Abstract
Context: The influence of co-morbid conditions on the outcome of acute methanol poisoning in mass poisoning outbreaks is not known.
Objective: The objective of this is to study the impact of burden of co-morbidities, complications, and methanol-induced brain lesions on hospital, follow-up, and total mortality.
Methods: All patients hospitalized with methanol poisoning during a mass poisoning outbreak were followed in a prospective cohort study until death or final follow-up after 6 years. The age-adjusted Charlson co-morbidity index (ACCI) score was calculated for each patient. A multivariate Cox regression model was used to calculate the adjusted hazards ratio (HR) for death. The survival was modeled using the Kaplan–Meier method.
Results: Of 108 patients (mean age with SD 50.9 ± 2.6 years), 24 (54.4 ± 5.9 years) died during hospitalization (mean survival with SD 8 ± 4 days) and 84 (49.9 ± 3.0 years; p = .159) were discharged, including 27 with methanol-induced brain lesions. Of the discharged patients, 15 (56.3 ± 6.8 years) died during the follow-up (mean survival 37 ± 11 months) and 69 (48.5 ± 3.3 years; p = .044) survived. The hospital mortality was 22%, the follow-up mortality was 18%; the total mortality was 36%. Cardiac/respiratory arrest, acute respiratory failure, multiorgan failure syndrome, and arterial hypotension increased the HR for hospital and total (but not follow-up) mortality after adjustment for age, sex, and arterial pH (all p < .05). All patients who died in the hospital had at least one complication. A higher ACCI score was associated with greater total mortality (HR 1.22; 1.00–1.48 95% CI; p = .046). Of those who died, 35 (90%) had a moderate-to-high ACCI. The Kaplan–Meier curve demonstrated that patients with a high ACCI had greater follow-up mortality compared to ones with low (p = .027) or moderate (p = .020) scores. For the patients who died during follow-up, cancers of different localizations were responsible for 7/15 (47%) of the deaths.
Conclusions: The character and number of complications affected hospital but not follow-up mortality, while the burden of co-morbidities affected follow-up mortality. Methanol-induced brain lesions did not affect follow-up mortality. Relatively high cancer mortality rate may be associated with acute exposure to metabolic formaldehyde produced by methanol oxidation.
Introduction
Methanol mass poisoning outbreaks present a major public health problem associated with high lethality rates, therapeutic demands, treatment cost, and serious health sequelae in survivors [Citation1–Citation4]. Recent studies estimate hospital mortality during methanol “epidemics” above 20% and the prevalence of toxic long-term visual and brain damage at 40–50% in survivors [Citation5–Citation9]. Poisoning outcome is mainly determined by its severity (grade of metabolic acidosis, state of consciousness, and ability to hyperventilate), time to presentation after methanol ingestion, out-of-hospital ethanol administration, and serum ethanol concentration (as an alcohol dehydrogenase (ADH) inhibitor) at admission to the hospital [Citation10–Citation14].
Although a number of prognostic factors have been identified for hospital survival in acute methanol poisoning, no studies investigated the impact of co-morbidities and complications during hospitalization, as well as long-term health sequelae of poisoning, on hospital, follow-up, and total mortality [Citation15–Citation20]. How to account for the influence of concomitant medical conditions on a patient’s short- and long-term outcomes remains unclear. No data are available in the literature as to whether co-morbid conditions and complications may change the prognosis in methanol-poisoned patients independently of poisoning severity. These facts make accurate risk stratification and triage of newly diagnosed patients during mass methanol poisoning outbreaks challenging [Citation21].
Here we report the findings of a prospective cohort study that investigated the impact of the character and number of hospital complications as well as the burden of co-morbidities – defined by the age-adjusted Charlson co-morbidity index (ACCI) score and the number of co-morbid conditions – on the methanol poisoning outcome. We further examined the association of these factors with hospital and follow-up mortality, cause of death, and long-term health sequelae. The ACCI is an age-weighted prognostic score based on several disease categories that has been validated over the last 30 years and has reliably predicted survival in many patient populations [Citation22,Citation23].
We analyzed the associations on a disease-specific level in order to identify separate co-morbidities and treatment complications that especially increased the risk of death. Further, we investigated the relationship between the burden of multiple co-morbidities and complications and total, hospital, and follow-up mortality. We hypothesized that complications during hospitalization may affect hospital mortality independently of age, sex, and severity of acidemia at admission, and the burden of co-morbidities may have a significant influence on follow-up and total mortality after adjustment for these confounders.
Materials and methods
Study design and population
For this prospective longitudinal cohort study, we analyzed data from the Czech methanol mass poisoning outbreak, which took place in September–December 2012 [Citation24]. All patients hospitalized with confirmed acute methanol poisoning during this outbreak were included in the study. Patients who died before admission to the hospital were excluded. The clinical, toxicological, and biochemical data, including data on personal and family history, co-morbidities, and chronic alcohol abuse, were obtained from treatment providers by applying a standardized data collection form. Responses were sent to the Toxicological Information Center (TIC) on the day following hospital admission. Information on pre-hospital and hospital therapeutic interventions and the outcome was obtained from hospital discharge reports.
The patients who survived and were discharged from the hospital were informed about the prospective study and invited to participate. Those who gave their written informed consent were followed in a prospective and systematic manner until death or the last follow-up 6 years later (31 December 2018; the end of the study). These patients were examined four times (at discharge, then 2, 4, and 6 years after discharge) in one hospital according to the same predefined study protocol described in detail in our previous publications [Citation25–29]. The study was approved by the institutional Ethics Committee and was conducted in accordance with the principles of the Declaration of Helsinki.
Co-morbidity and mortality assessment
Basic demographic and medical parameters, including age, sex, clinical admission (Glasgow coma scale, visual toxicity, neurological, and other clinical signs and symptoms), laboratory data (serum methanol, ethanol, formic acid, arterial blood pH, anion gap, base deficit, bicarbonate, lactate, glucose, creatinine, and others), and treatment modalities (antidote administration, renal replacement therapy, folate substitution, alkalization, mechanical ventilation, vasopressors, and others), were assessed. Co-morbidity data were collected from the patients’ primary medical records and the results of the performed clinical examinations. Specific diagnoses were than grouped according to organ systems.
Co-morbidities were assigned to one of these categories: arterial hypertension, diabetes mellitus, cardiovascular diseases (including ischemic heart diseases, acute coronary syndrome, cardiac arrhythmias, and heart valve diseases), hyperlipoproteinemia, hyperuricemia, kidney diseases, cancer history, stroke history, hypothyroidism, respiratory diseases, anemia, rheumatic diseases, polyneuropathy, alcoholic hepatitis, and epilepsy. The patients were further classified as having 0–1, 2, 3, or 4-or-more co-morbidities. Chronic alcohol abuse and smoking were also considered. Complications during hospital treatment were assigned to one of the following categories: acute respiratory failure/mechanical ventilation, cardiac/respiratory arrest, hospital/aspiration pneumonia, arterial hypotension with low mean arterial blood pressure/vasopressors, delirium, multiorgan failure syndrome, and coma. The patients were categorized as having 0, 1–3, or 4-or-more complications during hospital treatment.
The ACCI score was calculated for each patient based on comorbid health conditions present at the time of hospitalization. A 0–2 ACCI score was considered low, 3–5 was moderate, and 6-or-more was high. Information on the outcome and cause of death was collected from discharge reports that were retrieved through the national total population registry. The linkage was mediated by a personal identity number, a 10-digit number unique to each Czech resident. For patients who died during hospitalization or follow-up, the cause of death was classified as: acute methanol poisoning, malignancy (cancer), cardiovascular disease, alcoholic cirrhosis of liver, or other co-morbid health conditions.
Information on long-term poisoning health sequelae was obtained from the results of follow-up clinical examinations during 6 years of observation. For example, visual sequelae of poisoning were diagnosed by complete ocular examination and standard ophthalmic tests, including visual acuity measurement, slit-lamp examination, intraocular pressure measurement, fundus examination, color vision, visual fields, optical coherence tomography with retinal nerve fiber layer measurements, and visual evoked potentials. Central nervous system sequelae (methanol-induced brain lesions, mainly bilateral necrosis of the putamen) were diagnosed using magnetic resonance (MR) imaging or computer tomography of the brain, single-photon emission computer tomography, and neurological and neuropsychological examinations [Citation30–32].
Calculations and data analysis
Descriptive statistics were used to summarize baseline clinical characteristics. Basic descriptive statistics (mean, median, confidence interval (CI), standard deviation (SD), skewness, and kurtosis) were computed for all variables, which were subsequently tested for normality using the Kolmogorov–Smirnov test. The Chi-squared test was used to compare frequency counts of demographic and clinical categorical variables. Explorative regression analyses were applied to test associations between poisoning outcome and each co-morbidity and complication. Significant results were further analyzed by applying a multivariate Cox regression model where we adjusted for age, sex, and poisoning severity (arterial blood pH) at admission. The hazard ratio (HR) and corresponding 95% CI were reported as measures of association and stability.
Survival was modeled using the Kaplan–Meier method with a log-rank test to compare the burden for each co-morbidity index. Overall survival was defined as time from admission to death or last follow-up. The statistical significance was set at p < .05. The statistical analysis was performed in Excel (Microsoft, Redmond, WA), and formal calculations were produced in QC Expert software 3.1 (Trilobyte, Pardubice, Czech Republic) and IBM SPSS version 24.0 (SPSS Inc., Chicago, IL).
Results
Patients and baseline characteristics
In August 2012, ten thousands liters of toxic spirits containing mixture of 33% of ethanol and 66% of methanol were produced by two entrepreneurs with windshield liquids and further distributed to several illegal producers of strong alcoholic beverages; all of the specimens were mixed and bottled outside the facilities of legal producers. The liquor looked identical to original bottles of rum, vodka, and local spirits. All samples of toxic alcohol contained mixtures of methanol and ethanol, but the final proportion varied substantially, from 20% methanol/80% ethanol to 50% methanol/50% ethanol, in different kinds of strong alcoholic beverages with an alcohol content of around 40% alcohol by volume [Citation24]. During methanol mass poisoning outbreak, 108 patients were hospitalized with confirmed acute methanol poisoning. Twenty additional patients died outside the hospital and were excluded from the study. Of the 108 hospitalized patients, 24 patients (mean age with SD 54.4 ± 5.9 years) died from acute methanol poisoning during hospitalization, with a mean survival time with SD of 8 ± 4 d; 84 patients (49.9 ± 3.0 years; p = .159), including 27 patients with methanol-induced brain lesions on MR, survived and were discharged. Of these patients, 15 subjects (mean age 56.3 ± 6.8 years) died during the follow-up period, with a mean survival time of 37 ± 11 months; 69 subjects (48.5 ± 3.3 years; p = .044) survived until the end of the 6-year study period. Therefore, hospital mortality was 22% and follow-up mortality was 18%; total mortality in the study population was 36%. The youngest patient was 16 years old at the time of methanol poisoning and the oldest was 79 years old. Of those who survived acute methanol poisoning and were discharged from the hospital, 54 patients (64%) were included in the prospective clinical follow-up study. The follow-up mortality data in the other 30 (36%) patients were retrieved from the national total population registry. These 30 patients were older than those who agreed with follow-up (mean age with SD 54.9 ± 4.7 versus 47.1 ± 3.7 years, p = .012), with insignificant difference in gender distribution (p = .341) and in the number of cases with chronic alcoholism (p = .187). At admission, difference in serum methanol, ethanol, and arterial blood pH between two groups was insignificant (all p > .05). The follow-up survival time in the patients who died in this group did not differ from the follow-up survival of those who agreed with the study (31 ± 13 versus 41 ± 17 months, p = .439). The difference in the number of patients who died during the follow-up in both groups was insignificant (10/54 versus 5/30, p = .832). Basic demographic, clinical, and laboratory data of the patients from the study population are presented in .
Table 1. Basic demographic, clinical and laboratory data of 108 patients with acute methanol poisoning (n, %, means with SD).
Patients and co-morbidities
At least one co-morbidity was present in 99 (92%) and at least one treatment complication was present in 50 (46%) of the patients in the study population. The prevalence of various co-morbidities and complications is presented in . Alcoholic hepatitis (including three cases of alcoholic hepatitis with cirrhosis), cardiovascular diseases, and metabolic disorders were among the most frequent conditions. In most patients, four-or-more co-morbidities (up to eight diseases) were present, but there was a significant proportion of patients with only one or none co-morbidity. More than half of the patients had no complications during hospitalization, and all the patients who died in the hospital had at least one treatment complication.
Table 2. Prevalence of co-morbidities and treatment complications in 108 patients with acute methanol poisoning.
In most of the patients, the ACCI score was moderate-to-high at the time of hospitalization (74%), a result that reflects a significant total burden from co-morbidities. Notably, 96% of patients who died in the hospital had a moderate-to-high ACCI score. Arterial blood pH at admission was the only acute laboratory parameter that significantly impacted hospital mortality.
For follow-up mortality, age and ACCI score had a significant impact; 80% of patients who died during the 6-year observation period had a moderate-to-high ACCI score and were older than 50 years. The effect of poisoning severity and character and number of hospital complications on survivor follow-up mortality was not significant. The patients who survived poisoning with methanol-induced brain lesions were elder, with more severe poisoning (lower arterial pH and higher serum methanol), and had a greater number of treatment complications compared to those without brain lesions. The effect of chronic alcohol abuse and smoking was not significant for hospital, follow-up, or total mortality.
Associations between specific treatment complications, co-morbidities, and total and hospital mortality
Explorative analyses of the association between treatment complications and outcome revealed a significant association between hospital or total mortality and cardiac/respiratory arrest, acute respiratory failure, arterial hypotension with low mean arterial blood pressure, coma, or multiorgan failure syndrome, but not with hospital pneumonia or alcohol delirium. In separate co-morbidity categories, only kidney diseases and metabolic disorders were associated with poisoning outcome.
In a Cox regression model, cardiac/respiratory arrest, acute respiratory failure, and arterial hypotension with low mean arterial blood pressure were significant complications for both hospital and total mortality, while multiorgan failure syndrome was a significant complication for hospital mortality (). All patients who died in the hospital had at least one treatment complication, and the presence of any complication influenced total mortality in the study population after adjustment for sex, age, and poisoning severity. For the separate co-morbidity categories, after adjustment, only kidney diseases negatively affected hospital mortality, while hyperlipoproteinemia demonstrated a certain protective effect on both hospital and total mortality.
Table 3. Results of Cox regression analyses of hazard ratio (HR) of total mortality (n = 39), hospital mortality (n = 24), and separate complications and co-morbidities in 108 patients with acute methanol poisoning (unadjusted and adjusted for sex, age, arterial blood pH at admission; HR with 95% CI are shown).
The total number of co-morbidities, but not ACCI score, affected hospital mortality closely to the limit of significance after adjustment for confounders. However, a higher ACCI score was associated with greater total mortality in the study population. The risk of death was 22% higher for the patients with a high ACCI score.
Association between the burden of co-morbidities and 6-year follow-up mortality
During the 6-year follow-up, 15 of 84 patients died. The causes of death were the following: oncological diseases in seven (47%) cases (cancer of prostate, pancreatic cancer, esophageal cancer, lung cancer, and acute leukemia), cardiac diseases in four cases (coronary heart disease and cardiomyopathy), alcoholic liver cirrhosis in three cases, and complications from diabetes mellitus in one patient. The patients who died were older than those who survived (56.3 ± 6.8 versus 48.5 ± 3.3 years, respectively; p = .044). The patients who died during follow-up did not differ from those who survived with regards to gender, number of cases with chronic alcohol abuse, or smoking (all p > .05). The presence of methanol-induced brain lesions and long-term visual sequelae of poisoning did not differ between patients who died and survived (p = .277 and 0.490, respectively). Admission laboratory parameters that reflected poisoning severity and the presence and number of treatment complications had no impact on follow-up mortality (all p > .05).
The effect of separate treatment complications on mortality during the years following hospital discharge was not significant. Only the patients with an episode of alcohol delirium during hospitalization had an almost five-fold greater risk of death during the 6-year follow-up (). These patients had severe alcohol dependence at admission to the hospital, and delirium was caused by withdrawal syndrome after stopping treatment with the antidote.
Table 4. Results of Cox regression analyses of hazard ratio (HR) of 6-year follow-up mortality and separate complications and co-morbidities in 84 survivors of acute methanol poisoning (unadjusted and adjusted for sex, age, arterial blood pH at admission; HR with 95% CI are shown).
With univariate analysis, a higher ACCI score was associated with greater follow-up mortality. Patients with cardiovascular and respiratory diseases had an increased risk of death, but there was no effect of alcoholism and alcoholic hepatitis on follow-up mortality. When controlling for confounders, atrial fibrillation or other cardiac arrhythmias and respiratory diseases increased the risk of death. Methanol-induced brain lesions did not affect follow-up mortality.
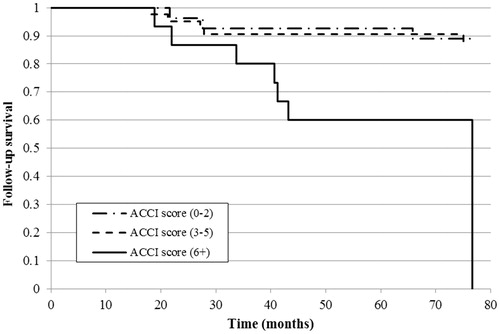
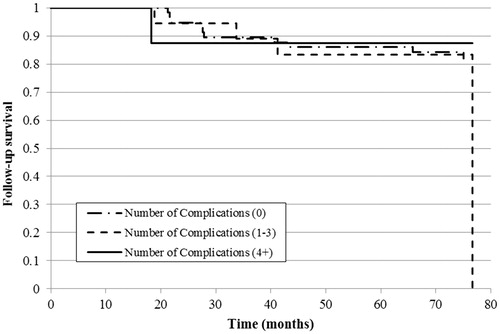
The Kaplan–Meier curve revealed a significant difference in overall survival in patients with a higher ACCI score (6-or-more) compared to low (p < .001) and moderate (p = .013) scores () and in patients without complications during hospital treatment compared to 1–3 or 4-or-more complications (both p < .001). However, there was no survival difference between patients with a moderate or high number of complications ().
Figure 1. Kaplan–Meier curve of overall survival estimate by ACCI score in the study population (n = 108).
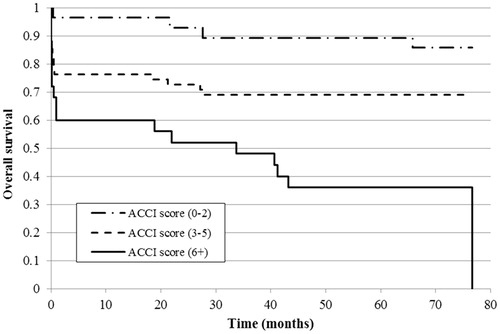
Figure 2. Kaplan–Meier curve of overall survival estimate by the number of treatment complications in the study population (n = 108).
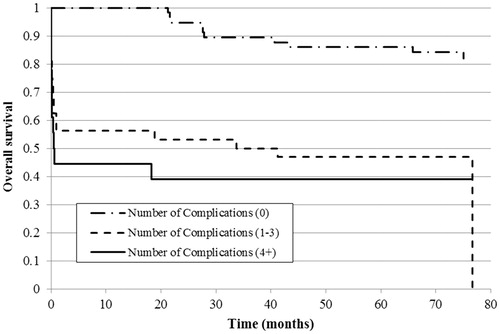
With regards to 30-d hospital survival, patients with a low ACCI score had significantly higher survival compared to those with moderate (p = .023) or high (p = .001) scores, whereas the difference between the last two groups was not significant (). The results were similar for hospital survival when considering the complication number: patients without hospital complications had significantly higher survival compared to 1–3 and 4-or-more complications (both p < .001), but the difference was not significant between the patients with moderate or high numbers of complications ().
Figure 3. Kaplan–Meier curve of 30-d hospital survival estimate by ACCI score in the study population (n = 108).
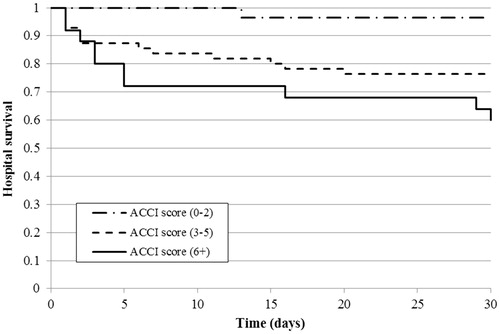
Figure 4. Kaplan–Meier curve of 30-d hospital survival estimate by the number of treatment complications in the study population (n = 108).
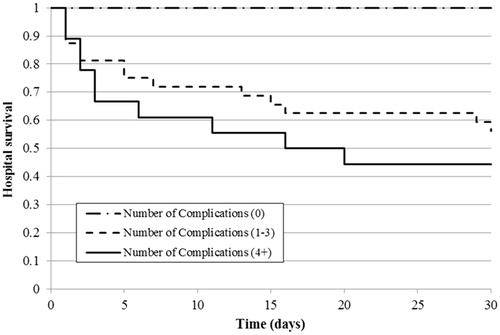
Finally, for follow-up survival, patients with a higher ACCI score (6-or-more) had significantly greater mortality compared to ones with a low (p = .027) or moderate (p = .020) score (). There was no effect from the number of complications during hospital treatment on six-year follow-up survival for hospital survivors ().
Discussion
Limited information is available regarding the impact of co-morbidities on the long-term outcome of acute methanol poisoning and the cause of death following discharge [Citation33]. Here we report a study that evaluated 6-year survival in a cohort of patients with acute methanol poisoning and the relationships between survival outcome and hospital treatment complications, co-morbidities, or poisoning sequelae. Our study demonstrated that the nature and number of treatment complications strongly affected hospital but not follow-up mortality. On the other hand, the co-morbidity burden affected both total and follow-up mortality; patients with a higher ACCI score had a significantly greater risk of death during the study period after adjustment for age, gender, and poisoning severity. There was no effect from chronic alcoholism or alcoholic hepatitis on hospital, follow-up, or total mortality, but the patients with an episode of alcohol delirium caused by withdrawal syndrome during hospitalization had a five-fold higher risk of death during the subsequent years. Long-term CNS sequelae (methanol-induced brain lesions) did not affect follow-up mortality. Finally, in the patients who died during the follow-up period, malignant neoplasms were responsible for approximately half of the deaths.
In our study population with a relatively young mean age, co-morbidities were common at the time of acute methanol poisoning, with alcoholic hepatitis, arterial hypertension, and hyperlipoproteinemia each observed in more than 40% of the patients. Despite the high prevalence of co-morbidities, 45% of patients had fewer than three co-morbidities, a fact that points to a relatively low individual burden of co-morbid conditions in the relatively young patients. Separate co-morbidity categories had a low impact on hospital mortality, whereas the effects of poisoning severity (metabolic acidosis) and treatment complications clearly dominated. In separate co-morbid conditions, only kidney diseases were associated with higher hospital mortality. These patients were treated with renal replacement therapy (RRT), intermittent hemodialysis, extended daily dialysis, or continuous methods of RRT [Citation34,Citation35]. As we previously demonstrated, the RRT modality did not have a significant effect on the outcome [Citation36].
While the ACCI score and number of co-morbidities had limited prognostic value for hospital mortality, the presence of treatment complications increased the risk of hospital death independently of age and poisoning severity. Therefore, episodes of cardiac/respiratory arrest, multiorgan failure syndrome, arterial hypotension with low mean arterial pressure that required high catecholamine doses, and acute respiratory failure had independent prognostic value. All patients who died in the hospital had at least one complication, and only 10% of patients with more than four complications survived. Coma at admission is a known prognostic factor for a poor outcome. In our study, this complication had no independent prognostic value after adjustment for confounders, because this condition was typically accompanied by acute respiratory failure with the need for intubation and mechanical ventilation, low mean arterial pressure that required vasopressors, and often cardiac/respiratory arrest with the need of cardiopulmonary resuscitation.
Hospital or aspiration pneumonia was a relatively frequent complication, but it had no effect on hospital mortality. Timely diagnosis and effective antibiotic therapy may explain this finding. Delirium as a result of alcohol withdrawal syndrome after cessation of hospital treatment with ethanol is another frequent complication in chronic alcohol abusers [Citation37–39]. Its impact on hospital mortality was also not significant; on the other hand, this complication was the only one with independent prognostic value for follow-up mortality. Other complications had no impact on survival during the years following hospital discharge. This finding could be because the patients with delirium were relatively elderly with a long history of severe alcoholism and a high burden of co-morbidities.
Patient mortality during the follow-up period was high; approximately one-fifth of the patients discharged from hospitals died within 26–48 months. In a study by Paasma et al, 6-year follow-up mortality was even higher, at least 30% [Citation33]. The causes of death in the cited study were most frequently alcohol intoxication (unknown alcohol kind or origin; 27%), followed by cardiac diseases (23%), trauma (11%), carbon monoxide poisoning (11%), and pneumonia (8%). Interestingly, in our study, almost half (47%) of the patients who died during the follow-up period succumbed to different cancers (mean survival of 44 ± 21 months after acute methanol poisoning). Their median age at the time of poisoning was 67 (range 49–69) years. Formaldehyde is the first product of methanol oxidation by ADH, and its half-life is very short due to activity of aldehyde dehydrogenase, which can be different due to genetic polymorphism and other reasons [Citation40]. The International Agency for Research on Cancer (IARC) classifies formaldehyde as a Group 1 human carcinogen [Citation41]. In 2011, the National Toxicology Program, an interagency program of the Department of Health and Human Services, named formaldehyde as a known human carcinogen [Citation42]. It is not known if high metabolic formaldehyde production from methanol in acute poisoning may exert carcinogenic potential that is realized in subsequent years. Relatively high cancer mortality rate may be associated with acute exposure to metabolic formaldehyde produced by methanol oxidation. Possible mechanisms of reactive oxygen species production increased by formaldehyde and oxidative stress may play a role as well [Citation43–45]. Alcoholic beverages per se, with low content of methanol, are classified by IARC as a Group 1 human carcinogen as well.
The second most common cause of death during the follow-up period was cardiovascular diseases (27%), a finding similar to Paasma et al. [Citation33]. Several epidemiological studies demonstrated a higher risk of mortality from cardiac diseases in subjects who excessively consumed alcohol [Citation46,Citation47]. On the other hand, despite the fact that 81% of the patients in the study population were chronic alcohol abusers, and the symptoms of alcoholic hepatitis were present in 64% of the patients, only three of 84 (4%) patients died from alcoholic liver cirrhosis during the 6-year follow-up period. There was no association between chronic alcohol abuse, alcoholic hepatitis, and total, hospital, and follow-up mortality. This fact may be explained by the high prevalence of alcoholism in the study population, the dominant impact of poisoning severity and the character of complications on hospital mortality, and the longer time span required for the progression of alcoholic hepatitis to cirrhosis and then to the decompensated form during the observation period in our study [Citation48]. Finally, methanol-induced brain lesions, mainly bilateral putamen necrosis, had no impact on 6-year follow-up survival. Methanol-induced acute neuroinflammation typically does not persist during the years following poisoning suggesting against the shift to chronic neuroinflammation, as it was demonstrated in our previous study [Citation49]. Only three out of 27 patients with brain lesions, two males (53 and 58 years old), and one female (52 years old), died during the follow-up period from alcoholic liver cirrhosis (two cases) or pancreatic cancer (one case).
Predicting follow-up mortality and causes of death during the years after discharge from hospitals in acute methanol poisoning patients is very important for planning screening, clinical examination, and therapeutic intervention measure programs, especially if therapy is needed [Citation50]. Currently, many patients who survive acute methanol poisoning are not involved in long-term medical surveillance programs. The fact that one-fifth of the acute methanol poisoning survivors died within approximately 5 years after discharge, and two-thirds of those deaths were due to malignant neoplasms or cardiovascular diseases highlights the importance of screening, prevention, timely diagnosis, and therapy for these diseases. Ultimately, these measures may translate into longer survival and lower total healthcare-associated costs.
Limitations of the study
The study has certain strengths and limitations. The strengths include the prospective design with a high participation rate, complete 6-year follow-up according to the same standardized clinical protocol in the same medical facility, and the ability to adjust the results for confounders, including acidemia severity at admission (a well-established prognostic factor for acute methanol poisoning). The population of 108 patients poisoned over a relatively short-time period during one mass poisoning outbreak, with two-thirds of survivors systematically followed at one center, represents a sufficient sample size for regression model estimates, but it should be considered as limited; a larger sample size might have provided more significant associations for separate co-morbidity and complication categories. Type II error can be possible due to a limited number of patients with methanol-induced brain lesions in estimating the effect of brain damage on the follow-up mortality.
Further strengths include the fact that clinical, biochemical, toxicological, and co-morbidity data were analyzed in depth and that a total of 16 categories of disorders were considered. We chose a 6-year follow-up period that was longer than the typical 5-year period considered as the criterion for long-term survival in oncological diseases. This period provided sufficient time to estimate the long-term outcome of poisoning; therefore, we operated with complete and reliable information on the long-term impact of co-morbidities and other survival parameters. We analyzed both the impact of specific entities (separate co-morbidity and complication categories) and the overall burden estimated by the ACCI score and the numerical indices (total number of co-morbidities and complications). However, the ACCI score might not reflect the true co-morbidity burden since it is based on International Classification of Diseases (ICD) codes without considering the degree, stage, or disease severity. Nevertheless, it is still a common, widely used simple and objective tool.
Disclosure statement
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this paper. The manuscript has been read and approved by all authors. The authors certify that the submission is not under review at any other publication. The authors certify that the authors have no other submissions and previous reports that might be regarded as overlapping with the current work. The authors declare no financial disclosures.
Additional information
Funding
References
- Hassanian-Moghaddam H, Nikfarjam A, Mirafzal A, et al. Methanol mass poisoning in Iran: role of case finding in outbreak management. J Public Health. 2015;37:354–359.
- Nurieva O, Diblik P, Kuthan P, et al. Progressive chronic retinal axonal loss following acute methanol-induced optic neuropathy: four-year prospective cohort study. Am J Ophthalmol. 2018;191:100–115.
- Rulisek J, Balik M, Polak F, et al. Cost-effectiveness of hospital treatment and outcomes of acute methanol poisoning during the Czech Republic mass poisoning outbreak. J Crit Care. 2017;39:190–198.
- Zhang G, Grews K, Wiseman H, et al. Application to include fomepizole on the WHO model list of essential medicines. 2015 [cited 2015 Aug 3]. Available from: http://www.who.int/selection_medicines/committees/expert/19/applications/Fomepizole_4_2_AC_Ad.pdf
- Hovda KE, Hunderi OH, Tafjord AB, et al. Methanol outbreak in Norway 2002–2004: epidemiology, clinical features and prognostic signs. J Intern Med. 2005;258:181–190.
- Paasma R, Hovda KE, Tikkerberi A, et al. Methanol mass poisoning in Estonia: outbreak in 154 patients. Clin Toxicol. 2007;45:152–157.
- Zakharov S, Pelclova D, Urban P, et al. Long-term visual damage after acute methanol poisonings: longitudinal cross-sectional study in 50 patients. Clin Toxicol. 2015;53:884–892.
- Nurieva O, Hubacek JA, Urban P, et al. Clinical and genetic determinants of chronic visual pathway changes after methanol – induced optic neuropathy: four-year follow-up study. Clin Toxicol. 2018;17:1–11.
- Zakharov S, Kotikova K, Vaneckova M, et al. Acute methanol poisoning: prevalence and predisposing factors of haemorrhagic and non-haemorrhagic brain lesions. Basic Clin Pharmacol Toxicol. 2016;119:228–238.
- Barceloux DG, Bond GR, Krenzelok EP, et al. American Academy of clinical toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol Clin Toxicol. 2002;40:415–446.
- Paasma R, Hovda KE, Hassanian-Moghaddam H, et al. Risk factors related to poor outcome after methanol poisoning and the relation between outcome and antidotes – a multicenter study. Clin Toxicol. 2012;50:823–831.
- Zakharov S, Pelclova D, Urban P, et al. Use of out-of-hospital ethanol administration to improve outcome in mass methanol outbreaks. Ann Emerg Med. 2016;68:52–61.
- Zakharov S, Kurcova I, Navratil T, et al. Is the measurement of serum formate concentration useful in the diagnostics of acute methanol poisoning? A prospective study of 38 patients. Basic Clin Pharmacol Toxicol. 2015;116:445–451.
- Zakharov S, Nurieva O, Kotikova K, et al. Positive serum ethanol concentration on admission to hospital as the factor predictive of treatment outcome in acute methanol poisoning. Monatsh Chem. 2017;148:409–419.
- Desai T, Sudhalkar A, Vyas U, et al. Methanol poisoning: predictors of visual outcomes. JAMA Ophthalmol. 2013;131:358–364.
- Drangsholt E, Vangstad M, Zakharov S, et al. The hypothesis of circulus hypoxicus and its clinical relevance in patients with methanol poisoning – an observational study of 35 patients. Basic Clin Pharmacol Toxicol. 2018;123:749–755.
- Hassanian-Moghaddam H, Pajoumand A, Dadgar SM, et al. Prognostic factors in methanol poisoning. Hum Exp Toxicol. 2007;26:583–586.
- Megarbane B, Borron SW, Baud FJ. Current recommendations for treatment of severe toxic alcohol poisonings. Intensive Care Med. 2005;31:189–195.
- Nurieva O, Kotikova K, Urban P, et al. Prevalence, dynamics, and biochemical predictors of optic nerve remyelination after methanol-induced acute optic neuropathy: a two-year prospective study in 54 patients. Monatsh Chem. 2016;147:239–249.
- Sanaei-Zadeh H, Zamani N, Shadnia S. Outcomes of visual disturbances after methanol poisoning. Clin Toxicol. 2011;49:102–107.
- Hassanian-Moghaddam H. Prioritizing the intoxicated patients for extracorporeal treatments in methanol poisoning. Crit Care Med. 2015;43:e210–e211.
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251.
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682.
- Zakharov S, Pelclova D, Urban P, et al. Czech mass methanol outbreak 2012: epidemiology, challenges and clinical features. Clin Toxicol. 2014;52:1013–1024.
- Zakharov S, Nurieva O, Kotikova K, et al. Factors predicting optic nerve axonal degeneration after methanol-induced acute optic neuropathy: a two-year prospective study in 54 patients. Monatsh Chem. 2016;147:251–261.
- Urban P, Zakharov S, Diblik P, et al. Visual evoked potentials in patients after methanol poisoning. Int J Occup Med Environ Health. 2015;29:471–478.
- Vaneckova M, Zakharov S, Klempir J, et al. Imaging findings after methanol intoxication (cohort of 46 patients). Neuro Endocrinol Lett. 2015;36:737–744.
- Bezdicek O, Michalec J, Vaneckova M, et al. Cognitive sequelae of methanol poisoning involve executive dysfunction and memory impairment in cross-sectional and long-term perspective. Alcohol. 2017;59:27–35.
- Peterová K, Brožová H, Klempíř J, et al. Gait and balance impairment after acute methanol poisoning. Basic Clin Pharmacol Toxicol. 2018;122:176–182.
- Vaneckova M, Zakharov S, Klempir J, et al. Methanol intoxication on magnetic resonance imaging – case reports. Cesk Slov Neurol N. 2014;77:235–239.
- Bezdicek O, Klempir J, Liskova I, et al. Sequelae of methanol poisoning for cognition. Cesk Slov Neurol N. 2014;77/110:320–325.
- Zakharov S, Kotikova K, Nurieva O, et al. Leukotriene-mediated neuroinflammation, toxic brain damage, and neurodegeneration in acute methanol poisoning. Clin Toxicol. 2017;55:249–259.
- Paasma R, Hovda KE, Jacobsen D. Methanol poisoning and long term sequelae – a six years follow-up after a large methanol outbreak. BMC Clin Pharmacol. 2009;9:5.
- Zakharov S, Pelclova D, Navratil T, et al. Intermittent hemodialysis is superior to continuous veno-venous hemodialysis/hemodiafiltration to eliminate methanol and formate during treatment for methanol poisoning. Kidney Int. 2014;86:199–207.
- Zakharov S, Pelclova D, Navratil T, et al. Efficiency of acidemia correction on intermittent versus continuous hemodialysis in acute methanol poisoning. Clin Toxicol. 2017;55:123–132.
- Zakharov S, Rulisek J, Nurieva O, et al. Intermittent versus continuous renal replacement therapy in acute methanol poisoning: comparison of clinical effectiveness in mass poisoning outbreaks. Ann Intensive Care. 2017;7:77.
- Zakharov S, Pelclova D, Navratil T, et al. Fomepizole versus ethanol in the treatment of acute methanol poisoning: comparison of clinical effectiveness in a mass poisoning outbreak. Clin Toxicol. 2015;53:797–806.
- Zakharov S, Navratil T, Pelclova D. Fomepizole in the treatment of acute methanol poisonings: experience from the Czech mass methanol outbreak 2012–2013. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:641–649.
- Zakharov S, Navratil T, Salek T, et al. Fluctuations in serum ethanol concentration in the treatment of acute methanol poisoning: a prospective study of 21 patients. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:666–676.
- Hubacek JA, Jirsa M, Bobak M, et al. Aldehyde dehydrogenase 2 polymorphism affects the outcome of methanol poisoning in exposed humans. Clin Genet. 2018;94:445–449.
- International Agency for Research on Cancer (June 2004). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 88 (2006): Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol. Available from: http://monographs.iarc.fr/ENG/Monographs/vol88/index.php
- National Toxicology Program (June 2011). Report on Carcinogens, Twelfth Edition. Department of Health and Human Services, Public Health Service, National Toxicology Program. Available from: http://ntp.niehs.nih.gov/go/roc12
- Hlusicka J, Loster T, Lischkova L, et al. Role of activation of lipid peroxidation in the mechanisms of acute methanol poisoning. Clin Toxicol. 2018;56:893–903.
- Hlusicka J, Loster T, Lischkova L, et al. Markers of nucleic acids and proteins oxidative damage in acute methanol poisoning. Monatsh Chem. 2019;150:477–487.
- Hlusicka J, Loster T, Lischkova L, et al. Reactive carbonyl compounds, carbonyl stress and neuroinflammation in methyl alcohol intoxication. Monatsh Für Chem. 2019; in press.
- Leong DP, Smyth A, Teo KK, et al. Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case–control study. Circulation. 2014;130:390–398.
- Mostofsky E, Chahal HS, Mukamal KJ, et al. Alcohol and immediate risk of cardiovascular events: a systematic review and dose-response meta-analysis. Circulation. 2016;133:979–987.
- D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231.
- Zakharov S, Hlusicka J, Nurieva O, et al. Neuroinflammation markers and methyl alcohol induced toxic brain damage. Toxicol Lett. 2018;298:60–69.
- Zamani N, Hassanian-Moghaddam H, Roberts D, et al. Consensus statements on the approach to patients in a methanol poisoning outbreak. Clin Toxicol. 2019; accepted for publication. doi:10.1080/15563650.2019.1636992