjects and between 80 and 1000 mg/L in drug-related deaths [Citation21,Citation37]. The lower limit of quantitation (LLOQ) of GHB by use of these sensitive and specific analytical techniques is often 0.5 mg/L or less, depending on the method used.
Human pharmacokinetics
GHB has a short plasma elimination half-life as verified in a recent randomized double-blind cross-over study [Citation38]. The mean half-life was 36 ± 9 min (± SD) after 20 mg/kg and 39 ± 7 min after a dose of 35 mg/kg. The range of half-lives for both doses of GHB was 25–58 min (N = 32 male subjects), which calls for timely sampling of biological specimens in clinical and forensic medicine when drug-related crimes and/or victims of sexual assault are investigated.
The pharmacokinetics (PK) of GHB has been well studied in both healthy volunteers and in various patient groups treated with sodium oxybate. For safety reasons, the doses of GHB administered usually range from 20 to 100 mg/kg body weight [Citation39–42]. The study by Abanades et al. [Citation43] was particularly useful because four different doses of GHB (40, 50, 60, and 72 mg/kg) were administered to eight healthy volunteers. The main PK parameters derived from their study are summarized in .
Table 1. Pharmacokinetic parameters of GHB in plasma from a double-blind randomized cross-over study involving eight male volunteers, who received sodium oxybate in the doses indicated. The results are (mean ± SD) from the study by Abanades et al. [Citation43].
After oral administration, GHB is rapidly absorbed from the gut reaching peak concentrations in plasma (Cmax) by 0.6–0.9 h postdosing (). The mean Cmax increased with increasing GHB dose from 79.1 mg/L (40 mg/kg) to 130.1 mg/L (72 mg/kg). As expected, area under the curve (AUC) also increased with dose from 106.5 mg/L × h (40 mg/kg) to 301.1 mg/L × h (72 mg/kg). The mean plasma elimination half-life of GHB was short being only 0.57–0.73 h (34–44 min) and was independent of the dose of GHB.
Only about 1–2% of the administered dose was excreted unchanged in the urine, which was collected at intervals for up to 6 h postdosing (). The low urinary excretion of unchanged GHB confirmed earlier work by Ferrara et al. [Citation41], who administered 25 mg/kg GHB every 12 h to alcohol-dependent patients and recovered ∼1% of the dose in the urine.
After low therapeutic doses of GHB, the concentration–time profiles are best described by first-order kinetics, whereas after abuse doses of the drug capacity-limited (zero-order) kinetics seems more appropriate, because the metabolizing enzymes are seemingly saturated at high substrate concentrations [Citation39,Citation44,Citation45].
shows the PK profiles of GHB derived from a controlled dosing study involving 18 men and 18 women, each of whom received 4.5 g GHB as sodium oxybate [Citation45]. The left graph in is drawn on a linear scale and the right plot after making a semi-log transformation. The shape of the concentration–time profiles of GHB was not significantly different for the male and female subjects. When the kinetics of elimination were assumed to be first-order (right plot), the mean plasma elimination half-life was ∼39 min (0.65 h) and this was independent of gender. Evaluation of the same data using a zero-order kinetic model (left plot) gave an elimination rate constant (k0) of 18 mg/L/h on average [Citation44].
Figure 2. Pharmacokinetic profiles of GHB in plasma from a controlled dosing study with 18 men and 18 women. The subjects received an oral dose of 4.5 g GHB as sodium oxybate [Citation45]. The left plot shows concentration-time data plotted on a linear scale (zero-order elimination rate 18 mg/L/h) and the right plot is a semi-logarithmic scale (elimination half-life 39 min). The graph was re-drawn from reference [Citation44].
![Figure 2. Pharmacokinetic profiles of GHB in plasma from a controlled dosing study with 18 men and 18 women. The subjects received an oral dose of 4.5 g GHB as sodium oxybate [Citation45]. The left plot shows concentration-time data plotted on a linear scale (zero-order elimination rate 18 mg/L/h) and the right plot is a semi-logarithmic scale (elimination half-life 39 min). The graph was re-drawn from reference [Citation44].](/cms/asset/8a68aa36-1b10-4626-82c1-947a7eb683dd/ictx_a_1519194_f0002_b.jpg)
Assuming that the mean plasma half-life of GHB is 30–45 min, a simple calculation shows that after 180–270 min (six half-lives) the drug is more or less cleared from the bloodstream. Accordingly, the concentrations of GHB remaining in the blood by 3–4.5 h postdosing cannot be distinguished from endogenous levels, which range from 1 to 5 mg/L according to various studies.
After intake, GHB distributes into the total body water (TBW) compartment and there is no evidence of any binding to plasma proteins. The distribution volume (Vd) of GHB falls within the range expected (0.4–0.6 L/kg) for water-soluble drugs. However, the concentration–time profiles in blood sometimes suggest a two-compartment model with an initial Vd of 0.40 L/kg (first compartment) increasing to 0.60 L/kg (second compartment) after complete absorption and distribution in all body fluids and tissues. GHB is a drug that shows capacity-limited metabolism and the Michaelis–Menten equation can be used to characterize the plasma elimination kinetics [Citation44].
Endogenous concentrations of GHB in blood
When interpreting the concentrations of GHB determined in blood and other body fluids it is essential to consider the dual nature of this substance, endogenous metabolite and recreational drug of abuse. Low concentrations of GHB (<0.5–5 mg/L) are measurable in different biological specimen types even without any prior exposure to the drug. These endogenous concentrations do not seem to depend on gender, ethnicity, or the medication a person was taking at the time [Citation46–50]. Certain food stuff and alcoholic beverages contain small amounts of GHB, although these are diluted with the TBW, which represents 50–60% of body weight, so these sources of the drug do not pose any danger to public health [Citation51–53].
Human blood contains ∼80% w/w water and has a density of 1.055 g/mL in healthy individuals with normal hematocrit value. The expected distribution ratio of GHB between plasma (92% w/w water) and whole blood (80% w/w water) is 1.15:1 based on differences in water content (92/80 = 1.15) of the specimens [Citation54].
Endogenous concentrations of GHB tend to be higher in autopsy blood compared with blood drawn from living subjects, and this should be considered when toxicological results are interpreted [Citation55]. In decomposed bodies, a few studies have reported GHB concentration exceeding 100 mg/L when central (heart) blood was the specimen analyzed [Citation56,Citation57]. The higher GHB concentrations in central compared with peripheral blood could have several explanations. The condition of the body, ongoing putrefaction processes, is obviously important as is the addition of a fluoride preservative to the blood sample [Citation58]. The mean endogenous concentration of GHB in charred cadavers (4.53 mg/L) was lower than in noncharred bodies (11.7 mg/L), and denaturation of enzymes by heat was suggested to account for this difference [Citation55]. The role of PM redistribution from tissues, such as the liver, to the blood circulation has not been thoroughly investigated for GHB. However, in one study endogenous GHB concentrations were not significantly different for cardiac blood (4.0 mg/L) and femoral venous blood (4.6 mg/L) when postmortem interval was 48 h or less [Citation59].
Practical cut-off concentrations
Because of the endogenous nature of GHB, a good laboratory practice requires that toxicologists adopt an analytical “cut-off” concentration above which positive results are reported. This is a safeguard to avoid reporting false positive results and accusations of GHB use or abuse [Citation60]. These analytical cut-offs are higher than the highest endogenous concentration and are different for different biological specimen types.
reports endogenous concentrations of GHB in different biological matrices for antemortem and postmortem specimens [Citation48,Citation61]. These values were derived from a review of the literature and our own experience from routine forensic casework. Also, shows the analytical cut-off concentrations recommended before positive GHB results are reported [Citation62]. Note that the “cut-off” concentrations are higher than the highest endogenous concentration listed for the various biological specimen types analyzed [Citation63].
Table 2. Comparison of endogenous concentrations of GHB in antemortem (AM) and postmortem (PM) biological specimens and suggested practical cut-off concentrations to use when analytical results are interpreted.
The situation with autopsy specimens is more complicated and it is necessary to consider the blood-sampling site, (cardiac or femoral vein), the postmortem interval, any evidence of decomposition of the body as well as addition of fluoride as enzyme inhibitor. Finding that the concentration of GHB in femoral venous blood is just above the 30 mg/L threshold (2) might be consistent with GHB intake during life but this conclusion is augmented with elevated levels found in an alternative specimen, such as urine, VH or CSF [Citation56]. GHB concentrations seem to increase in body fluids after death, especially in central cardiac blood, which might necessitate a higher analytical cut-off for this type of specimen (see the footnote of ). In bodies that are badly decomposed considerable caution is warranted before positive blood GHB concentrations are reported [Citation57].
When judging whether a positive GHB result correctly reflects exogenous exposure or intake of the drug as opposed to being a postmortem artifact, one needs to consider all available information in the case including the age and gender of the deceased, the cause and manner of death, and other circumstances such as medical records and history of substance abuse. The analytical cut-offs in depend on the endogenous concentrations of GHB for the various biological specimen types. The higher the endogenous concentration the higher the cut-offs, which are also higher for postmortem specimens compared with antemortem specimens.
Concentrations of GHB in clinical and forensic practice
Apprehended drivers
reports the age, gender, and concentrations of GHB in blood samples from people arrested in Sweden for driving under the influence of drugs [Citation27]. The average GHB concentration in male traffic offenders was 90 mg/L, which was not significantly different from females with a mean concentration 99 mg/L (p > 0.05). However, the prevalence of male traffic offenders was appreciably higher than females (94% vs 6%, p < 0.001), although there was no statistically significant gender difference in their mean age (p > 0.05).
Table 3. Summary statistics of age, gender and GHB concentrations in venous blood from people arrested in Sweden for driving under the influence of drugs [Citation27].
When interpreting GHB concentrations in traffic offenders, it is important to consider that on average 30–90 min (mean 60 min) elapses after a driver is arrested and before a blood sample is taken. In injured drivers, the time delay could be much longer. The short elimination half-life of GHB makes it obvious that the blood concentrations were appreciably higher at the time of the driving compared with the time of sampling blood for toxicological analysis [Citation27].
Sexual assault cases
Little information is available about GHB concentrations in blood from victims of sexual assault or date rape, because of the drug’s rapid clearance from the bloodstream, e.g. after surreptitious administration [Citation64]. In many such cases, a considerable time elapses before victims contact the police authorities so that arrangements can be made for a medical examination and sampling of blood and urine for toxicological analysis [Citation65]. During this time delay, the concentration of GHB in blood often decreases reaching endogenous levels, because of the drug’s short elimination half-life. Forensic evidence of a drug-related crime is often lacking and analytical reports in such cases often conclude that GHB was not detected [Citation66,Citation67].
Another problem with investigating the surreptitious administration of GHB is that victims suffer from anterograde amnesia, which makes it difficult to remember exactly what happened to them, except that they suddenly became incapacitated and then engaged in a nonconsensual sexual act [Citation25,Citation68].
Drug-related deaths
summarizes the concentrations of GHB determined in femoral blood in 51 deaths that forensic pathologists attributed to being a drug intoxication or some other cause, such traffic crash or sharp or blunt force trauma (N = 20). The mean age of victims was not significantly different in relation to cause of death (p > 0.05), although the median GHB concentration in blood was considerably higher in the intoxication deaths (280 mg/L vs. 72 mg/L, p < 0.001).
Table 4. Summary statistics comparing postmortem concentrations of GHB in femoral blood when death was attributed to intoxication or other causes, such as trauma. Note that GHB was not necessarily the only drug present in these drug-related deaths [Citation21].
shows the concentrations of GHB in femoral blood and bladder urine in a case series of drug intoxication deaths compared with other causes of death, such as trauma. The mean age of victims was close to 30 years regardless of the cause of death (p > 0.05). However, the mean and (median) concentrations of GHB in blood were significantly higher (p < 0.001) in the intoxication deaths 637 mg/L (260 mg/L) compared with other causes 73 mg/L (52 mg/L). Furthermore, both the mean and median urinary concentrations of GHB were higher than the concentrations in femoral venous blood regardless of the cause of death (p < 0.001).
Table 5. Comparison of GHB concentrations in femoral blood and bladder urine in deaths attributed by medical examiners to drug intoxication or other causes of death, such as trauma [Citation69].
The relative frequency distributions of GHB concentrations in blood samples from living subjects, such as impaired drivers, and GHB-related deaths show a significant overlap. In apprehended drivers, the GHB concentrations in blood ranged from the analytical cut-off (e.g. 5 mg/L) up to 600 mg/L [Citation27]. In autopsy cases, the mean and median concentrations of GHB in femoral blood were higher in drug intoxication (poisoning) deaths (range 30–9200 mg/L) compared with other causes of death (range 30–210 mg/L).
Analysis of GHB in alternative specimens
Toxicological analysis of drugs in alternative or less conventional biological specimens has emerged as an important area of clinical and forensic toxicology [Citation69]. Identification of a drug or toxin in more than one type of specimen or matrix is a recommended practice because the quality of autopsy blood samples can vary widely, which might compromise the results. Furthermore, the identification of a drug in different types of biological fluids gives more compelling evidence that a person was exposed to that substance or had ingested the drug during life. In clinical situations collecting hair strands, saliva, sweat, or nail clippings is considered less invasive compared with sampling venous blood.
Cerebrospinal fluid
CSF is the clear watery liquid that surrounds the brain and spinal cord of humans and animals. CSF is mostly water (95–99%) and its biochemical composition is similar to that of plasma or interstitial fluid [Citation70]. CSF is usually obtained by lumbar puncture (spinal tap) in living patients, whereas during autopsy, the sample is taken from the cisterna magna (cisterna CSF). The total volume of CSF available in adults is ∼135 mL and because this is a relatively clean biological fluid it is ideal for forensic drug analysis [Citation71,Citation72]. The sampling and analysis of CSF are useful whenever a dead body is decomposed, because CSF is more protected from the spread of bacteria from the gut.
Moriya and Hashimoto [Citation59] investigated the presence of endogenous GHB in CSF in nine autopsy cases when the postmortem interval was less than 48 h. The GHB concentrations ranged from undetected (less than 0.5 mg/L) to 4.6 mg/L (mean 1.8 mg/L, median 1.9 mg/L. The mean GHB concentration was significantly lower (p < 0.01) in CSF compared with femoral blood from the same autopsy case. The authors concluded that CSF was a useful alternative biological matrix for analysis of GHB and that 10 mg/L was an appropriate analytical cut-off concentration for reporting positive cases, the same as that used with urine.
More recently, Andresen-Streichert et al. [Citation62] measured endogenous GHB in different biological specimens (heart blood, peripheral blood, urine, CSF, and VH) in 64 autopsies when the deceased had no prior history of GHB exposure during life. In 52 cases, an autopsy was performed between 1 and 11 days after death, and the concentrations of GHB in CSF ranged from <0.6–24.0 mg/L (mean 4.2, median 3.2 mg/L). In four cases, the concentration exceeded the 10 mg/L cut-off proposed earlier by Moriya and Hashimoto. The GHB concentrations in blood in these cases were 10.3 mg/L, 10.4 mg/L, and 10.8 mg/L, and one was as high as 24 mg/L.
Tittarelli et al. [Citation73] determined GHB in samples of CSF from three men treated with sodium oxybate for type 1 narcolepsy (NT1). The dose regime was either 2.5 g, 3.0 g, or 7.0 g given in two daily half-doses at 10.30 pm and 2.30 am. The GHB concentrations in samples of CSF taken 6 h after the second half dose were 5.62 mg/L (2.5 g dose), 6.1 mg/L (3.0 g), and 17.7 mg/L (7.0 g), respectively. These concentrations can be compared with a range from <0.5 mg/L to 0.55 mg/L in CSF from drug-free control subjects The relatively low concentrations of GHB in CSF from patients treated with Xyrem® can be explained by metabolism occurring over the 6 h interval between drug intake and sampling of CSF. In the same study with NT1 patients, the glucuronide metabolite of GHB (GHB-Gluc) was determined in CFS and compared with a drug-free control group. The concentrations of GHB metabolite were not significantly different between patients receiving sodium oxybate and the controls [Citation73].
Busardò et al. [Citation35] measured GHB concentrations in CSF in three narcoleptic patients on daily doses of sodium oxybate given as two half doses. Six hours after the second half dose lumber CSF contained 21.07 mg/L, 17.74 mg/L, and 5.4 mg/L of GHB when the patients had taken 9 g, 9 g, and 5 g of sodium oxybate, respectively. In the same study, GHB-Gluc concentrations in CSF agreed well with values reported earlier [Citation73].
Saliva
Oral fluid or saliva has received considerable attention as an alternative specimen for the analysis of drugs in both clinical and forensic toxicology [Citation74,Citation75]. Saliva is mainly composed of water (99%) and is produced by three pairs of glands, the submandibular, the sublingual, and the parotid glands [Citation76]. Specimens for analysis can be produced by tongue and lip movements and then spat or ejected into a small glass vial, which is made airtight. Several milliliters of oral fluid can be produced in this way although also available are specially designed sampling devices, such as a cotton wool swab and spatula [Citation77]. Some people, such as those taking anticholinergic drugs, suffer from a dry mouth and might have difficulty producing the required volume of oral fluid for analysis. The sampling of saliva is simplified and more standardized when a commercially available device is used, because these also indicate when the required volume of sample is collected [Citation78].
Only the nonprotein bound free fraction of a drug in plasma can enter the saliva, which is relevant to consider when concentrations of drugs determined in saliva are compared with concentrations in plasma [Citation79]. Factors influencing salivary pH might be important to consider for some drugs, depending on their pKa and other factors [Citation80].
In 120 individuals (75 females and 45 males), mean, median, and range of endogenous GHB concentrations in oral fluid were 1.29 mg/L, 1.13 mg/L, and 0.15–3.33 mg/L, respectively [Citation46]. Statistical analysis using the Mann-Whitney test indicated that GHB concentrations in saliva neither depended on the donor’s age, gender, medical condition, and use of medications nor recent intake of food or drink. This study concluded that saliva was a viable biological specimen for analysis of GHB and in some situations might be more suitable than blood, such as at remote locations or epidemiological surveys of impaired driving. However, the authors did not draw any conclusion regarding the analytical cut-off concentration that differentiates endogenous levels in saliva from exogenous intake.
After administration of GHB, the concentrations in saliva decreased at about the same rate as in blood indicating similar PK profiles, so the use of saliva as an alternative matrix does not extend the detection window for GHB [Citation81]. Furthermore, the saliva/blood concentration ratios of GHB were mostly less than unity, which implies an overall lower salivary GHB concentration.
Abanades et al. [Citation82] were among the first to analyze GHB in saliva and reported a mean (± SD) predosing (endogenous) concentration of 0.12 ± 0.02 mg/L. After administration of a single oral dose of 50 mg/kg GHB as sodium oxybate the concentrations of GHB in saliva peaked at 0.5 h postdosing (Cmax range, 11.6–49.0 mg/L) decreasing to reach 0.5 mg/L by 6 h. The concentration–time course of GHB in oral fluid followed closely that of plasma, except that the concentrations in saliva were roughly 33% lower and saliva-to-plasma ratios were always less than unity (1.0). Brenneisen et al. [Citation40] also investigated PK of GHB in plasma, saliva, and urine in eight subjects after a low dose corresponding to 25 mg/g body weight. In contrast to the other studies cited the saliva showed a higher peak concentration of GHB at 10–15 min postdosing compared with blood concentrations.
The available evidence verifies that saliva is a viable matrix for analysis of GHB but does not seem to offer any advantage over blood in terms of extending the window of detection [Citation81,Citation82]. However, obtaining samples of saliva is less invasive than drawing blood, which makes this fluid more convenient for off-site testing, such as drug screening in various populations, such as motorists [Citation83,Citation84]. Despite more water in saliva (99%) compared with whole blood (80%) the endogenous GHB concentrations were mostly lower in saliva [Citation46].
Vitreous humor
VH is commonly used in postmortem toxicology for analysis of ethanol and other abused drugs, such as 6-acetylmorphine [Citation85,Citation86]. VH is a useful matrix because it is isolated within the eye globe, the retina, and the lens, which makes it less prone to putrefaction process and spread of bacterial from the gut [Citation87]. Hence VH is considered a promising matrix for toxicological analysis of many drugs. Moreover, a suitable specimen of VH can be obtained with syringe and needle without the need to perform a complete autopsy [Citation88,Citation89]. VH mainly consists of water and a few milliliters can be obtained from each eyeball and then pooled to give a larger volume of specimen for toxicological analysis.
Kintz et al. [Citation56] were among the first to determine GHB in VH and compare the concentrations with other postmortem specimens, including cardiac blood, femoral blood, and bile. Concentrations determined in VH were always above 50 mg/L when GHB was incriminated in drug-related deaths. Endogenous concentrations of GHB in vitreous were always much lower than 50 mg/L, which was therefore considered a practical cut-off concentration to distinguish endogenous GHB in VH from ingestion of GHB during life [Citation56].
Elliot [Citation63] determined the endogenous concentrations of GHB in VH and reported a range of 1–3 mg/L in fatalities that were unrelated to prior usage or exposure to GHB during life. Marinetti et al. [Citation90] investigated the effect of different storage conditions on concentrations of GHB in VH from 26 autopsies and reported values less than 5 mg/L. One case had a concentration of 7 mg/L, which might, therefore, be a more appropriate concentration to differentiate antemortem ingestion from postmortem synthesis.
Castro et al. [Citation91] reviewed the endogenous production of GHB in postmortem toxicology and also advocated use of VH as a matrix for analysis, because various PM artifacts were less of a problem compared with blood samples [Citation91].
Further evidence supporting analysis of vitreous is exemplified by a police investigation of a GHB-related death in Italy [Citation92]. A woman was thought to have been a victim of sexual assault and samples of peripheral blood and VH were taken at the crime scene and again 34 h later when a forensic autopsy was performed. Over this time period, the concentration of GHB in peripheral blood increased by 66.3% compared with an 8.1% increase in VH. This finding might suggest that GHB underwent PM redistribution in blood, considering the rise in concentration observed. An alternative explanation is that GHB in blood increased over the 34 h time period between death and autopsy, but this artifact is less of a problem for VH. Whatever the reason, the concentrations of GHB in VH are more stable than in blood, which supports the use of vitreous as a biological specimen in PM toxicology.
Busardò et al. [Citation93] investigated the stability of endogenous GHB in VH from 22 deaths using samples taken at the crime scene and later when the forensic autopsy was performed. The concentration of endogenous GHB in VH showed only a small increase in concentration from 2.5 mg/L to 3.0 mg/L (geometric mean values), whereas the concentrations in peripheral blood increased from 3.6 mg/L to 7.4 mg/L.
Andresen-Streichert et al. [Citation62] measured endogenous GHB concentrations in VH samples from 54 deaths when GHB exposure during life could be ruled out. The PMI ranged from 1 to 11 days and the concentrations of GHB ranged from <0.6 to 39.0 mg/L (mean 9.6 mg/L, median. 7.5 mg/L). The concentrations of GHB in VH in this study were somewhat higher than in previous studies, although neither PMI nor signs of putrefaction correlated with endogenous concentrations of GHB.
Breast milk
The passage of drugs from the circulating blood into breast milk is important to consider in neonatal care, because nursing mothers treated with various drugs might also feed their infant [Citation94]. Water-soluble drugs, such as ethanol and GHB, easily pass from the bloodstream into the milk of lactating women [Citation95]. The concentrations determined in milk are roughly the same as in the bloodstream, which are continuously decreasing through metabolism. The concentrations of drugs in breast milk are very low and if ingested are further diluted with TBW [Citation96].
The concern about GHB and breastfeeding arose in connection with a nursing mother being treated with Xyrem® for narcolepsy, and who wanted to breast-feed her infant. This medication, which is the sodium salt of GHB, distributes into body fluids and tissues including milk of lactating women. Breast milk is composed mostly of water (∼90%) so GHB easily passes from the bloodstream into the milk. The PK profiles of GHB in blood and breast milk are similar in terms of maximum concentration reached and time necessary for metabolism and clearance of the drug [Citation97].
Busardò et al. [Citation98] determined GHB in breast milk collected from a 32-year-old female suffering from narcolepsy and being medicated with sodium oxybate (4.5 g twice daily). The concentrations of endogenous GHB were determined in breast milk collected from 20 healthy breastfeeding mothers not being treated with drugs. Using a validated GC–MS method of analysis, the endogenous concentration of GHB in milk ranged from 0.13 to 1.03 mg/L (mean 0.36 mg/L). These concentrations were significantly lower than in breast milk form women treated with sodium oxybate, which was 23.2 mg/L at 1 h postdosing. At 5 h postdosing, the GHB concentration in breast milk was 0.99 mg/L and not significantly different from the endogenous level in the control group of women. As a general guideline, it was proposed that nursing mothers should wait at least 5 h after taking Xyrem® before breastfeeding their infant. However, this recommendation was based on a single case so further studies are needed to confirm this suggestion.
A study by Barker et al. [Citation99] determined the concentration of GHB in breast milk collected from two women treated with sodium oxybate. In one patient, samples of breast milk were collected for two consecutive nights after 3 g and 4.5 g of sodium oxybate twice nightly. In the other patient, 2.25 g and 3 g of the drug were taken twice nightly. Morning samples of breast milk were collected from both women and these were analyzed by GC-MS. The concentrations of GHB were 2–5 times higher than endogenous concentrations and remained higher for up to 4 h afterward. The authors recommended that first morning milk should not be used in a baby feed, but if this was discarded subsequent milk production was considered safe. A weakness of the study by Barker et al. [Citation99] was the lack of a control group of breastfeeding women in which endogenous GHB concentrations were determined. This was commented upon in a letter-to-the-editor of the journal where the article was published [Citation98].
Brain tissue
GHB easily passes the blood–brain barrier (BBB) and is distributed throughout the brain tissue where it acts as a depressant drug exerting its effects via the GABAB receptor complex, although other mechanisms, such as a specific GHB receptor, have been postulated [Citation100]. Use of brain tissue for analysis of drugs of abuse seems to be undergoing a renaissance and attention has been given to GHB [Citation101,Citation102]. The brain is protected by the skull and is therefore considered less prone to microbial contamination unless the body is severely decomposed and putrefied [Citation21]. The analysis of GHB in brain tissue was suggested as a practical alternative to the analysis of blood samples and that the concentrations in brain might be less prone to artifact formation [Citation102,Citation103].
A study from 1978 determined endogenous GHB in human brain in seven deaths unrelated to exposure or abuse of the drug during life [Citation104]. The endogenous concentrations ranged from below analytical detection limit up to 2.1 mg/kg for different brain regions. The highest concentration (0.8 mg/kg) was found in the frontal cortex [Citation22]. A study by Ferrara et al. [Citation105] reported the first case of GHB determination in brain tissue in an overdose death involving heroin. The brain tissue analyzed contained GHB and morphine concentrations of 40.0 mg/kg and 0.43 mg/kg brain, respectively.
In a drug-related death, Kalasinsky et al. [Citation106] reported 221 mg/kg GHB in brain tissue from the frontal cortex, which was significantly higher than concentrations previously reported. Since GHB was not detected in hair strands, chronic use of the drug could be excluded. The victim died from an acute fatal overdose of GHB and a general toxicology screening for other drugs was negative, which confirms that GHB was culpable in the death. Mazarr-Proo and Kerrigan [Citation107] quantified GHB in brain tissue and reported a concentration of 102 mg/kg in a 35-year-old male recreational drug user. Since high concentrations of GHB were also detected in other biological fluids and matrices, the cause of death was attributed to GHB intoxication, and the manner of death was accidental.
Mehling et al. [Citation20] found a GHB concentration of 110 mg/kg in brain tissue from a 6-year-old girl, who was sexually assaulted and later died. By exclusion of other causes of death, the fatality was attributed to GHB intoxication and the assailant was charged with murder.
A 38-year-old man was found dead and near to the body was an empty plastic bottle marked “glue remover.” Inspection of the label showed it contained 99.9% GBL, a precursor of GHB. The concentration of GHB in brain was 826 mg/kg, which is good evidence of a massive ingestion of glue remover, probably in a suicide attempt or accidental overdose with GHB [Citation28].
In a re-appraisal of brain samples in forensic toxicology, Thomsen et al. [Citation102] proposed a GHB cut-off concentration of 10 mg/kg, which they felt would distinguish endogenous levels from exogenous intake. The GHB concentrations in brain tissue from three fatalities were 12.7 mg/kg, 78.4 mg/kg, and 166 mg/kg. Sampling and analysis of brain were considered less prone to artifacts caused by decomposition. Forensic practitioners from Denmark have published a series of articles advocating the use of brain tissue for analysis of many licit and illicit drugs, including GHB. They consider that sampling brain offers certain advantages when blood is unavailable or when, for various reasons, the results might be compromised [Citation102]. Brain tissue is certainly an option for toxicological analysis, but this specimen is not widely used in most laboratories, probably because reference concentrations for most drugs have not yet been established [Citation108].
In adults the brain weighs about 1.1–1.5 kg, although drugs are not necessarily evenly distributed throughout the tissue, depending on water and lipid solubility of the active substance and site and mechanism of action in the brain [Citation109]. Many drugs act at specific receptor sites and accumulate in these particular brain regions. GHB is a small uncharged molecule that easily passes the BBB penetrating cell membranes and interacting with various receptor sites (e.g. GABA-B) [Citation110].
Hair
Since the early 1990s, forensic practitioners have utilized hair strands as a biological matrix for the analysis of therapeutic agents and drugs of abuse [Citation111,Citation112]. After intake most drugs enter the growing hair follicles, depending on the dose taken and the frequency of administration [Citation113]. The hair color and its melanin content might play some role for uptake of drugs and deserves consideration when results are interpreted. Likewise, the rate of hair growth can differ between individuals depending on the person’s age, ethnicity, and gender [Citation114,Citation115]. Furthermore, care is needed with decontaminated (washing) procedures, which are crucial for removing drug residues, which might be deposited from the environment or form use of various shampoos and/or coloring agents, which can add to or lower the concentrations of drugs in hair [Citation116]. Many of the problems and pitfalls to consider when results of drug analysis in hair strands are interpreted were the subject of a comprehensive review by Cuypers and Flanagan [Citation113].
Hair analysis is the ideal biological matrix to document a single past exposure to GHB in cases where there is a long delay between drug intake or administration and when conventional fluids and tissue give negative results [Citation117]. Assuming that hair grows at an average rate of 1 cm per month (range 0.7–1.4 cm), this makes it possible to backtrack to the time when an alleged attack or assault took place. Segmenting the hair shaft into 0.5 cm portions or less (down to 0.3 cm), and the use of sensitive analytical methods can disclose the presence of exogenous GHB in hair [Citation118–120].
Owing to the dual nature of GHB, endogenous metabolite and recreational drug of abuse, major efforts have been exerted to establish endogenous concentrations in hair to serve as baseline levels [Citation121]. Studies have shown that GHB concentrations in hair vary in relation to hair color and ethnicity and are usually in the range 0–5 ng/mg [Citation121]. The first segment of hair closest to the scalp should not be used in the analysis of GHB, because of a possible contamination with sweat, which can elevate endogenous concentrations in hair up to 20 ng/mg hair [Citation122].
To simplify the interpretation of GHB analysis in hair strands, it was suggested that each subject serves as his or her own control [Citation120]. The guidelines issued by United Nations Office on Drugs and Crime (UNODC), recommends that if the concentration of GHB in a hair segment is 10 times higher than in another segment, then this is compelling evidence of exogenous intake or surreptitious administration of this drug. However, a difference of 10 to 1 may be too conservative, because there is some evidence to support a lower ratio, such as 3:1 as evidence of GHB intake from hair analysis [Citation118].
Hair testing is a useful tool in PM toxicology to verify antemortem exposure to GHB, because hair is less prone to PM artifacts, such as those associated with decomposition and diffusion [Citation123]. The concentrations of GHB in hair strands are therefore more stable and less likely to increase in relation to PM interval, and other factors, such as autolysis/putrefaction processes [Citation124].
The phase 2 metabolite of GHB (GHB-Gluc) was first verified in hair strands by Petersen et al. [Citation125]. This prompted further studies of GHB-Gluc in blood, hair, and other specimens in the hope that it might extended the window of detection of the drug. This line of research was analogous with studies of ethyl glucuronide as a biomarker of drinking alcoholic beverages.
Wang et al. [Citation117] used UPLC-MS/MS to study the incorporation of GHB and GHB-Gluc into hair from two recreational users of GHB and ten control subjects not previously exposed to the drug. In the control group, GHB-Gluc ranged from < LOQ (0.48 ng/mg) to 1.2 ng/mg, whereas in the two GHB users, GHB-Gluc was only slightly elevated in one of then (1.7–3.1 ng/mg) and was below LOQ in the other. These results raise concern about the utility of analyzing GHB-Gluc in hair and more research is needed on this subject.
Busardò et al. [Citation35] developed a method for analysis of GHB, GHB-Gluc, GBL and GABA in hair by UHPLC-MS/MS. A 10 cm hair sample was collected from a 45-year-old man treated with sodium oxybate for 6 months. The sample of hair was cut into 10 segments of 1 cm each and GHB-Gluc was detected in all segments ranging in concentration from 0.32 to 0.86 ng/mg. The GHB-Gluc content of hair did not depend on which segment was analyzed nor whether the mean for the first six segments was compared with the last four. In the same study, GHB concentration were in the range 7.35–9.23 ng/mg in the first six segments and decreased to between 0.89 and 2.89 ng/mg in the remaining four segments.
More recently, Mehling et al. [Citation126] determined both GHB and GHB-Gluc concentrations in hair strands from three narcoleptic patients treated with sodium oxybate. The parent drug and glucuronide metabolite and their ratio were in the same range as in the two previously published studies.
The evidence available indicates that analysis of GHB-Gluc does not provide any additional diagnostic information compared with analysis of GHB in blood, plasma, or hair [Citation126,Citation127]. This phase II metabolite of GHB is interesting, but less useful as a biomarker for recent drug intake compared with ethyl glucuronide, the corresponding metabolite of ethanol, which is widely used as a biomarker of recent drinking [Citation128,Citation129]. The sulfate metabolite of GHB is another phase II metabolite, but this pathway has not been as well studied as the GHB-Gluc pathway [Citation130].
The procedures established for analysis and interpretation of GHB in hair is the same when dealing with AM and PM specimens [Citation121]. Several studies confirm a fairly broad range of endogenous GHB concentrations in hair strands, ranging from 0 to 12 ng/mg [Citation131]. This probably reflects differences in wash frequency, hygiene, and treatment regimes, such as the type of shampoo, colouring, and/or bleaching agents used and decontamination methods [Citation113].
For these reasons, it is difficult to propose a practical and reliable cut-off concentration for GHB in a single hair strand as compelling evidence of exogenous intake or surreptitious administration in sexual assault cases [Citation122]. The best recommendation so far is to divide the hair shaft into ∼1 cm segments, analyze each of them, and then compare and contrast the results for each segment [Citation119]. In this way, hair from the same individual acts as a control and a sudden increase in GHB concentration in one or more of the hair segments compared with those in close proximity speaks towards exposure or administration of the drug at an earlier time.
Dried blood spots
The use of DBS as an alternative biological matrix for analysis of drugs like GHB has been reported in a few publications [Citation132,Citation133]. DBS are a practical medium for analysis of a wide range of licit and illicit drugs. The concentrations of GHB measured in DBS agreed well with those in venous blood samples with an analytical bias of only −2.8%, according to a Bland–Altman plot [Citation134].
Urine
Drugs and their metabolites in urine are usually at higher concentrations than in blood or plasma, which makes urine a good specimen for making an initial broad screening analysis [Citation87,Citation135]. Formed in the kidneys, the urine is an ultrafiltrate of the blood and healthy individuals produce ∼1 mL urine per min, which is collected and stored in the bladder until voided. The urge to void usually occurs after a volume of ∼300–400 mL is collected. Urine is essentially water (95–99%) with a specific gravity of 1.003–1.004 along with metabolic waste products, such as urea from nitrogen and amino acid metabolism and creatinine, which is a byproduct of muscle metabolism. Indeed, the analysis of creatinine in urine serves as a biomarker to identify dilute specimens. A creatinine content of less than 200 mg/L is used as a threshold to identify dilute specimens and a value below 50 mg/L is considered evidence of manipulation or substitution, such as by dilution with water or another liquid [Citation136,Citation137].
Many studies verify that GHB is a normal constituent of urine produced by healthy individuals and the concentration is mostly in the range of 0.5–2 mg/L, although values as high as 5 mg/L have been reported [Citation138,Citation139]. In a study of endogenous GHB concentrations in random urine voids from 670 healthy individuals, the concentrations ranged from 0.34 to 5.7 mg/L (median 3.0 mg/L) [Citation58]. Another study analyzed GHB in urine from 1126 healthy female students and found concentrations ranging from below LLOQ of the method to 5.5 mg/L (mean 0.84 mg/L. The median concentration was 0.68 mg/L) [Citation140]. These concentrations did not seem to depend on urinary pH, the donor’s body mass index, racial origin or use of medication. Only a weak positive correlation (R2 = 0.3) was found between endogenous GHB concentration and specific gravity of the urine sample. In another study of 50 urine samples from healthy female volunteers, the average endogenous GHB concentration was 1.46 mg/L, which led to the suggestion of using 5 mg/L as a practical cut-off concentration for use in forensic casework [Citation138].
Endogenous concentrations of GHB in urine ranged from 0.17 to 3.03 mg/L (N = 20) in diabetics and these were not significantly different from healthy individuals; range 0.16–2.14 mg/L (N = 30). In pregnancy, the mean urinary GHB concentration was 0.36 mg/L (N = 66) compared with 0.24 mg/L (N = 69) in nonpregnant women [Citation47]. Urinary GHB does not seem to depend on a person’s age, gender, smoking, and/or drinking habits and therefore a cut-off of 10 mg/L was considered appropriate to distinguish endogenous synthesis from exogenous intake [Citation50]. However, in postmortem toxicology, this cut-off concentration should be set higher, and several groups of investigators suggest a cut-off of 30 mg/L (2).
Endogenous concentrations of GHB were determined in urine from 207 drug-free individuals by a GC-MS method and the results ranged from below LLOQ up to 2.7 mg/L (median 0.24 mg/L) [Citation141]. The mean was 0.27 mg/L (range 0–2.7 mg/L) in males (N = 130) compared with a mean of 0.29 mg/L (range 0–0.98 mg/L) in females (N = 77). In another study of people aged 6–59 years (N = 55) the median concentration of endogenous GHB in urine was 1.3 mg/L (mean 1.65 mg/L) and the range was from 0.9 to 3.5 mg/L) [Citation142].
The tubes used to collect urine samples for shipment to the laboratory should contain sodium fluoride (1–2% w/v) as a preservative and enzyme inhibitor [Citation143]. The presence of fluoride is important and helps to stabilize GHB concentrations, such as whenever there is a long delay between voiding and performing the analysis. For long-term storage, the urine specimens should be stored frozen [Citation144].
When urine samples with sodium fluoride as a preservative were stored at room temperature for 12 months, the mean and median endogenous concentrations of GHB were 1.8 mg/L and 1.6 mg/L, respectively. The urinary concentration was less than 5 mg/L in 95% of cases (highest concentration 7 mg/L). These results lend further support to use of 10 mg/L as a suitable cut-off concentration for urine voided by living subjects [Citation145].
After administration of GHB, the concentration–time course in urine is similar to that in blood, except that the curves are shifted in time and urine concentrations are higher [Citation39,Citation146]. The absorption of GHB into the blood and excretion into the urine takes a certain time and as this occurs the concentration of GHB in blood is changing rapidly during the absorption phase. For about the first 60 min after intake, the concentrations in blood are initially higher than urinary GHB, whereas at all later times blood GHB is lower than urinary GHB for the entire postabsorptive phase [Citation39]. The difference in concentration between blood and urine GHB is partly explained by differences in water content between specimens blood (80%) and urine (100%), which suggest a theoretical urine/blood ratio for GHB of 1.25:1 [Citation146].
Comparing GHB concentrations in urine and blood furnishes useful information in postmortem toxicology, because the metabolism and clearance of drugs takes place in the liver, whereas there is no evidence that GHB is metabolized in the urinary bladder. Furthermore, GHB concentrations in urine, VH and CSF tend to be more stable than in blood as shown in several studies [Citation59,Citation147].
The close similarity in absorption, distribution, metabolism, and excretion patterns of ethanol and GHB have been discussed elsewhere [Citation2,Citation148]. The concentration in urine should reflect the concentration in blood at a mid-point in time since the previous void. In many forensic cases, GHB might be reported as “not detected” in a blood sample (below cut-off), whereas a high concentration was present in bladder urine. This proves the person was exposed to GHB during life, but a long survival time has meant that blood concentrations have dropped under the analytical cut-off used for reporting positive results.
Obtaining a positive urine drug test result gives evidence of prior intake or exposure to that substance, although neither the dose administered nor the time of last intake can be deduced from the drug concentration in urine. Both the parent drug and its metabolites are usually at higher concentrations in urine compared to blood, which lengthens the window of detection by several hours, depending on the type of drug analyzed. For some drugs (e.g. acetone, ethanol, methanol, and GHB), the concentration determined in urine can give a hint about the concentration in blood at an earlier time, such as during the time that urine was formed in the kidney and stored in the bladder [Citation149,Citation150].
Conclusions
This review amplifies current knowledge about the concentrations of GHB in different types of biological specimen, both endogenous levels and after recreational use or surreptitious administration of the drug. Body fluids for the analysis of GHB must be obtained as quickly as possible after a poisoned patient is admitted to hospital or after a person is arrested for a drug-related crime because of the short plasma elimination half-life and rapid clearance from the blood circulation.
Sampling urine lengthens the window of detection of GHB by 3–4 h compared with blood, but with longer delays between last intake of the drug and obtaining specimens for analysis, hair strands and/or nails might be the only option [Citation151]. These keratinous matrices can be collected weeks or months after an alleged sexual assault took place. In the case of hair sampling, one of the requirements is segmental analysis and comparison of the GHB content along the shaft and specifically around the time of an alleged crime [Citation121]. Efforts to extend the window of detection of GHB, such as by analysis of phase II metabolites (GHB-glucuronide) have not been very successful [Citation127].
To reach an evidence-based opinion about abuse of GHB or involvement of the drug in certain crimes or overdose deaths, one needs to carefully consider all available evidence in the case. This includes demographics of the victim/suspect and integrity of the biological specimens analyzed, including the risk of various postmortem artifacts. Recently, Professor Hans Maurer (Homberg) pointed out: “Evidence-based case interpretation is more than comparing exactly determined blood levels with reference level lists” [Citation152].
Disclosure statement
There was no external funding applied for or received to prepare this manuscript and neither of the authors consider that they have any conflicts of interest in publishing this review in an international scientific journal.
References
- Ciolino LA, Mesmer MZ, Satzger RD. The chemical interconversion of GHB and GBL: forensic issues and implications. J Forensic Sci. 2001;46:1315–1323.
- Busardo FP, Jones AW. GHB pharmacology and toxicology: acute intoxication, concentrations in blood and urine in forensic cases and treatment of the withdrawal syndrome. Curr Neuropharmacol. 2015;13:47–70.
- Vickers MD. Gamma hydroxybutyric acid. Clinical pharmacology and current status. Proc R Soc Med. 1968;61:821–824.
- Hoes MJ, Vree TB, Guelen PJ. Gamma-hydroxybutyric acid as hypnotic. Clinical and pharmacokinetic evaluation of gamma-hydroxybutyric acid as hypnotic in man. Encephale 1980;6:93–99.
- Helrich M, McAslan TC, Skolnik S. Correlation of blood levels of 4-hydroxybutyrate with state of consciousness. Anesthesiology 1964;25:771–775.
- Takahara J, Yunoki S, Yakushiji W, et al. Stimulatory effects of gamma-hydroxybutyric acid on growth hormone and prolactin release in humans. J Clin Endocrinol Metab. 1977;44:1014–1017.
- Brailsford AD, Bartlett C, Kicman AT, et al. Increases in serum growth hormone concentrations associated with GHB administration. J Anal Toxicol. 2017;41:54–59.
- Wong CG, Chan KF, Gibson KM, et al. Gamma-hydroxybutyric acid: neurobiology and toxicology of a recreational drug. Toxicol Rev. 2004;23:3–20.
- Brunt TM, van Amsterdam JG, van den Brink W. GHB, GBL and 1,4-BD addiction. Curr Pharm Des. 2014;20:4076–4085.
- Schep LJ, Knudsen K, Slaughter RJ, et al. The clinical toxicology of gamma-hydroxybutyrate, gamma-butyrolactone and 1,4-butanediol. Clin Toxicol. 2012;50:458–470.
- Thai D, Dyer JE, Jacob P, et al. Clinical pharmacology of 1,4-butanediol and gamma-hydroxybutyrate after oral 1,4-butanediol administration to healthy volunteers. Clin Pharmacol Ther. 2007;81:178–184.
- Palmer RB. Gamma-butyrolactone and 1,4-butanediol: abused analogues of gamma-hydroxybutyrate. Toxicol Rev. 2004;23:21–31.
- Lora-Tamayo C, Tena T, Rodriguez A, et al. Intoxication due to 1,4-butanediol. Forensic Sci Int. 2003;133:256–259.
- Poldrugo F, Snead OC. 3rd,. 1,4 Butanediol, gamma-hydroxybutyric acid and ethanol: relationships and interactions. Neuropharmacology 1984;23:109–113.
- Kapitany-Foveny M, Zacher G, Posta J, et al. GHB-involved crimes among intoxicated patients. Forensic Sci Int. 2017;275:23–29.
- Ortmann LA, Jaeger MW, James LP, et al. Coma in a 20-month-old child from an ingestion of a toy containing 1,4-butanediol, a precursor of gamma-hydroxybutyrate. Pediatr Emerg Care. 2009;25:758–760.
- De Paepe P, Calle PA, Buylaert WA. Coma induced by intoxication. Handb Clin Neurol. 2008;90:175–191.
- Ingels M, Rangan C, Bellezzo J, et al. Coma and respiratory depression following the ingestion of GHB and its precursors: three cases. J Emerg Med. 2000;19:47–50.
- Thomas G, Bonner S, Gascoigne A. Coma induced by abuse of gamma-hydroxybutyrate (GBH or liquid ecstasy): a case report. BMJ. 1997;314:35–36.
- Mehling LM, Johansen SS, Wang X, et al. Drug facilitated sexual assault with lethal outcome: GHB intoxication in a six-year-old girl. Forensic Sci Int. 2016;259:e25–e31.
- Kugelberg FC, Holmgren A, Eklund A, et al. Forensic toxicology findings in deaths involving gamma-hydroxybutyrate. Int J Legal Med. 2010;124:1–6.
- Zvosec DL, Smith SW, Hall BJ. Three deaths associated with use of Xyrem. Sleep Med. 2009;10:490–493.
- Abramowitz MZ. GHB and date rape. Br J Psychiatry. 2004;185:176–177.
- Busardo FP. The importance of hair testing in GHB facilitated sexual assault cases. J Forensic Leg Med. 2016;39:74–75.
- Winstock A. New health promotion for chemsex and γ-hydroxybutyrate (GHB) ). BMJ. 2015;351:h6281
- Couper FJ, Logan BK. GHB and driving impairment. J Forensic Sci. 2001;46:919–923.
- Jones AW, Holmgren A, Kugelberg FC. Driving under the influence of gamma-hydroxybutyrate (GHB). Forensic Sci Med Pathol. 2008;4:205–211.
- Corkery JM, Loi B, Claridge H, et al. Gamma hydroxybutyrate (GHB), gamma butyrolactone (GBL) and 1,4-butanediol (1,4-BD; BDO): A literature review with a focus on UK fatalities related to non-medical use. Neurosci Biobehav Rev. 2015;53:52–78.
- Zvosec DL, Smith SW, Porrata T, et al. Case series of 226 gamma-hydroxybutyrate-associated deaths: lethal toxicity and trauma. Am J Emerg Med. 2011;29:319–332.
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511.
- Busardo FP, Kyriakou C, Napoletano S, et al. Clinical applications of sodium oxybate (GHB): from narcolepsy to alcohol withdrawal syndrome. Eur Rev Med Pharmacol Sci 2015;19:4654–4663.
- Gallimberti L, Canton G, Gentile N, et al. Gamma-hydroxybutyric acid for treatment of alcohol withdrawal syndrome. Lancet. 1989;2:787–789.
- Caputo F, Bernardi M. Sodium oxybate to treat alcohol dependence: 20 years of clinical experience. Addict Biol. 2013;18:901–903.
- Ingels AS, Wille SM, Samyn N, et al. Screening and confirmation methods for GHB determination in biological fluids. Anal Bioanal Chem. 2014;406:3553–3577.
- Busardo FP, Kyriakou C, Marchei E, et al. Ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) for determination of GHB, precursors and metabolites in different specimens: application to clinical and forensic cases. J Pharm Biomed Anal. 2017;137:123–131.
- Castro AL, Tarelho S, Dias M, et al. A fast and reliable method for GHB quantitation in whole blood by GC-MS/MS (TQD) for forensic purposes. J Pharm Biomed Anal. 2016;119:139–144.
- Jones AW, Holmgren A, Kugelberg FC. Gamma-hydroxybutyrate concentrations in the blood of impaired drivers, users of illicit drugs, and medical examiner cases. J Anal Toxicol. 2007;31:566–572.
- Liechti ME, Quednow BB, Liakoni E, et al. Pharmacokinetics and pharmacodynamics of γ-hydroxybutyrate in healthy subjects. Br J Clin Pharmacol. 2016;81:980–988.
- Brailsford AD, Cowan DA, Kicman AT. Pharmacokinetic properties of γ-hydroxybutyrate (GHB) in whole blood, serum, and urine. J Anal Toxicol. 2012;36:88–95.
- Brenneisen R, Elsohly MA, Murphy TP, et al. Pharmacokinetics and excretion of gamma-hydroxybutyrate (GHB) in healthy subjects. J Anal Toxicol. 2004;28:625–630.
- Ferrara SD, Zotti S, Tedeschi L, et al. Pharmacokinetics of gamma-hydroxybutyric acid in alcohol dependent patients after single and repeated oral doses. Br J Clin Pharmacol. 1992;34:231–235.
- Scharf MB, Lai AA, Branigan B, et al. Pharmacokinetics of gammahydroxybutyrate (GHB) in narcoleptic patients. Sleep 1998;21:507–514.
- Abanades S, Farre M, Segura M, et al. Gamma-hydroxybutyrate (GHB) in humans: pharmacodynamics and pharmacokinetics. Ann N Y Acad Sci. 2006;1074:559–576.
- Jones AW, Eklund A, Kronstrand R. Concentration-time profiles of gamma-hydroxybutyrate in blood after recreational doses are best described by zero-order rather than first-order kinetics. J Anal Toxicol. 2009;33:332–335.
- Borgen LA, Okerholm R, Morrison D, et al. The influence of gender and food on the pharmacokinetics of sodium oxybate oral solution in healthy subjects. J Clin Pharmacol. 2003;43:59–65.
- De Paoli G, Walker KM, Pounder DJ. Endogenous γ-hydroxybutyric acid concentrations in saliva determined by gas chromatography-mass spectrometry . J Anal Toxicol. 2011;35:148–152.
- Raknes G, Aronsen L, Fuskevag OM. Urinary concentrations of gamma-hydroxybutyric acid and related compounds in pregnancy. J Anal Toxicol. 2010;34:394–399.
- Andresen H, Sprys N, Schmoldt A, et al. Gamma-hydroxybutyrate in urine and serum: additional data supporting current cut-off recommendations. Forensic Sci Int. 2010;200:93–99.
- Mari F, Politi L, Trignano C, et al. What constitutes a normal ante-mortem urine GHB concentration? J Forensic Leg Med. 2009;16:148–151.
- Moriya F, Nishimura H, Furumiya J, et al. Effects of drinking and smoking on endogenous levels of urinary gamma-hydroxybutyric acid, a preliminary study. Leg Med. 2006;8:231–234.
- Elliott SP, Fais P. Further evidence for GHB naturally occurring in common non-alcoholic beverages. Forensic Sci Int. 2017;277:e36–e38.
- Brown AJ. Low-carb diets, fasting and euphoria: Is there a link between ketosis and gamma-hydroxybutyrate (GHB)? Med Hypotheses. 2007;68:268–271.
- Elliott S, Burgess V. The presence of gamma-hydroxybutyric acid (GHB) and gamma-butyrolactone (GBL) in alcoholic and non-alcoholic beverages. Forensic Sci Int. 2005;151:289–292.
- Jones AW, Hahn RG, Stalberg HP. Distribution of ethanol and water between plasma and whole blood; inter- and intra-individual variations after administration of ethanol by intravenous infusion. Scand J Clin Lab Invest. 1990;50:775–780.
- Nishimura H, Moriya F, Hashimoto Y. Mechansim of gamma-hydroxybutyic acid production during the early postmortem period. Forensic Toxicol. 2009;27:55–60.
- Kintz P, Villain M, Cirimele V, et al. GHB in postmortem toxicology. Discrimination between endogenous production from exposure using multiple specimens. Forensic Sci Int. 2004;143:177–181.
- Korb AS, Cooper G. Endogenous concentrations of GHB in postmortem blood from deaths unrelated to GHB use. J Anal Toxicol. 2014;38:582–588.
- Beránková K, Mutňanská K, Balíková M. Gamma-hydroxybutyric acid stability and formation in blood and urine. Forensic Sci Int. 2006;161:158–162.
- Moriya F, Hashimoto Y. Site-dependent production of gamma-hydroxybutyric acid in the early postmortem period. Forensic Sci Int. 2005;148:139–142.
- Biedermann A, Taroni F, Bozza S, et al. Critical analysis of forensic cut-offs and legal thresholds: A coherent approach to inference and decision. Forensic Sci Int. 2018;288:72–80.
- Zorntlein SW, Kopp A, Becker J, et al. In vitro production of GHB in blood and serum samples under various storage conditions. Forensic Sci Int. 2012;214:113–117.
- Andresen-Streichert H, Jensen P, Kietzerow J, et al. Endogenous gamma-hydroxybutyric acid (GHB) concentrations in post-mortem specimens and further recommendation for interpretative cut-offs. Int J Legal Med. 2015;129:57–68.
- Elliott SP. Further evidence for the presence of GHB in postmortem biological fluid: implications for the interpretation of findings. J Anal Toxicol. 2004;28:20–26.
- Marinetti L, Montgomery MA. The use of GHB to facilitate sexual assault. Forensic Sci Rev. 2010;22:41–59.
- Dinis-Oliveira RJ, Magalhaes T. Forensic toxicology in drug-facilitated sexual assault. Toxicol Mech Methods. 2013;23:471–478.
- EMCDDA. Sexual assaults facilitated by drugs and alcohol. Lisbon: European Monitoring Centre for Drugs and Drug Addiction; 2008.
- EMCDDA. GHB and its precursor GBL: an emerging trend case study. Lisbon: European Monitoring Centre for Drugs and Drug Addiction; 2008.
- Pichini S, Marchei E, Pacifici R, et al. Chemsex intoxication involving sildenafil as an adulterant of GHB. Drug Test Anal. 2017;9:956–959.
- Jenkins AJ, editor. Drug testing in alternative biological specimens. New York: Humana Press; 2008, 1–186.
- Di Terlizzi R, Platt S. The function, composition and analysis of cerebrospinal fluid in companion animals: part I – function and composition. Vet J. 2006;172:422–431.
- Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:309–316.
- Spector R, Robert Snodgrass S, Johanson CE. A balanced view of the cerebrospinal fluid composition and functions: focus on adult humans. Exp Neurol. 2015;273:57–68.
- Tittarelli R, Pichini S, Pedersen DS, et al. Ultra-high-performance liquid chromatography tandem mass spectrometry determination of GHB, GHB-glucuronide in plasma and cerebrospinal fluid of narcoleptic patients under sodium oxybate treatment. Forensic Sci Int. 2017;274:70–74.
- Drummer OH. Drug testing in oral fluid. Clin Biochem Rev. 2006;27:147–159.
- Schramm W, Smith RH, Craig PA, et al. Drugs of abuse in saliva: a review. J Anal Toxicol. 1992;16:1–9.
- Proctor GB. The physiology of salivary secretion. Periodontol 2000. 2016;70:11–25.
- Henson BS, Wong DT. Collection, storage, and processing of saliva samples for downstream molecular applications. Methods Mol Biol 2010;666:21–30.
- Langel K, Engblom C, Pehrsson A, et al. Drug testing in oral fluid-evaluation of sample collection devices. J Anal Toxicol. 2008;32:393–401.
- Langel K, Gjerde H, Favretto D, et al. Comparison of drug concentrations between whole blood and oral fluid. Drug Test Anal. 2014;6:461–471.
- Mucklow JC, Bending MR, Kahn GC, et al. Drug concentration in saliva. Clin Pharmacol Ther. 1978;24:563–570.
- Kintz P, Goulle JP, Cirimele V, et al. Window of detection of gamma-hydroxybutyrate in blood and saliva. Clin Chem. 2001;47:2033–2034.
- Abanades S, Farre M, Segura M, et al. Disposition of gamma-hydroxybutyric acid in conventional and nonconventional biologic fluids after single drug administration: issues in methodology and drug monitoring. Ther Drug Monit. 2007;29:64–70.
- Krotulski AJ, Mohr ALA, Friscia M, et al. Field detection of drugs of abuse in oral fluid using the Alere™ DDS®2 Mobile Test System with Confirmation by Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS). J Anal Toxicol. 2018;42:170–176.
- Logan BK, D’Orazio AL, Mohr ALA, et al. Recommendations for toxicological investigation of drug-impaired driving and motor vehicle fatalities-2017 update. J Anal Toxicol. 2018;42:63–68.
- Bevalot F, Cartiser N, Bottinelli C, et al. Vitreous humor analysis for the detection of xenobiotics in forensic toxicology: a review. Forensic Toxicol. 2016;34:12–40.
- Metushi IG, Fitzgerald RL, McIntyre IM. Assessment and comparison of vitreous humor as an alternative matrix for forensic toxicology screening by GC-MS. J Anal Toxicol. 2016;40:243–247.
- Skopp G. Postmortem toxicology. Forensic Sci Med Pathol. 2010;6:314–325.
- Dinis-Oliveira RJ, Carvalho F, Duarte JA, et al. Collection of biological samples in forensic toxicology. Toxicol Mech Methods. 2010;20:363–414.
- Flanagan RJ, Connally G, Evans JM. Analytical toxicology: guidelines for sample collection postmortem. Toxicol Rev. 2005;24:63–71.
- Marinetti LJ, Isenschmid DS, Hepler BR, et al. Analysis of GHB and 4-methyl-GHB in postmortem matrices after long-term storage. J Anal Toxicol. 2005;29:41–47.
- Castro AL, Dias M, Reis F, et al. Gamma-hydroxybutyric acid endogenous production and post-mortem behaviour - The importance of different biological matrices, cut-off reference values, sample collection and storage conditions. J Forensic Leg Med. 2014;27:17–24.
- Busardo FP, Portelli F, Montana A, et al. When a death apparently associated to sexual assault is instead a natural death due to idiopathic hypereosinophilic syndrome: the importance of gamma-hydroxybutyric acid analysis in vitreous humor. Leg Med. 2017;26:92–97.
- Busardo FP, Mannocchi G, Giorgetti R, et al. Stability of endogenous GHB in vitreous humor vs peripheral blood in dead bodies. Forensic Sci Int. 2017;274:64–69.
- Hotham N, Hotham E. Drugs in breastfeeding. Aust Prescr. 2015;38:156–159.
- Haastrup MB, Pottegard A, Damkier P. Alcohol and breastfeeding. Basic Clin Pharmacol Toxicol. 2014;114:168–173.
- Ho E, Collantes A, Kapur BM, et al. Alcohol and breast feeding: calculation of time to zero level in milk. Biol Neonate. 2001;80:219–222.
- Busardo FP, Bertol E, Mannocchi G, et al. Determination of GHB levels in breast milk and correlation with blood concentrations. Forensic Sci Int. 2016;265:172–181.
- Busardo FP, Pichini S. GHB concentration in breast milk of narcoleptic women treated with sodium oxybate. How important it is to be careful when interpreting values. Sleep Med. 2017;40:129.
- Barker EC, Puchowicz M, Letterio J, et al. GHB levels in breast milk of women with narcolepsy with cataplexy treated with sodium oxybate. Sleep Med. 2017;36:172–177.
- Carter LP, Koek W, France CP. Behavioral analyses of GHB: receptor mechanisms. Pharmacol Ther. 2009;121:100–114.
- Mardal M, Johansen SS, Thomsen R, et al. Advantages of analyzing postmortem brain samples in routine forensic drug screening-case series of three non-natural deaths tested positive for lysergic acid diethylamide (LSD). Forensic Sci Int. 2017;278:e14–e18.
- Thomsen R, Rasmussen BS, Johansen SS, et al. Postmortem concentrations of gamma-hydroxybutyrate (GHB) in peripheral blood and brain tissue – differentiating between postmortem formation and antemortem intake. Forensic Sci Int. 2017;272:154–158.
- Nedahl M, Johansen SS, Linnet K. Reference brain/blood concentrations of citalopram, duloxetine, mirtazapine and sertraline. J Anal Toxicol. 2018;42:149–156.
- Doherty JD, Hattox SE, Snead OC, et al. Identification of endogenous gamma-hydroxybutyrate in human and bovine brain and its regional distribution in human, guinea pig and rhesus monkey brain. J Pharmacol Exp Ther. 1978;207:130–139.
- Ferrara SD, Tedeschi L, Frison G, et al. Fatality due to gamma-hydroxybutyric acid (GHB) and heroin intoxication. J Forensic Sci. 1995;40:501–504.
- Kalasinsky KS, Dixon MM, Schmunk GA, et al. Blood, brain, and hair GHB concentrations following fatal ingestion. J Forensic Sci. 2001;46:728–730.
- Mazarr-Proo S, Kerrigan S. Distribution of GHB in tissues and fluids following a fatal overdose. J Anal Toxicol. 2005;29:398–400.
- Nielsen MKK, Nedahl M, Johansen SS, et al. Validation of a fully automated solid-phase extraction and ultra-high-performance liquid chromatography-tandem mass spectrometry method for quantification of 30 pharmaceuticals and metabolites in post-mortem blood and brain samples. Drug Test Anal. 2018;10:1147–1157.
- Stimpfl T, Drugs-of-abuse testing in brain. In: Jenkins AJ, editor. Drug testing in alternative biological specimens. New York: Humana Press;2008. p. 157–180.
- White CM. Pharmacologic, Pharmacokinetic, and Clinical Assessment of Illicitly Used gamma-Hydroxybutyrate. J Clin Pharmacol. 2017;57:33–39.
- Kintz P, Salomone A, Vincenti M, editors. Hair analysis in clinical and forensic toxicology. London: Academic Press;2015.
- Villain M, Cirimele V, Kintz P. Hair analysis in toxicology. Clin Chem Lab Med. 2004;42:1265–1272.
- Cuypers E, Flanagan RJ. The interpretation of hair analysis for drugs and drug metabolites. Clin Toxicol. 2018;56:90–100.
- Pragst F, Balikova MA. State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta. 2006;370:17–49.
- Wang X, Drummer OH. Review: interpretation of drug presence in the hair of children. Forensic Sci Int. 2015;257:458–472.
- Petzel-Witt S, Pogoda W, Wunder C, et al. Influence of bleaching and coloring on ethyl glucuronide content in human hair. Drug Test Anal. 2018;10:177–183.
- Wang X, Linnet K, Johansen SS. Development of a UPLC–MS/MS method for determining γ-hydroxybutyric acid (GHB) and GHB glucuronide concentrations in hair and application to forensic cases. Forensic Toxicol. 2016;34:51–60.
- Bertol E, Mari F, Vaiano F, et al. Determination of GHB in human hair by HPLC-MS/MS: Development and validation of a method and application to a study group and three possible single exposure cases. Drug Test Analysis. 2015;7:376–384.
- Busardo FP, Vaiano F, Mannocchi G, et al. Twelve months monitoring of hair GHB decay following a single dose administration in a case of facilitated sexual assault. Drug Test Anal. 2017;9:953–956.
- Kintz P, Cirimele V, Jamey C, et al. Testing for GHB in hair by GC/MS/MS after a single exposure. Application to document sexual assault. J Forensic Sci. 2003;48:195–200.
- Busardo FP, Pichini S, Zaami S, et al. Hair testing of GHB: an everlasting issue in forensic toxicology. Clin Chem Lab Med. 2018;56:198–208.
- Kintz P. A novel approach to document single exposure to GHB: hair analysis after sweat contamination. J Anal Toxicol. 2016;40:563–564.
- Kintz P. Value of hair analysis in postmortem toxicology. Forensic Sci Int. 2004;142:127–134.
- Castro AL, Tarelho S, Dias M, et al. Comparison of endogenous GHB concentrations in blood and hair in death cases with emphasis on the post mortem interval. Int J Legal Med. 2016;130:959–965.
- Petersen IN, Tortzen C, Kristensen JL, et al. Identification of a new metabolite of GHB: gamma-hydroxybutyric acid glucuronide. J Anal Toxicol. 2013;37:291–297.
- Mehling LM, Wang X, Johansen SS, et al. Determination of GHB and GHB-beta-O-glucuronide in hair of three narcoleptic patients-comparison between single and chronic GHB exposure. Forensic Sci Int. 2017;278:e8–e13.
- Piper T, Mehling LM, Spottke A, et al. Potential of GHB phase-II-metabolites to complement current approaches in GHB post administration detection. Forensic Sci Int. 2017;279:157–164.
- Palmer RB. A review of the use of ethyl glucuronide as a marker for ethanol consumption in forensic and clinical medicine. Semin Diagn Pathol. 2009;26:18–27.
- Skipper GE, Weinmann W, Thierauf A, et al. Ethyl glucuronide: a biomarker to identify alcohol use by health professionals recovering from substance use disorders. Alcohol Alcohol. 2004;39:445–449.
- Hanisch S, Stachel N, Skopp G. A potential new metabolite of gamma-hydroxybutyrate: sulfonated gamma-hydroxybutyric acid. Int J Legal Med. 2016;130:411–414.
- Busardo FP, Pichini S. The everlasting issue of GHB cut-offs in biological samples: How important it is to be careful when interpreting values. Forensic Sci Int. 2017;279:e14–e15.
- Stove CP, Ingels AS, De Kesel PM, et al. Dried blood spots in toxicology: from the cradle to the grave?. Crit Rev Toxicol. 2012;42:230–243.
- Sadones N, Capiau S, De Kesel PM, et al. Spot them in the spot: analysis of abused substances using dried blood spots. Bioanalysis 2014;6:2211–2227.
- Sadones N, Archer JR, Ingels AS, et al. Do capillary dried blood spot concentrations of gamma-hydroxybutyric acid mirror those in venous blood? A comparative study. Drug Test Anal. 2015;7:336–340.
- Drummer OH. Requirements for bioanalytical procedures in postmortem toxicology. Anal Bioanal Chem. 2007;388:1495–1503.
- Arndt T. Urine-creatinine concentration as a marker of urine dilution: reflections using a cohort of 45,000 samples. Forensic Sci Int. 2009;186:48–51.
- Chaturvedi AK, Sershon JL, Craft KJ, et al. Effects of fluid load on human urine characteristics related to workplace drug testing. J Anal Toxicol. 2013;37:5–10.
- Crookes CE, Faulds MC, Forrest AR, et al. A reference range for endogenous gamma-hydroxybutyrate in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2004;28:644–649.
- Elian AA. Determination of endogenous gamma-hydroxybutyric acid (GHB) levels in antemortem urine and blood. Forensic Sci Int. 2002;128:120–122.
- Brailsford AD, Cowan DA, Kicman AT. Urinary gamma-hydroxybutyrate concentrations in 1126 female subjects. J Anal Toxicol. 2010;34:555–561.
- LeBeau MA, Montgomery MA, Morris-Kukoski C, et al. A comprehensive study on the variations in urinary concentrations of endogenous gamma-hydroxybutyrate (GHB). J Anal Toxicol. 2006;30:98–105.
- Yeatman DT, Reid K. A study of urinary endogenous gamma-hydroxybutyrate (GHB) levels. J Anal Toxicol. 2003;27:40–42.
- Gambino R. Sodium fluoride: an ineffective inhibitor of glycolysis. Ann Clin Biochem. 2013;50:3–5.
- Fjeld B, Burns ML, Karinen R, et al. Long-term stability of GHB in post-mortem samples and samples from living persons, stored at −20 degrees C, using fluoride preservatives. Forensic Sci Int. 2012;222:47–51.
- Kerrigan S. In vitro production of gamma-hydroxybutyrate in antemortem urine samples. J Anal Toxicol. 2002;26:571–574.
- Schrock A, Hari Y, Konig S, et al. Pharmacokinetics of GHB and detection window in serum and urine after single uptake of a low dose of GBL – an experiment with two volunteers. Drug Test Anal. 2014;6:363–366.
- Moriya F, Hashimoto Y. Endogenous gamma-hydroxybutyric acid levels in postmortem blood. Leg Med (Tokyo). 2004;6:47–51.
- Jones AW, Holmgren A, Kugelberg FC, et al. Relationship between postmortem urine and blood concentrations of GHB furnishes useful information to help interpret drug intoxication deaths. J Anal Toxicol. 2018;42. Available from: https://doi.org/10.1093/jat/bky041
- Jones AW, Holmgren P. Urine/blood ratios of ethanol in deaths attributed to acute alcohol poisoning and chronic alcoholism. Forensic Sci Int. 2003;135:206–212.
- Jones AW. Ethanol distribution ratios between urine and capillary blood in controlled experiments and in apprehended drinking drivers. J Forensic Sci. 1992;37:13208J– 132034.
- Cappelle D, De Keukeleire S, Neels H, et al. Keratinous matrices for the assessment of drugs of abuse consumption: A correlation study between hair and nails. Drug Test Anal. 2018;10:1110–1118.
- Maurer HH. Impact of pharmacogenomic variations and/or drug-drug (food) interctions on interpretation of clinical and forensic toxicology cases with CNS acting drugs. TIAFT Bull. 2018;48:27–30.