Abstract
Background: Electronic cigarettes (e-cigarettes), the smokeless alternative to conventional tobacco cigarettes, have become increasingly popular. E-cigarettes vaporise e-liquid, a solution of highly concentrated nicotine, propylene glycol (PG) and vegetable glycerine (VG). With the popularity of e-cigarettes, e-liquid refills have become easily accessible and several cases of intoxication due to the ingestion of e-liquid have been reported. We provide an overview of these cases, their pathophysiology and patients’ characteristics.
Methods: We carried out a retrospective evaluation of the scientific literature reporting on cases of liquid nicotine intoxication, using the following inclusion criteria: (1) the article is or contains a case report, (2) describes an intoxication with e-liquid, (3) the substance contains nicotine, and (4) intake is oral, intravenous or subcutaneous.
Results: We found 26 case reports describing a total of 31 patients who suffered from e-liquid intoxication. All intoxications up to the age of six were reported as unintentional, whereas nearly all cases from ages 13 to 53 were due to suicide attempts. The three most prevalent symptoms of e-liquid intoxication were tachycardia, altered mental status and vomiting. Eleven cases resulted in the death of the patient. In the survivors, the highest plasma concentration of nicotine was 800 µg L−1, while the lowest concentration in the non-survivors was 1600 µg L−1.
Conclusions: There is a mismatch between the generally accepted lethal oral nicotine dose of 60 mg, resulting in approximately 180 µg L−1 plasma concentration, and the 4.4- to 8.9-fold higher lethal plasma concentrations we found in cases of e-liquid intoxication. In these severe intoxications, plasma cotinine concentration does not act as a more reliable indicator of nicotine intoxication than nicotine itself. The ages of the patients display a bimodal distribution. In patients above the age of 10, intoxication results mainly from suicide attempts rather than accidental ingestion. The role of PG and VG in e-liquid intoxications is remarkably unclear. However, the similarity across nicotine and PG toxicity symptoms leads us to believe a cumulative effect cannot be excluded.
Introduction
Electronic cigarettes or “e-cigarettes”, also known as smokeless cigarettes, are an alternative to regular cigarettes. They are battery-powered devices similar to normal tobacco cigarettes in use. However, e-cigarettes do not burn tobacco. Instead they vaporise e-liquid, a solution that is typically composed of nicotine, propylene glycol (PG) and vegetable glycerine (VG) often supplemented with various flavouring compounds and/or ethanol [Citation1–3]. The main benefit of e-cigarettes is that they enable nicotine intake without exposure to many other, often hazardous, chemicals which are normally present in tobacco cigarettes [Citation4]. Moreover, e-cigarettes have become a popular supporting tool for those who wish to quit smoking.
In 2003, Hon, a Chinese pharmacist, invented and patented the currently used e-cigarette in China (patent no. US8511318B2) [Citation5]. E-cigarettes were introduced to the Western market in 2004 [Citation6]. The nicotine containing solution can be refilled and a wide variety of such e-liquid refill cartridges, each containing different amounts of nicotine and flavouring agents, are currently available on the market. Moreover, thanks to the internet, e-liquid refills are easily accessible. During the last decade, regulatory parameters for e-liquids with respect to, for example, labelling, packaging, health warning, sale and advertising are set forward worldwide [Citation7–9], which likely will decrease discrepancies between actual content and labelling as found previously [Citation3,Citation10]. However, regulation has not been installed everywhere and often differs between countries [Citation9]. For example, maximum nicotine concentration in e-liquids is set at 20 mg/ml in 32 EU countries [Citation11], whereas many other countries have not put forward an upper limit, and e-liquid preparations with nicotine concentrations up to 60 mg/ml can be found online. Also, minimum age for e-liquid purchase varies (e.g., Belgium: age 16; United States: 18; Honduras: 21) and is often not restricted at all.
Exposure to highly concentrated substances in e-liquids through unwarranted use such as oral consumption, goes hand in hand with a substantial risk of severe toxicity [Citation12]. Life-threatening nicotine intoxication in young children due to accidental ingestion of e-liquid has been reported [Citation13]. Another concern is nicotine intoxication resulting from suicide attempts using e-liquids [Citation14]. With the introduction of e-cigarettes, e-liquids containing highly concentrated nicotine have become widely available. However, the clinical consequences of nicotine intoxication through e-liquid ingestion remain unclear. Moreover, at present, there is no consensus on the lethal dose of nicotine since different values have been reported. For oral intake, 60 mg of nicotine is often suggested as the lethal dose in the literature; although, research has shown that adults survive dosages much higher than this (up to 500 mg) [Citation15]. Furthermore, the implications of combined nicotine and PG/VG intake, the main constituents of e-liquids, are uncertain. The symptoms most frequently seen in nicotine intoxication are vomiting, agitation, pallor, hypertension, tachycardia and headache [Citation16]. Symptoms of PG intoxication vary between hyperosmolality and lactic acidosis to haemolysis, renal failure and CNS depression [Citation17]. VG related symptoms are usually headache, nausea, diuresis and hyperglycaemia, mostly due to dehydration [Citation18,Citation19].
Two epidemiological studies from 2014 and 2019, respectively [Citation13,Citation20], examined exposures involving e-cigarettes reported to the National Poison Data System by all [Citation13] or a single [Citation20] U.S. Poison Center(s) and described trends in these exposures and clinical descriptions. They found that most reports of e-liquid nicotine intoxication involved accidental ingestion by children below the age of five leading to minimal toxicity and only rarely resulted in mortality [Citation13,Citation20].
We took a different approach and studied case reports describing e-liquid intoxication, while being aware of the potential bias towards overrepresentation of severe cases compared to the epidemiological approach. The main objective of this study is to analyse case reports describing oral liquid-nicotine intoxication and to attempt to correlate symptoms to the various components of e-liquids, in order to better understand the pathophysiology and consequences of e-liquid ingestion. We analysed patient characteristics, including age and cause of intoxication, compared derived dose-outcome relationships from concentrations of nicotine and of its main metabolite cotinine, evaluated clinical signs and discussed underlying pathophysiology. We found that severe cases of e-liquid poisoning mainly resulted from suicide attempts in the ages of 13–53 years.
Materials and methods
Data collection
For this literature review, we have carried out a retrospective evaluation of the existing scientific literature reporting on cases of e-liquid intoxication as defined and/or indicated by the authors. We have searched PubMed, Embase, WebMD and Google Scholar using the following search terms: nicotine, liquid nicotine, electronic cigarette liquid, e-cigarette liquid and e-liquid combined with intoxication, ingestion, injection and tachycardia. We have included and evaluated English and non-English articles. We included articles that met the following requirements: (1) the article is or contains a case report, (2) describes an intoxication with e-liquid, (3) the substance contains nicotine, and (4) intake is oral, intravenous, or subcutaneous. One case was presented twice [Citation21,Citation22], for this case we combined the data from both articles.
Data analysis
All case reports in this study have been read and interpreted by at least two authors of the current paper. For translating articles not written in English or Dutch, we have used Google Translate and consulted native speakers. For the statistical analysis of the data on nicotine and cotinine plasma, blood, and urine concentrations, GraphPad Prism 7.04 software was used (GraphPad Software, La Jolla, CA). Data were clustered into two groups based on the patients’ outcome (survival; death). Numerical data are presented as mean ± SD. Welch’s T-test was used to determine statistical significance (p < .05).
Results
We found 26 case reports describing a total of 31 patients who suffered from e-liquid nicotine intoxication either due to ingestion or injection. Twenty case reports have been published in English, whereas translation was required for six articles. The published case reports originate from 11 different countries ( and ). Most case reports are from the US (n = 6), followed by South Korea (n = 4), Poland (n = 3) and the Netherlands (n = 3). When looking at the number of case reports per continent, most reports originate from Europe. The articles describe a total of 31 intoxications, of which 17 (55%) are male and 14 (45%) are female (). The mean ages of the male and female population at intoxication are 27 and 20 years, respectively. The ages of the patients described in the case reports display a bimodal distribution ().
Figure 1. Distribution of cases of e-liquid poisoning by age. Motive for intake is indicated by different shading.
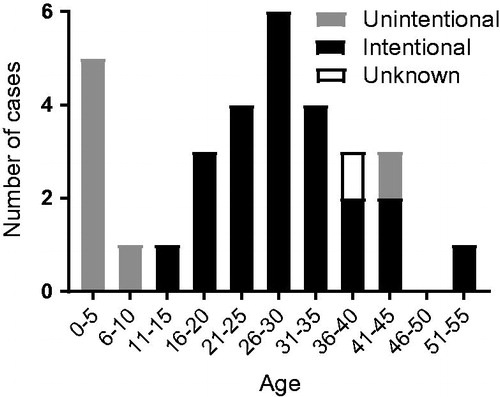
Table 1. Overview of case reports reviewed.
Table 2. Overview of the origin of case reports describing acute e-liquid poisoning including the number of patients.
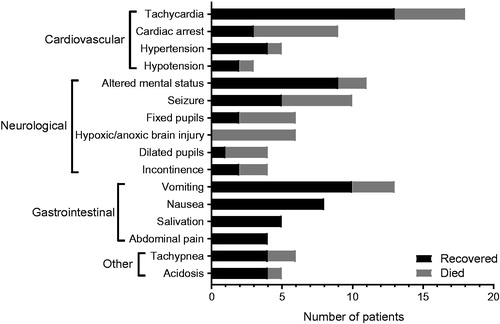
All nicotine e-liquid intoxications up to the age of 10 were reported as unintentional. From ages 13 to 53, nearly all cases were the result of intentional e-liquid intake, most likely suicide attempts. The symptoms of e-liquid intoxication can be divided into groups: “cardiovascular”, “neurological”, “gastrointestinal”, and “other” symptoms (). Tachycardia (n = 18), vomiting (n = 13), and altered mental status (n = 11) are the three most prevalent symptoms. Other symptoms, which were reported less frequently, are: pulseless electrical activity (n = 2), dizziness (n = 2), headache (n = 2), paresis (n = 2), purple colour (n = 1), trunk ataxia (n = 1), and grunting (n = 1). Eleven of the 31 presented cases of e-liquid intoxication resulted in the death of the patient.
Figure 2. Reported symptoms in patients with acute e-liquid poisoning classified by cardiovascular, neurological, gastrointestinal and other symptoms. Outcome is indicated by different shading. Only symptoms that occurred in three or more cases were included in the figure.
The plasma nicotine concentration had been specifically determined in 11 cases (). In cases reporting multiple measurements of nicotine concentration the first, which was also the highest, measurement was used. The mean plasma nicotine concentration among the survivors (n = 6) was 307 ± 312 µg L−1 (median: 222 μg L−1). Among the five patients that died, the mean plasma nicotine concentration was 3360 ± 1692 µg L−1 (p < .05 vs. survivors) (median: 3000 µg L−1). As illustrated by , the highest plasma concentration of nicotine in the surviving group was 800 µg L−1, while the lowest plasma concentration in the group of patients that had not survived the intoxication was 1600 µg L−1. We omitted one patient, a non-survivor, from since the patient’s exceptionally high plasma nicotine concentration of 85,200 µg L−1 exceeded the mean nicotine concentration in this group by 48.4 times SD [Citation44].
Figure 3. (A) Individual and mean nicotine concentration in the plasma in µg L−1 in cases with acute e-liquid poisoning subdivided in the outcome recovered or death. Symbols: triangle, oral intake; squares, intravenous intake. Black horizontal dotted lines (at 180 and 4000 µg L−1) indicate levels of lethal value as found in literature [Citation15]. Grey horizontal bar indicates our own lethal value range. The mean is displayed by the short black lines (at 307 and 3360 µg L−1). (B) Individual and mean cotinine concentration in the plasma in µg L−1 in cases with acute e-liquid poisoning subdivided in the outcome. Symbols: triangle, oral intake; squares, intravenous intake. n.s.: not significant. The mean is displayed by the short black lines (at 1224 and 1044 µg L−1).
![Figure 3. (A) Individual and mean nicotine concentration in the plasma in µg L−1 in cases with acute e-liquid poisoning subdivided in the outcome recovered or death. Symbols: triangle, oral intake; squares, intravenous intake. Black horizontal dotted lines (at 180 and 4000 µg L−1) indicate levels of lethal value as found in literature [Citation15]. Grey horizontal bar indicates our own lethal value range. The mean is displayed by the short black lines (at 307 and 3360 µg L−1). (B) Individual and mean cotinine concentration in the plasma in µg L−1 in cases with acute e-liquid poisoning subdivided in the outcome. Symbols: triangle, oral intake; squares, intravenous intake. n.s.: not significant. The mean is displayed by the short black lines (at 1224 and 1044 µg L−1).](/cms/asset/2ad1a83e-4a05-4129-a3f3-2d3343497823/ictx_a_1636994_f0003_b.jpg)
Plasma concentrations of cotinine, the major metabolite of nicotine, had been specifically determined in 12 cases (). In cases reporting multiple measurements of cotinine concentration we used the values from the first, which were also the highest, measurements. The mean plasma cotinine concentration in the surviving group was 1224 ± 971 µg L−1. The patients that did not survive the intoxication had a mean plasma cotinine concentration of 1044 ± 731 µg L−1 (n.s. vs. survivors).
Discussion
We reviewed 26 case reports on nicotine e-liquid intoxications from 11 different countries. The age of patients ranged between 10 months and 53 years, showing a bimodal distribution. Nicotine and cotinine concentrations were determined in 11 and 12 cases, respectively. Plasma nicotine concentration was significantly higher in patients that had died compared to patients that survived (p < .05), whereas cotinine concentrations showed no significant difference. The most frequently reported symptoms (tachycardia, altered mental status, and vomiting) can be explained by a variety of physiological processes relating to the primary active agents in e-liquids which are nicotine, PG and VG. Unfortunately, we were unable to provide comprehensive insights into potential interactions regarding comorbidities and/or intake of other toxic components, such as alcohol, since the case reports rarely provided a full patient history.
Nicotine binds to a variety of nicotinic cholinergic receptors throughout the body, which may explain the many different manifestations of e-liquid intoxication observed. Acute nicotine intoxication can induce both stimulation and inhibition of cholinergic receptors, which usually results in a biphasic clinical manifestation [Citation16]. First, sympathetic symptoms such as tachycardia, hypertension, and involuntary muscle contractions occur. Subsequently, parasympathetic symptoms can appear due to sympathetic receptor desensitisation.
Tachycardia is the most prevalent symptom in the studied case reports. By activation of cholinergic receptors of the paravertebral stellate ganglia leading to the heart, nicotine induces sympathetic activity in the myocardium and vascular smooth muscle cells [Citation45]. Smoking one cigarette usually increases the heart rate by 15–20 beats per minute and causes an increase in systolic as well as diastolic blood pressure by roughly 12 mmHg. Higher doses of nicotine can result in the development of severe tachycardia and acute hypertension [Citation45].
Altered mental status is observed while smoking cigarettes and relates to release of serotonin, dopamine, and other neuroactive agents. Being aware of the notion that high concentrations of nicotine may associate with severe cognitive and behavioural alterations [Citation46], it is tempting to speculate that these symptoms as described in several case reports result from high nicotine levels. Although, one should be aware that the act of cigarette smoking and its resulting tobacco smoke exposure, cannot be directly compared to, often intentional, liquid nicotine intoxication.
The described seizures and myoclonus have two possible explanations, depending on a physician’s definition of these terms. First, nicotinic cholinergic receptors are, besides paravertebrally, situated at the neuromuscular junction. Nicotine acts as a substitute for acetylcholine and as such stimulates more rapid depolarisation of the muscle fibres. In high concentrations, it can induce involuntary contraction of the muscles, even resulting in seizure-like movements [Citation47]. Second, at very high dosage, nicotine can cause epileptic activity in the brain. This mechanism is not yet fully understood, but may be related to desensitisation of the cholinergic receptors after prolonged exposure. The cholinergic receptors in the central nervous system normally stimulate GABAergic interneurons, which in turn have an inhibitory effect on the pyramidal cells. A decrease in cholinergic receptors reduces the GABAergic activity and has an excitatory effect on the pyramidal cells, resulting in seizure [Citation48,Citation49]. The desensitisation of the nicotinic cholinergic receptors eventually induces a similar effect at the neuromuscular junction. Lengthened exposure to nicotine leads to a reduction in available receptors at the muscle cell membrane, followed by decreased cell depolarisation. In this way, partial or complete muscle paralysis may occur [Citation16].
Finally, as is frequently the case in oral intoxications, gastrointestinal symptoms appeared in nearly all of the described patients. These included vomiting, nausea, salivation and abdominal pain. The gastrointestinal symptoms are partially caused by excessive sympathetic stimulation mentioned earlier [Citation16], nicotine stimulation of the area postrema (specifically the chemoreceptor trigger zone), and irritation of the mucosal tissue which activates a natural detoxification reaction [Citation50]. The eventual cause of death due to nicotine intoxication is most likely related to cardiac or cerebral pathology, but can be difficult to determine precisely.
Clinical symptoms described from our reviewed case reports are in line with those reported in an earlier epidemiological study [Citation20], albeit that the amounts of ingested e-liquid in our cases, which involve many events of intentional intake, are likely higher. This may explain absence or low incidence of mortality in the epidemiological studies [Citation13,Citation20] compared to the current study.
There is no consensus on the lethal dose of nicotine. In this study, we provide a clear overview of nicotine plasma concentrations in survivors versus patients that died. In our dataset, the lethal nicotine concentration is between 800 and 1600 µg L−1, which is 4.4- to 8.9-fold higher than the generally accepted lethal oral dose of 60 mg or less that would lead to a plasma concentration of approximately 180 µg L−1 [Citation15]. This latter plasma concentration is an estimate, since there are individual differences in nicotine metabolism, due to genetic polymorphism and other interindividual differences [Citation38]. For example, women generally metabolise nicotine more rapidly than men [Citation51]. Nevertheless, this results in minor differences and does not explain the gap between ±180 µg L−1 and 800–1600 µg L−1. Thus, there is a mismatch between the lethal dose consistently stated in textbooks, databases and safety sheets (30–60 mg), and the lethal doses we found in cases of nicotine intoxication. Such discrepancy has been noticed before, but the 60 mg-value is still widely accepted [Citation15]. Our data thus supports the suggestion of Mayer [Citation15] that revision of the 60 mg lethal dose is necessary.
Nicotine is a water- and lipid-soluble liquid alkaloid, partly present in blood as free base [Citation52]. According to the literature, plasma nicotine half-life is short (40–120 minutes) [Citation53,Citation54]. After oral ingestion, nicotine is absorbed by the mucosa in mouth and bowel and metabolised predominantly in the liver [Citation55]. The first-pass effect decreases the bioavailability of nicotine by 30–40% [Citation38]. Peak plasma concentrations are reached around 60 minutes after oral ingestion, however, in high dosages Tmax is potentially prolonged. Nicotine undergoes rapid biotransformation, mainly in the liver, to one of the six identified primary metabolites, of which cotinine is most abundant. Up to 80% of nicotine is converted to this lactam derivate via cytochrome oxidase routes, primarily by CYP P450 2A6 [Citation56]. In the case reports that we studied, the time-interval between the intake of e-liquid and the collection of biological samples for analysis, ranged from 1 hour to 25 hours. Since the half-life of nicotine is short in biological samples (T½ of two hours) [Citation57], the analytical results of nicotine could be inaccurate depending upon the timing of the sampling. Due to the short half-life of nicotine, cotinine (T½ of 13–19 hours) is widely used as a marker for exposure to nicotine [Citation58], whereas in terms of clinical practice, cotinine has no cardiovascular activity on cigarette smokers by itself [Citation59]. In terms of quantity, cotinine is the most stable metabolite and its concentration is considered directly proportional to the amount of nicotine absorbed [Citation58]. However, according to reports on nicotine poisoning, clinical patterns and survival rates do not always associate with cotinine concentration [Citation38]. Despite the consensus in literature on cotinine being a more reliable indicator of nicotine intoxication than nicotine itself, our results from e-liquid intoxications show the opposite. This could possibly be due to different kinetics of nicotine in the case of e-liquid intoxications compared to nicotine poisoning through (ab)use of other nicotine-containing products. Another possible explanation is that in severe intoxication cases, rapidly occurring liver damage precludes nicotine metabolisation into cotinine. In addition, circulating nicotine and cotinine levels are determined by the rate of nicotine metabolism, which may differ between individuals [Citation38,Citation51].
E-liquids are often based on PG and VG, compounding in various ratios, as primary solvents. The PG/VG ratio can vary between a sole PG or VG base, and may influence nicotine delivery as well as toxicity of the e-liquid [Citation2]. However, the extent to which the PG/VG ratio in e-liquids influences acute effects is unknown.
PG is an alcohol derivative also referred to as propane-1,2-diol. It is widely used as a drug solvent, an additive in foods, and as a moisturiser and emulsifier in cosmetic products [Citation60]. PG is absorbed in the small intestine, metabolised in the liver by alcohol dehydrogenase into lactic acid and finally turned into pyruvic acid through glycolysis. Both lactic acid and pyruvic acid, being normal constituents of the citric acid cycle, are finally converted into the non-toxic end products water and carbon dioxide [Citation61]. 12–45% of PG is eliminated by the kidneys unchanged or as glucuronide conjugate [Citation62].
In 1982, PG received the “Generally regarded as safe” (GRAS) status by the US Food and Drug Administration (FDA) [Citation63]. Nevertheless, PG toxicity has been reported in some cases [Citation17]. High doses of PG pose a risk especially in infants, children, and elderly [Citation64,Citation65], due to a relatively impaired alcohol dehydrogenase system and kidney disease, respectively. When PG is ingested in very high doses, the excess lactic and pyruvic acid will not be metabolised and will accumulate, potentially causing metabolic acidosis. Additionally, high dosage of PG can cause accumulation of PG itself, in turn leading to hyperosmolarity [Citation62]. The combination of metabolic acidosis and hyperosmolarity may result in serious illness, such as haemolysis, renal failure, CNS depression with seizures and various presentations of cardiovascular decompensation [Citation17].
The role of PG in e-liquid intoxications is remarkably unclear. Although PG is usually a main component of e-cigarette liquid [Citation60], no study has been conducted on the long-term toxic effects of PG in e-cigarettes/e-liquids [Citation66]. As reviewed by Barnes et al. [Citation67], 75% of the cases on symptomatic PG intoxication report plasma concentrations higher than 250 µg L−1. Only one of our case reports measured a PG concentration: 300 µg L−1 after 14 hours, by which the authors estimated the initial plasma concentration to be more than 1000 µg L−1 [Citation30]. This could indicate a dual intoxication of PG and nicotine. As only one of the case reports provided the plasma concentration of PG, we were unable to relate any of the symptoms to PG with full certainty. However, the similarity across nicotine and PG toxicity symptoms leads us to believe a cumulative effect cannot be excluded. No evidence for a possible interaction has been published so far, but in view of e-liquid intoxication, it may be a subject for further studies.
VG, also propane-1,2,3-triol or glycerol, is a simple trihydric alcohol which is naturally present in human tissue as it forms the skeleton of triglycerides [Citation68]. After absorption in the small intestine, VG is metabolised in the liver by glycerol kinase into glycerol-3-phosphate, which then becomes esterified with fatty acids in the citric acid cycle [Citation69]. VG is eliminated by the kidneys, either unchanged or metabolised [Citation70]. Due to its sweet taste, VG is often used in the food industry. It is also widely used in medical applications because of its potent osmotic properties. For example, it is provided as a treatment for cerebral oedema as VG has a dehydrating effect on the brain [Citation18]. VG is considered to be safe for oral ingestion [Citation71]; however, its effects in an overdose are not well researched. Known adverse effects of VG taken orally are mostly due to dehydration: headache, nausea, diuresis, and hyperglycaemia. In very severe cases, symptoms such as apathy, cyanosis, renal failure, and cardiac arrhythmia are reported. Haemolysis seems to be a reaction strictly related to intravenous injection [Citation19]. None of the case reports provided a concentration of VG, and since severe symptoms are only reported for high doses of up to 1.4 g/kg bodyweight [Citation19], we believe that VG has no obvious effect in these cases.
In this study, PG and/or VG in e-liquids could have affected the acidosis seen in several case reports. The reason being that acidic PG metabolites potentially cause metabolic acidosis [Citation62] and that the osmotic dehydration produced by VG may lead to ketoacidosis [Citation70]. Interestingly, the bioavailability of nicotine is dependent upon pH, which varies substantially across different e-liquids. More alkaline e-liquids contain a larger fraction of unionised nicotine, a form that is absorbed more easily and rapidly through biological membranes [Citation2].
Ethanol can be added to the PG/VG base as a solvent for flavourings or other additives. Despite extensive regulations on reporting all components, ethanol has frequently been found as an unlabelled ingredient in e-liquids available in the US [Citation3]. It might be possible that ethanol contributed to the observed acidosis in our case reports as well, although the concentration in the used e-liquids was presumably too low for this effect.
Our study comes with several limitations. There are some concerns about the comparability of the nicotine levels found in the case reports. As described, the moment of biological sample collection varies from one to 25 hours after intake of e-liquid. Besides, several different analytical methods have been reported, using GC, HPLC, GC/MS or LC/MS/MS for the analysis of nicotine and metabolites [Citation44]. Furthermore, we used the highest reported values in the case reports, but cannot determine whether these are equal to the peak concentrations that have occurred in the patients.
Studying only case reports could have led to bias in our results since case reports generally describe more severe cases. Also, the quantity of occurred symptoms may not be fully correct, since some of these symptoms can only be described by patients themselves (such as nausea or abdominal pain), while several patients in our case reports were found post-mortem. This could also have affected the number of other symptoms (such as tachypnoea, hypertension, and altered mental status), which can only be detected during life. Adding to this, we had a concern about the interpretation of some of the symptoms, in particular seizure and myoclonus. As described before, nicotine intoxication can cause both symptoms to occur by two different pathways. We are concerned that, in acute situations, these two symptoms might present themselves similarly and are difficult to distinguish from one another. Therefore, the occurrence ratio of seizure and myoclonus might be different than presented by the case reports.
Conclusions and recommendations
E-liquid cartridges, often containing high doses of liquid nicotine, are easily accessible and unwarranted use can result in severe intoxication. The ages of the patients display a bimodal distribution. In patients above the age of 10, intoxication results mainly from suicide attempt rather than accidental ingestion. In these severe intoxications, we found that cotinine does not act as a more reliable indicator of nicotine intoxication than nicotine itself. Furthermore, there is a mismatch between the generally accepted lethal nicotine dose of 60 mg (resulting in a plasma concentration of approximately 180 μg L−1) and the 4.4- to 8.9-fold higher lethal plasma concentrations that we found in cases of e-liquid intoxication. The role of PG in e-liquid intoxications is remarkably unclear. However, the similarity across nicotine and PG toxicity symptoms leads us to believe a cumulative effect cannot be excluded. VG and ethanol may also play a part in the manifestation of e-liquid intoxication.
As e-liquids are very easily accessible and purchased by many people, we urge to raise more awareness about the dangers of these substances when ingested or injected. We expect this will especially prevent more cases of accidental ingestion in young children. On a global scale, stricter regulation is necessary to ensure accurate descriptions of contents on e-liquid cartridges. This is also important for healthcare professionals, who need to know the amount of substances ingested by patients. As for further research, we suggest examining possible PG/VG-nicotine interactions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Twyman L, Watts C, Chapman K, et al. Electronic cigarette use in New South Wales, Australia: reasons for use, place of purchase and use in enclosed and outdoor places. Aust N Z J Public Health. 2018;42:491–496.
- DeVito EE, Krishnan-Sarin S. E-cigarettes: impact of E-liquid components and device characteristics on nicotine exposure. Curr Neuropharmacol. 2018;16:438–459.
- Poklis JL, Wolf CE, 2nd, Peace MR. Ethanol concentration in 56 refillable electronic cigarettes liquid formulations determined by headspace gas chromatography with flame ionization detector (HS-GC-FID). Drug Test Anal. 2017;9:1637–1640.
- Pellegrino TB, Mangiaracina G, et al. Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter (PM). Ann Ig. 2012;24:279–288.
- Hon L, inventor; Fontem Holdings 1 BV, assignee. Electronic cigarette. United States patent US 8511318B2. 2013 Aug 20.
- Noble MJ. The new dangers of electronic cigarettes. Clin Pediatr Emerg Med. 2017;18:163–172.
- Food and Drug Administration, HHS. Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Final rule. Fed Regist. 2016;81:28973–29106.
- The European Parliament and the Council of the European Union. Directive 2014/40/EU of the European Parliament and of the Council of 3 April 2014 on the approximation of the laws, regulations and administrative provisions of the Member States concerning the manufacture, presentation and sale of tobacco and related products and repealing Directive 2001/37/EC Text with EEA relevance; [updated 2014 May 3; cited 2019 May 17]. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ%3AJOL_2014_127_R_0001
- Kennedy RD, Awopegba A, De León E, et al. Global approaches to regulating electronic cigarettes. Tob Control. 2017;26:440–445.
- Girvalaki C, Tzatzarakis M, Kyriakos CN, et al. Composition and chemical health hazards of the most common electronic cigarette liquids in nine European countries. Inhal Toxicol. 2018;30:361–369.
- Country laws regulating E-cigarettes: a policy scan. Baltimore (MD): Institute for Global Tobacco Control; [updated 2018 Nov 2; cited 2019 May 17]. Available from: https://www.globaltobaccocontrol.org/e-cigarette_policyscan
- Sommerfeld K, Łukasik-Głębocka M, Kulza M, et al. Intravenous and oral suicidal e-liquid poisonings with confirmed nicotine and cotinine concentrations. Forensic Sci Int. 2016;262:e15–e20.
- Vakkalanka JP, Hardison LS, Jr, Holstege CP. Epidemiological trends in electronic cigarette exposures reported to U.S. Poison Centers. Clin Toxicol. 2014;52:542–548.
- Park EJ, Min YG. The emerging method of suicide by electronic cigarette liquid: a case report. J Korean Med Sci. 2018;33:e52.
- Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch Toxicol. 2014;88:5–7.
- Soghoian S. Nicotine. In: Weitz M, Naglieri C, editors. Goldfrank’s toxicologic emergencies. 10th ed. New York (NY): McGraw-Hill Education; 2015. p. 1138–1143.
- O'Donnell J, Mertl SL, Kelly WN. Propylene glycol toxicity in a pediatric patient: the dangers of diluents. J Pharm Pract. 2000;13:214–224.
- Choulis NH, Aronson JK. Miscellaneous drugs and materials, medical devices, and techniques. In: Ray SD, editor. Side effects of drugs annual. Vol. 28. Amsterdam: Elsevier; 2005. p. 587–601.
- Andresen H, Bingel U, Streichert T, et al. Severe glycerol intoxication after Menière's disease diagnostic–case report and overview of kinetic data. Clin Toxicol. 2009;47:312–316.
- Hughes A, Hendrickson RG. An epidemiologic and clinical description of e-cigarette toxicity. Clin Toxicol. 2019;57:287–293.
- Duijst W, Datema J, Dekker R. Acute nicotinevergiftiging met dodelijke afloop [Acute nicotine intoxication with fatal outcome]. PiL. 2017;21:43–45.
- Van der Meer DH, Pranger AD, Jansen I, et al. Fatal intoxication with nicotine for e-cigarette. Ned Tijdschr Geneeskd. 2017;161:D1591.
- Bartschat S, Mercer-Chalmers-Bender K, Beike J, et al. Not only smoking is deadly: fatal ingestion of e-juice—a case report. Int J Legal Med. 2015;129:481–486.
- Basset RA, Osterhoudt K, Brabazon T. Nicotine poisoning in an infant. N Engl J Med. 2014;370:2249–2250.
- Cervellin G, Luci M, Bellini C, et al. Bad news about an old poison. A case of nicotine poisoning due to both ingestion and injection of the content of an electronic cigarette refill. Emerg Care J. 2013;9:18.
- Chen BC, Bright SB, Trivedi AR, et al. Death following intentional ingestion of e-liquid. Clin Toxicol. 2015;53:914–916.
- Christensen LB, van‘t Veen T, Bang J. Three cases of attempted suicide by ingestion of nicotine liquid used in e-cigarettes. Clin Toxicol. 2013;51:290.
- Eberlein CK, Frieling H, Köhnlein T, et al. Suicide attempt by poisoning using nicotine liquid for use in electronic cigarettes. Am J Psychiatry. 2014;171:891.
- Eggleston W, Nacca N, Stork CM, et al. Pediatric death after unintentional exposure to liquid nicotine for an electronic cigarette. Clin Toxicol. 2016;54:890–891.
- Garat A, Nisse P, Kauv M, et al. Lactic acidosis due to voluntary e-liquid ingestion. Toxicol Analyt Clin. 2016;28:329–332.
- Gill N, Sangha G, Poonai N, et al. E-cigarette liquid nicotine ingestion in a child: case report and discussion. CJEM. 2015;17:699–703.
- Gomółka E, Radomska M, Bielska DE. Ostre zatrucie płynem do e-papierosów – opis przypadku [Acute poisoning with e-cigarette liquid – case report]. Przegl Lek. 2016;73:795–797.
- Howard C. A new source for nicotine exposures in pediatric patients: electronic cigarettes. J Emerg Nurs. 2016;42:451–453.
- Jalkanen V, Värelä V, Kalliomäki J. Sähkötupakkanesteen itsetuhoinen käyttö [Case report: two severe cases of suicide attempts using nicotine containing electronic cigarette liquid]. Duodecim. 2016;132:1480–1483.
- Martin-Kleisch A, Leclercq M, Zulfiqar AA. Intoxication voluntaire au liquide de cigarette électronique [Voluntary intoxication with liquid from electronic cigarette]. Ann Fr Med Urgence. 2016;6:428–430.
- Morley S, Slaughter J, Smith PR. Death from ingestion of e-liquid. J Emerg Med. 2017;53:862–864.
- Noble MJ, Longstreet B, Hendrickson RG, et al. Unintentional pediatric ingestion of electronic cigarette nicotine refill liquid necessitating intubation. Ann Emerg Med. 2017;69:94–97.
- Paik JH, Kang S, Durey A, et al. Symptomatic bradycardia due to nicotine intoxication. Rev Bras Ter Intens. 2018;30:121–126.
- Räsänen M, Helanterä I, Kalliomäki J, et al. A case report of successful kidney donation after brain death following nicotine intoxication. Transplant Proc. 2017;49:229–231.
- Schipper EM, de Graaff LC, Koch BC, et al. A new challenge: suicide attempt using nicotine fillings for electronic cigarettes. Br J Clin Pharmacol. 2014;78:1469–1471.
- Seo AD, Kim DC, Yu HJ, et al. Accidental ingestion of E-cigarette liquid nicotine in a 15-month-old child: an infant mortality case of nicotine intoxication. Korean J Pediatr. 2016;59:490–493.
- Thornton SL, Oller L, Sawyer T. Fatal intravenous injection of electronic nicotine delivery system refilling solution. J Med Toxicol. 2014;10:202–204.
- Waldman W, Sein JA. Nagle zatrzymanie krazenia w przebiegu zatrucia nikotyna – opis przypadku i przeglad pismiennictwa [Cardiac arrest during intoxication with nicotine – a case report and a review of literature]. Przegl Lek. 2012;69:606–608.
- You G, Rhee J, Park Y, et al. Determination of nicotine, cotinine and trans‐3′‐hydroxycotinine using LC/MS/MS in forensic samples of a nicotine fatal case by oral ingestion of e‐cigarette liquid. J Forensic Sci. 2016;61:1149–1154.
- Benowitz NL. Nicotine safety and toxicity. New York (NY): Oxford University Press; 1998.
- Herman AI, DeVito EE, Jensen KP, et al. Pharmacogenetics of nicotine addiction: role of dopamine. Pharmacogenomics. 2014;15:221–234.
- Boron WF, Boulpaep EL. Medical physiology. 3rd ed. Philadelphia (PA): Elsevier; 2017.
- Dobelis P, Hutton S, Lu Y, et al. GABAergic systems modulate nicotinic receptor-mediated seizures in mice. J Pharmacol Exp Ther. 2003;306:1159–1166.
- Alkondon M, Pereira EF, Eisenberg HM, et al. Nicotine receptor activation in human cerebral cortical interneurons: a mechanism for inhibition and disinhibition of neuronal networks. J Neurosci. 2000;20:66–75.
- Silvette H, Hoff EC, Larson PS, et al. The actions of nicotine on central nervous system functions. Pharmacol Rev. 1962;14:137–173.
- Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–2303.
- Gorrod JW, Wahren J. Nicotine and related alkaloids: absorption, distribution, metabolism and excretion. London: Chapman & Hall; 1993.
- Baselt RC. Nicotine. Disposition of toxic drugs and chemicals in man. 9th ed. Seal Beach (CA): Biomedical Publications; 2011.
- Benowitz NL, Jacob P. Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clin Pharmacol Ther. 1993;53:316–323.
- Mishra A, Chaturvedi P, Datta S, et al. Harmful effects of nicotine. Indian J Med Paediatr Oncol. 2015;36:24–31.
- Benowitz NL, Hukkanen J, Jacob P. Nicotine chemistry, metabolism, kinetics and biomarkers. In: Henningfield JE, London ED, Pogun S, editors. Nicotine psychopharmacology. Berlin: Springer; 2009. p. 29–60.
- Miller EI, Norris HK, Rollins DE, et al. A novel validated procedure for the determination of nicotine, eight nicotine metabolites and two minor tobacco alkaloids in human plasma or urine by solid-phase extraction coupled with liquid chromatography-electrospray ionization-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:725–737.
- Raja M, Garg A, Yadav P, et al. Diagnostic methods for detection of cotinine level in tobacco users: a review. J Clin Diagn Res. 2016;10:ZE04–ZE06.
- Benowitz NL, Kuyt F, Jacob P, et al. Cotinine disposition and effects. Clin Pharmacol Ther. 1983;34:604–611.
- Göney G. Electronic cigarette (E-cigarette) using: toxicological aspects. Eurasian J Pulmonol. 2017;19:1–7.
- National Toxicology Program. NTP-CERHR monograph on the potential human reproductive and developmental effects of propylene glycol (PG). NTP CERHR MON; 2004;12:I–III6.
- Lim TY, Poole RL, Pageler NM. Propylene glycol toxicity in children. J Pediatr Pharmacol Ther. 2014;19:277–282.
- Generally Recognized as Safe (GRAS) status of propylene glycol. Silver Spring (MD): U.S. Food & Drug Administration; [updated 2015 Sept 29; cited 2018 Oct 1]. Available from: http://wayback.archive-it.org/7993/20171031062706; https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm261045.htm
- Demey HE, Daelemans RA, Verpooten GA, et al. Propylene glycol-induced side effects during intravenous nitroglycerin therapy. Intensive Care Med. 1988;14:221–226.
- Ethylene glycol and propylene glycol toxicity: what is propylene glycol?. Atlanta (GA): Agency for Toxic Substances & Disease Registry; [updated 2007 Oct 3; cited 2018 Oct 1]. Available from: https://www.atsdr.cdc.gov/csem/csem.asp?csem=12&po=14
- Bertholon JF, Becquemin MH, Annesi-Maesano I, et al. Electronic cigarettes: a short review. Respiration. 2013;86:433–438.
- Barnes BJ, Gerst C, Smith JR, et al. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy. 2006;26:23–33.
- Linderoth B, Lind G. Retrogasserian glycerol rhizolysis in trigeminal neuralgia. In: Quinones-Hinojosa A, editor. Schmidek and sweet operative neurosurgical techniques. Vol. 2. 6th ed. Philadelphia (PA): Elsevier Health Sciences; 2012. p.1393-1408.
- Jin ES, Sherry AD, Malloy CR. Metabolism of glycerol, glucose, and lactate in the citric acid cycle prior to incorporation into hepatic acylglycerols. J Biol Chem. 2013;288:14488–14496.
- Stamper RL, Drake MV. Hyperosmotic agents. In: Stamper RL, Lieberman MF, editors. Becker-Shaffer’s diagnosis and therapy of the glaucomas. 8th ed. Missouri (MO): Mosby Elsevier; 2009. p. 431–435.
- Dinakar C, O'Connor GT. The health effects of electronic cigarettes. N Engl J Med. 2016;375:1372–1381.
