Abstract
Objective
To assess characteristics of exposures to contaminated poppy and identify trends in exposure and poppy-related deaths.
Methods
Cross-sectional analysis of adverse events associated with exposure to poppy products (primarily poppy seeds) from the American Association of Poison Control Centers’ National Poison Data System (NPDS), 2000–2018, supplemented with analysis of overdoses and deaths related to poppy from the U.S. Food and Drug Administration (FDA) Center for Food Safety and Applied Nutrition’s Adverse Event Reporting System (CAERS) (2004–2018), and the FDA Adverse Event Reporting System (FAERS) (1968–2018).
Results
There were 591 NPDS exposure cases involving poppy between 2000 and 2018 including 392 in persons aged 13+. Rates of intentional exposures in NPDS increased among the age 13+ group over the study period. Most intentional exposures occurred in males in their teens and twenties. NPDS included 18 overdoses and three deaths likely attributable to poppy, most involving poppy seed tea. CAERS and FAERS included five additional deaths likely attributable to opioids in poppy.
Conclusions
Including previously reported cases, there are now at least 19 U.S. deaths associated with poppy seeds in the literature. We recommend that practitioners working in opioid treatment and recovery be alert to use of poppy to treat pain and symptoms of withdrawal.
Introduction
The opium poppy, Papaver somniferum, naturally produces opiate alkaloids including morphine, codeine, and thebaine [Citation1]. For this reason, Papaver somniferum cannot be legally cultivated in the United States. Its seeds may be imported as food or craft products, as they are not expected to contain opiate alkaloids, but the other parts of the plant (referred to as “poppy straw”) and its sap (referred to as poppy “latex”) are considered controlled substances under Schedule II of the Controlled Substances Act because of their opiate content [Citation2]. Poppy seeds can only be cultivated in select countries, and Turkey, the Czech Republic, the Netherlands, China, and Slovakia were the top exporters in 2018 [Citation3].
Poppy seeds may become contaminated with opiate alkaloids from poppy straw and latex in the field and during harvest, necessitating washing and processing to clean the seeds. Cleaning processes recommended by the European Commission have proved effective at reducing morphine contamination from 50 to 220 mg/kg down to four mg/kg or less [Citation4]. However, not all seeds are cleaned effectively, and some are even marketed as “unwashed” or “unprocessed,” conveying a higher likelihood of opiate contamination. Between 2012 and 2017, the European Food Safety Authority collected and tested 1,164 poppy seed samples from 10 countries and detected a mean of 57.8 mg/kg morphine (median = 13.6; 95th percentile = 253; max = 596) [Citation5].
Contaminated poppy seeds can pose serious risks to consumers. Such risks are exacerbated when consumers brew large amounts of seeds into tea, adding acidic ingredients such as lemon juice that help to extract the opiates. This practice is sometimes used for the purpose of intoxication or to provide claimed health benefits including the treatment of pain, anxiety, and opioid withdrawal [Citation6]. Recipes for poppy seed tea on Mercola.com and ChewTheWorld.com recommend combining 200–300 g of poppy seeds with 400 mL of water [Citation7,Citation8]. A 2018 study analyzing the opiate content of 22 samples of poppy seed tea made with poppy ingredients purchased online in the U.S. found up to 2,788 mg morphine per kg seeds after extraction, with a mean and median of 480.8 and 97.3 mg/kg morphine, respectively [Citation1]. If prepared as poppy tea using the 200–300 g recommended online, five to eight of the 22 samples would provide doses of more than 50 mg morphine per 400 mL recipe. A dose of 50 morphine mg equivalents per day has been demonstrated to increase the risk of overdose among patients prescribed morphine for pain treatment [Citation9].
In March 2010, the U.S. Department of Justice published a Drug Alert Watch regarding poppy tea, citing the deaths of five adult males who were reported by medical examiners to have ingested poppy tea shortly before their deaths [Citation10]. More recently, a 2019 literature review compiling cases of acute intoxication from opium poppy worldwide found 20 cases of accidental intoxication among recreational users of poppy since 1968, including 10 since 2010, four of which (all fatalities) occurred in the U.S [Citation11]. Three more publications from 2018 and 2020, not captured in the 2019 review, identify six additional cases of overdose, dependence, or death from poppy seed tea in the U.S. (five males ages 17 to 33, one female age 76) [Citation12–15].
Poppy has been largely overlooked as a small component of the ongoing U.S. opioid epidemic, but early evidence suggests that adverse events resulting from consumption of contaminated poppy seeds may be on the rise [Citation11]. In this study, we assess adverse event reports related to poppy from the American Association of Poison Control Centers’ (AAPCC) National Poison Data System (NPDS), supplemented with an analysis of overdoses and deaths related to poppy from two additional sources: the U.S. Food and Drug Administration’s (FDA) Center for Food Safety and Applied Nutrition’s Adverse Event Reporting System (CAERS) database and the FDA Adverse Event Reporting System (FAERS), which is the agency’s adverse event reporting system for drugs and biologics. With this study, we aim to assess characteristics of exposures to contaminated poppy and to identify trends in exposure and poppy-related deaths over the past two decades.
Methods
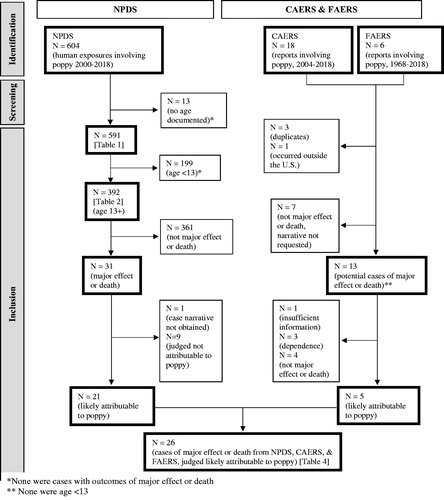
We incorporated data from three different sources in this analysis. demonstrates how our samples from each database were identified and screened, which exposures were included in our analyses, and how the databases were combined for certain analyses.
Data sources
The National Poison Data System is a database maintained by the American Association of Poison Control Centers containing de-identified case data based on calls for telephone consultation services by members of the public and health care professionals to 55 poison control centers that serve the populations of all 50 states, Washington, DC, American Samoa, Federated States of Micronesia, Guam, Puerto Rico, and the U.S. Virgin Islands [Citation15]. Case records in NPDS reflect the information provided by the public or health care professional, and AAPCC is not able to verify the accuracy of every report [Citation16]. We searched the NPDS database in May 2019, using the poppy seed unique identifier code, for human exposure cases (defined by NPDS as “actual or suspected contact with any substance which has been ingested, inhaled, absorbed, applied to, or injected into a human” [Citation16] that occurred between January 1, 2000 and December 31, 2018. All cases identified in this search were included in our sample. Data fields provided by NPDS included date of exposure; location of poison center; number of products; formulation and quantity of each product; age, sex, and weight of subject; chronicity, duration, reason, site, management site, and medical outcome for each exposure; clinical effects and clinical effect duration; route of exposure; and treatments recommended and/or administered. We requested detailed case narratives from individual poison centers for all exposures with the medical outcomes “Major effect” or “Death.”
This study also includes reports from two databases managed by the U.S. Food and Drug Administration: the Center for Food Safety and Applied Nutrition’s Adverse Event Reporting System (CAERS), which contains spontaneous adverse event reports for foods, dietary supplements, and cosmetics submitted by consumers, health care providers, and industry, and the FDA Adverse Event Reporting System (FAERS), a parallel database with adverse event reports on drugs and biologics. Like NPDS, reports reflect the information submitted and FDA is not able to verify the accuracy of every report. We searched the CAERS database in April 2019 using the search term “poppy” for cases that occurred between 2004 (first year of available data) and 2018. Data fields provided by CAERS included event date; product name, type, and description; patient age and sex; reactions; and outcomes. We searched the FAERS database in November 2019 using the terms “poppy seed” and “herbals\poppy seed” for cases that occurred between 1968 and 2018 (the FAERS public dashboard does not allow users to search between specific date ranges, so we searched the entire database which includes cases dating back to 1968). Data fields provided by FAERS included event date; date reported; sex; reason for use; reactions; severity; and outcome. Our searches of CAERS and FAERS identified 18 cases from CAERS and 6 cases from FAERS. We then used Freedom of Information Act requests to obtain case narratives for all U.S. cases that appeared to have potentially resulted in a major effect (using the NPDS definition: “symptoms as a result of the exposure which were life-threatening or resulted in significant residual disability or disfigurement” [Citation16]) or death after consumption of poppy seeds.
Data analysis
We calculated descriptive statistics (counts and percentages) based on NPDS data using Microsoft Excel 2010 and selected age 13 for stratification because data from the 2018 NPDS Annual Report show a distinct transition from unintentional to intentional exposures to any substance between the 6 and 12 year age group and the 12–19 year group [Citation17]. We excluded from our analyses of characteristics of exposures () cases for which age was not documented in NPDS. Analyses by intentionality used the definitions in the NPDS Data Dictionary [Citation16]. Variables we reported were sex, age, region, intentionality, number of products consumed, formulation, chronicity, symptoms, therapies, management site, and outcome. We adjusted exposure rates by age and region by dividing unadjusted rates by the population of each group using data from the 2010 U.S. Census [Citation18,Citation19].
Table 1. Intentionality and Outcome of NPDS Exposures Indicating Poppy, 2000–2018, by Agea.
Table 2. Characteristics of NPDS Exposures Indicating Poppy, Age 13+, 2000–2018, by Intentionalitya.
Table 3. Route of Exposure, Symptoms, Therapies, and Management Site in NPDS Single-Product Exposures Indicating Poppy, Age 13+, 2000–2018a.
To assess trends in NPDS poppy exposures between 2000 and 2018, we calculated the rate of exposures per 100,000,000 population each year using annual U.S. census data (population by age group) from 2000–2018 [Citation20]. We then created annual adjustment factors for the decline in overall volume of NPDS exposure cases by dividing the number of exposures per 1,000 population reported to NPDS each year by the number of exposures per 1,000 population in 2000 (our first year of data) [Citation17]. Annual rates of poppy exposure were divided by these annual adjustment factors to produce rates adjusted for the decline in reporting to NPDS.
Due to the small number of cases reported in CAERS and FAERS, we did not produce descriptive statistics or rates based on our findings from these databases. Instead, we only included CAERS and FAERS data in our analysis of cases associated with major effect or death.
Case narratives from NPDS, CAERS, and FAERS that were associated with a major effect. Duplicate cases found between databases, identified based on age, sex, and date of exposure, were removed. Exposures were considered likely overdoses attributable to exposure to poppy if (1) blood or urine tested positive for opioids; (2) the patient responded positively to administration of naloxone; OR (3) the patient exhibited symptoms of opioid overdose, including respiratory depression, pinpoint pupils, hypotension, cyanosis, hypoxia, pulmonary edema, and coma AND if there was no more plausible explanation for these outcomes or symptoms, including ingestion of another opioid. Another author (EG) identified all discrepancies in coding and these were resolved through discussion between PL and SD until consensus was reached.
Results
Our NPDS search identified 604 exposure cases involving poppy over the study period. Of the 604 NPDS exposures, 13 (2.2%) were excluded because age was not documented. Of the remaining 591 exposures, 392 (66.3%) were in persons 13 and over. Two hundred and twenty-one (56.4%) of the exposures in this group were categorized as intentional or withdrawal (hereinafter after referred to as “intentional”), compared with only three such exposures in the under age 13 group (). A substantial majority (92.5%) of exposures in the younger age group were categorized as unintentional. “Major effect” (27 cases) and “Death” (four cases) were not common, and all such cases occurred in the older age group. Only two of the 78 “Moderate effect” cases occurred in the younger age group. “No effect” was more common in the younger group (28.6% of cases vs. 7.9% of older cases).
Rates of NPDS exposures involving poppy between 2000 and 2018 are presented in . There was an apparent increase in intentional exposures in the age 13+ group and an apparent decrease in unintentional exposures in the same age group reported to NPDS between 2000 and 2018. The increasing trend among intentional exposures in the age 13+ group was still observed when cases with outcomes of “Minor effect,” “No effect,” “Judged as nontoxic,” “Not followed, minimal clinical effects possible,” and “Unrelated effect” were removed. Data from 2016 show a spike in the rate of exposures that was not attributed to any particular state or poison center. Due to low event rates, we were unable to perform statistical analysis of trends in rates of exposure over time. Because our primary interest was intentional exposure to contaminated poppy seeds, we excluded the younger age group from further analyses.
Figure 2. Annual rate of NPDS exposures indicating poppy, 2000–2018a. aCall rates were adjusted for the decline in overall volume of National Poison Data System exposure cases by normalizing reporting rates to 2000.
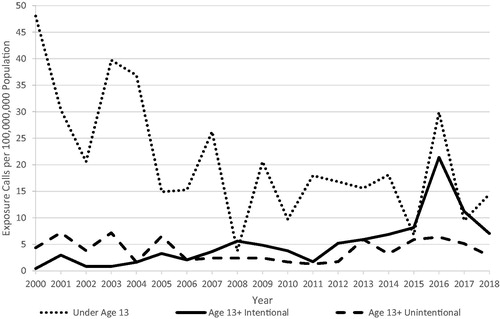
The rate of intentional NPDS exposures per 100,000 population was highest in the age 13–19 (0.19/100,000) and 20–29 (0.22/100,000) age groups (). Exposures occurred across all regions of the U.S. Exposures categorized as intentional were mostly male (72.4%) while exposures categorized as unintentional, adverse reaction, unknown, or other were mostly female (63.7%). Most exposures (74.0%) were single-product exposures, and a higher proportion of intentional exposures involved multiple products (34.8%) compared with unintentional exposures (14.6%). There was a higher proportion of exposures with liquid formulations of poppy among intentional exposures (29.0%) compared with unintentional exposures (7.6%). Most cases were considered acute (331, 84.4%) as opposed to chronic (24, 6.1%) or acute-on-chronic (23, 5.9%).
Among the 290 NPDS reports of individuals age 13+ who were only exposed to poppy (i.e., “single-product exposures”), the route of exposure for the vast majority of cases was ingestion (94.4%), but nine exposures were parenteral, six inhalation, and one rectal (). In this same group, 193 (66.6%) cases included at least one symptom, 124 (42.8%) reported at least one therapy, and 151 (52.1%) were treated in or referred to a health care facility (HCF). The most common symptoms for those with at least one symptom were drowsiness (24.9%), vomiting (22.8%), and nausea (21.2%). Respiratory depression was reported in 12.4% and miosis in 6.7% (). The most common therapies administered among reports with at least one therapy performed were fluids (37.1%), dilute/irrigate/wash (26.6%), naloxone (24.2%), and oxygen (16.9%). Of at least 44 cases admitted to hospital (29.1% of those treated in or referred to a HCF), 26 (59.1%) were admitted to a critical care unit.
Of 31 individual NPDS case narratives with documented outcomes “Major effect” or “Death” requested, only one case, a death, was unobtainable. Twenty-one (including 3 deaths) were considered to be likely overdoses attributable to opiates found in poppy and are included in . Of an additional 13 case narratives for potential serious adverse events or deaths obtained from CAERS and FAERS, five were deaths likely attributable to opiates found in poppy and none was a duplicate of cases from NPDS (these five deaths appear in ); one was a death with insufficient information to determine causality; three were cases of reported dependence to poppy seed tea (not overdoses); and four were not serious adverse events.
Table 4. Poppy-related overdoses and deaths in the United States from CAERS (2004–2018), FAERS (1968–2018), and NPDS (2000–2018).
After reviewing case narratives for cases associated with major effect or death from NPDS, CAERS, and FAERS, we identified 18 cases of non-fatal overdose likely attributable to poppy, all of which were NPDS cases, and eight deaths, of which three came from NPDS cases, and five from CAERS/FAERS cases (). The median age of these 26 cases was 24 years. Five of the cases were female (19%) and the cases came from 18 different states. At least six had histories of depression, anxiety, mood disorder, and/or alcohol use disorder. Twenty case reports stated that the poppy was ingested as a tea, and one individual apparently ingested the seeds themselves. The dose of poppy was not typically reported, but four cases noted acute consumption of between 1.5 and five pounds of seeds soaked in water. The source of the poppy product was also typically unavailable, but multiple reports noted poppy seeds were purchased online, while one exposure involved seeds purchased from a local health food store. All but one case was considered intentional, including one known suicide and one known attempted suicide (data not shown). Twelve cases’ medical histories showed substances other than poppy, and nine cases’ lab tests showed substances other than opiates. Based on medical histories and lab tests, 14 cases involved multiple substances, most commonly benzodiazepines (n = 10) and marijuana (n = 4). Involvement of emergency medical services was confirmed in 15 cases and hospitalization in 20 cases, including 18 admitted to the intensive care unit.
Two of the cases included in the table warrant special mention due to their unique routes of exposure and case trajectories. In one case, a 54-year-old woman who reportedly ingested approximately one lb of raw poppy seeds daily for six days, presented with stomach cramps and vomiting, and died after experiencing a massive colonic obstruction per the coroner’s report. Lab tests were not reported for this case, but given that opiates are known to slow gut motility, and the patient may have continued to absorb opiates from the seeds in her colon, we consider it likely that opiates from poppy were the cause of death.
In the second case, a 36-year-old woman injected herself with a liquid made from crushed poppy seeds in water for treatment of her chronic pain. She immediately experienced flank pain, shaking coldness, headache, and vomiting, but survived. While this case was not considered likely attributable to the opiates in poppy, as the symptoms more likely resulted from infection or contamination associated with the preparation and injection, it highlights a less common but high-risk practice among poppy users. The authors are aware of one additional case resulting in death that indicated injection as the route of exposure and death in the pre-hospital setting, but were unable to obtain the full case narrative.
Discussion
This study included an analysis of data from multiple sources; adjustments for population size and declining PCC call volume; and multiple reviewers assessing causality independently. Our analysis of exposures in NPDS between 2000 and 2018 shows that use of contaminated poppy has led to a substantial number of adverse events, including overdoses and deaths, and the rates of adverse events related to poppy use may be rising. Additionally, our analysis of cases associated with major effect or death from NPDS, CAERS, and FAERS identifies 18 non-fatal overdoses and seven deaths likely attributable to opiates found in poppy, that—based on age, sex, date, and location of exposure—do not appear to have been previously reported in the medical literature or official government reports (one additional death included in our analysis—the 24-year-old male from AR—was already reported by Powers et al. [Citation1]. Combined with fatal cases reported by Poponea et al. [Citation13], Powers et al. [Citation1], Bailey et al. [Citation21], U.S. Department of Justice [Citation10], and Bishop-Freeman et al. [Citation14], there are now a total of at least 19 U.S. deaths associated with poppy seeds in the literature. This is likely a conservative estimate, as additional deaths involving poppy have been reported in the popular media [Citation22].
The case narratives revealed that the majority of serious adverse events related to poppy result from intentional abuse. Studies from the UK and Eurasia have found that home preparations of opium and poppy tea are seen as cheap alternatives to injecting opioids, less risky alternatives to obtaining drugs on the street, or alternative options when opioids are unavailable [Citation23]. These alternatives remain risky due to variability in opioid content, inability to standardize preparations, and consequent risk of accidental overdose.
We found that males in their teens and twenties make up the majority of reports of adverse events related to use of contaminated poppy, and that intentional use of poppy appears to be on the rise. The disproportionate exposure to poppy by males in NPDS is notable given that, in that database, males account for only 36.6% of all reported exposures in teens age 13–19, and 41.7% of exposures in adults age 20 and over [Citation17]. This finding is in line with results from the 2016 National Survey on Drug Use and Health, which show that males are more likely than females to use almost all types of illicit drugs [Citation24].
There are no other examples of food products providing potentially lethal doses of a Schedule II controlled substance in the U.S. food supply. Based on our findings, we recommend that practitioners working in opioid treatment and recovery actively discourage the use of poppy to treat pain and symptoms of withdrawal and instead prescribe approved pharmaceuticals where indicated. We acknowledge that FDA is subject to many competing demands on its limited resources. However, should the agency decide to take action based on the risks documented here, we recommend that FDA consider clarifying to industry and retailers that the marketing and sale of contaminated poppy seeds is illegal, and that poppy seeds must be cleaned and any opiate alkaloids reduced to trace levels before entering commerce. FDA could also issue guidance to industry advising good manufacturing practices to reduce opiate contamination in poppy seeds, including establishing a maximum permissible level such as recommended by the European Food Safety Authority [Citation25].
Limitations
Underreporting in the NPDS, CAERS, and FAERS data systems is a significant limitation of this study, and those events reported may be a biased subset of all reports. Furthermore, the size and directionality of any bias due to selective reporting bias may differ between databases. A previous study of caffeinated beverages found that CAERS typically captures fewer pediatric cases and those with more serious symptoms compared with NPDS [Citation26]. Another limitation was inconsistent reporting by those reporting adverse events as well as potential inconsistencies in recording of those data by technicians, particularly in the case narratives. For example, missing age data led to the exclusion of some cases, although this was relatively rare. The denominator of exposures also cannot be known, particularly given the stigma associated with opioid use. Finally, the reports in NPDS do not establish causality and even causality assessments based on the data provided in case narratives were inherently subjective, although incidents of disagreement between raters were few and resolved by consensus.
Conclusion
We are now aware of at least 19 fatal and 18 non-fatal overdoses associated with contaminated poppy seeds in the United States. Clinicians may be unaware of the risks posed by poppy and FDA could play a stronger role in reducing these risks.
Disclosure statement
Funded by support from Steve and Betty Hacala to the Center for Science in the Public Interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Powers D, Erickson S, Swortwood MJ. Quantification of morphine, codeine, and thebaine in home-brewed poppy seed tea by LC-MS/MS. J Forensic Sci. 2018;63(4):1229–1235.
- 21 U.S.C. §§ 802(17) & 812(c); 21 C.F.R. § 1308.12(b).
- The Observatory of Economic Complexity. Poppy seeds; [cited 2020 July 7]. Available from: https://oec.world/en/profile/hs92/2120791/.
- Commission Recommendation of 10 September 2014 on good practices to prevent and to reduce the presence of opium alkaloids in poppy seeds and poppy seed products. Official Journal of the European Union. 2014/662/EU:96–100.
- Knutsen HK, Alexander J. Update of the scientific opinion on opium alkaloids in poppy seeds. Efsa J. 2018;16(5):5243.
- Haber I, Pergolizzi J, LeQuang J. Poppy seed tea: a short review and case study. Pain Ther. 2019;8(1):151–155.
- Is poppy seed tea safe to drink? Mercola. April 13, 2019; [cited 2020 July 7]. Available from: https://articles.mercola.com/teas/poppy-seed-tea.aspx.
- How to make poppy seed tea recipe the right way: check it here. Chew the World; [cited 2020 July 7]. Available from https://chewtheworld.com/poppy-seed-tea-recipe/.
- U.S. Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage; [cited 2020 July 7]. Available from:. https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf.
- U.S. Department of Justice. Drug alert watch: opium tea. March 2, 2010; [cited 2020 July 7]. Available from: https://www.justice.gov/archive/ndic/pubs40/40404/sw-Opium_Tea.pdf.
- Martinez MA, Ballesteros S. Opium poisoning in modern times. An overview. Forensic Sci Intl. 2019;302:109848.
- Spyres MB, van Wijk XMR, Lapoint J, et al. Two cases of severe opiate toxicity after ingestion of poppy seed tea. Toxicol Commun. 2018;2(1):102–104.
- Poponea N, Kashian MT, Vollstaedt J. Opium poppy seed tea: a lesser known but deadly cocktail. Am J Resp Crit Care Med. 2018;197:A6895.
- Bishop-Freeman SC, Fox L, Winecker RE, Hudson JS. Death from poppy tea consumption. J Anal Toxicol. 2020;44:734–740.
- American Association of Poison Control Centers. National Poison Data System (NPDS), 2020; [cited 2020 July 7]. Available from: https://aapcc.org/data-system.
- American Association of Poison Control Centers. National Poison Data System (NPDS) Data Dictionary, Version 2016.07.11. July 11, 2016.
- Gummin DD, Mowry JB, Spyker DA, et al. 2018 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin Toxicol. 2019;57(12):1220–1413.
- U.S. Census Bureau. Statistical Abstract of the United States: 2012. Updated February 9, 2018. Accessed July 7, 2020. https://www.census.gov/library/publications/2011/compendia/statab/131ed/population.html.
- U.S. Census Bureau. Age and Sex Composition: 2010. May 2011; [cited 2020 July 7]. Available from: https://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf.
- U.S. Census Bureau. Age & Sex Tables (2000–2018). 2020; [cited 2020 Oct 2]. Available from: https://www.census.gov/data/tables/time-series/demo/popest/intercensal-2000-2010-national.html.
- Bailey K, Richards-Waugh L, Clay D, et al. Fatality involving the ingestion of phanezepam and poppy seed tea. J Anal Toxicol. 2010;34:527–532.
- Other deaths from poppy seed tea (and poppy pod tea). 2020; [cited 2020 July 7]. Available from: http://www.poppyseedtea.com/Other%20Deaths%20Page.html.
- Van Hout M, Hearne E. “Vintage meds”: a netnographic study of user decision-making, home preparation, and consumptive patterns of laudanum. Subst Use Misuse. 2015;50:598–608.
- Substance Abuse and Mental Health Services Administration. 2016 national survey on drug use and health: detailed tables. September 7, 2017; [cited 2020 Jul 7]. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2016/NSDUH-DetTabs-2016.pdf.
- European Food Safety Authority. Opium alkaloids in poppy seeds: assessment update. May 16, 2018; [cited August 17, 2020]. Available from: https://www.efsa.europa.eu/en/press/news/180516.
- Markon AO, Jones OE, Punzalan CM, et al. Caffeinated energy drinks: adverse event reports to the US Food and Drug Administration and the National Poison Data System, 2008 to 2015. Public Health Nutr. 2019;22(14):2531–2542.