Abstract
Background
Bites by the European adder (Vipera berus) in the UK are uncommon but potentially life threatening, and can be associated with marked limb swelling and disability. Following an interruption in Zagreb Imunološki zavod antivenom supply around 2012, the UK changed its national choice of antivenom for Vipera berus to ViperaTAb, an ovine Fab monospecific antivenom. In the absence of randomised controlled trials, we established an audit to review its use in clinical practice.
Methods
A prospective audit of ViperaTAb use was conducted from March 2016 until November 2020 by the UK National Poison Information Service (NPIS). Users of the NPIS online toxicology database, TOXBASE, considering the use of antivenom for V. berus envenoming were invited to discuss the case with the on-call clinical toxicology consultant. Information was collected prospectively on indications, administration, adverse reactions and outcome of patients administered ViperaTAb antivenom.
Results
One hundred and seventy patients were administered ViperaTAb antivenom over five years. One hundred and thirty-two were adults and 38 children (median age and range: 38, 2–87 years). Bites occurred across the UK, but most commonly in coastal regions of Wales and of South-West and East England. Median time to presentation was 2.1 (IQR 1.5–4.0) h and to antivenom administration from presentation was 2.0 (IQR 0.9–3.6) h. A minority of patients presented to hospital more than 12 h after being bitten (n = 19, 11.2%) or received antivenom more than 12 h after presenting to hospital (n = 17, 10.0%). Features of systemic envenoming were present in 64/170 (37.6%) patients, including 23 (13.5%) with anaphylaxis and 26 (15.3%) with hypotension (nine with both). Clinician assessment considered the initial antivenom to have been effective in 122/169 (72.2%) patients. Repeated dosing was common, occurring in 55/169 (32.5%), predominantly due to persisting or worsening local effects (46/51, 90.2%). There were three cases of probable early adverse reaction. No deaths occurred during the study. Complications of envenoming were rare but included four patients that underwent surgery, three patients each with acute kidney injury, mild coagulopathy, or thrombocytopenia (one severe). The median duration of hospital stay was 43.7 (IQR 22.5–66.5) h, longer for children than adults (52.5 vs 41.3 h).
Conclusion
ViperaTAb antivenom appears to be effective and safe and should be administered as soon as possible for patients meeting clinical criteria. Patients require close observation following antivenom to detect adverse reactions and progression or recurrence of envenoming. Close collaboration with expert NPIS consultant advice can help optimise antivenom timing, ensure repeated dosing is given appropriately, and avoid unnecessary surgical intervention. All hospitals, particularly those located in areas of relatively high incidence, should stock sufficient antivenom available at short notice, 24 h a day.
Keywords:
Introduction
The European adder (Vipera berus) is the only species of venomous snake native to the UK. Bites are uncommon but potentially life-threatening, with an estimated 50–100 cases occurring annually in the UK [Citation1,Citation2]. Of these, 20–60 cases are treated with antivenom. Envenoming is characterised by bite site pain, bruising, and swelling that is often pronounced and may affect the entire limb and trunk [Citation3]. A minority of patients develop signs (or features) of systemic envenoming, the most concerning of which is a non-immune anaphylactic reaction to venom which develops soon after the bite with angioedema, urticaria, hypotension and collapse. Other features of systemic envenoming include vomiting, diarrhoea, and less commonly cardiotoxicity, acute kidney injury, thrombocytopaenia, and coagulopathy [Citation3,Citation4].
The Zagreb Imunološki zavod antivenom, raised against Croatian Vipera ammodytes venom [Citation5] was used in the UK for many years [Citation1] before an interruption in production around 2012 [Citation6]. This resulted in a change in choice of national antivenom from Zagreb to ViperaTAb (Micropharm) antivenom for moderate-severe Vipera berus envenoming.
ViperaTAb is a monospecific ovine Fab antivenom raised against whole V berus venom (sourced from Russia) manufactured by MicroPharm Ltd, UK. Fab antigen binding complexes are smaller than F(ab′)2 or whole IgG fragments providing the theoretical advantage of enhanced tissue penetration and fewer adverse reactions. However, the increased volume of distribution and shorter half-life of Fab fragments increases the risk of recurrent local and systemic envenoming after antivenom administration [Citation7]. Recurrent venom antigenaemia with coagulopathy after treatment with an Fab antivenom was first demonstrated in Echis ocellatus/romani envenoming in Nigeria [Citation8] and subsequently in a comparative trial of Fab and F(ab′)2 antivenom for Crotalinae envenoming in the USA [Citation9].
As is the case for all antivenoms, the same initial dose of ViperaTAb is given to children and adults. One dose consists of two vials (8 mL, 200 mg), diluted in normal saline and infused intravenously (IV) over 30 min. The preclinical evaluation and 50% binding value for ViperaTAb is superior to that of Zagreb antivenom for V. berus venom [Citation10].
There have been no randomised controlled trials of ViperaTAb [Citation5]. Published observational clinical data from 645 patients in Scandinavia, where ViperaTAb has been in use since 1991 [Citation11–18], suggest efficacy with quick resolution in features of envenoming, comparatively short length of hospital stay, and fewer adverse reactions than with Zagreb antivenom [Citation5,Citation19].
Following the change in national antivenom supply, the UK’s National Poison Information Service (NPIS) prospectively audited the indications, administration, adverse reactions, and outcomes of patients receiving ViperaTAb in the UK over a period of five years.
Methods
A prospective audit was conducted by the NPIS, the UK’s national poison control centre, of all V. berus snakebites from 1 March 2016 until the last case in 2020 (06 November 2020). Records of calls to NPIS are routinely collected into a common database, the UK Poisons Information Database (UKPID).
Clinicians accessing the UK toxicology database TOXBASE for advice on the treatment of V. berus envenoming were prompted with a Pop-Up box to telephone the NPIS and discuss management with the toxicology consultant on call if they were considering antivenom. Demographic data and envenoming details were routinely recorded during these conversations. Advice on antivenom administration (performed under close observation due to the possibility of severe adverse reactions) was provided based on the clinical criteria set out in TOXBASE (Box 1). Formal gradation was not undertaken. Timing of bite, presentation to hospital, antivenom administration and discharge were used to ascertain time to antivenom administration and length of hospital stay. Follow-up telephone calls to support management were made by one consultant. Clinicians were asked whether they considered the antivenom to have been effective i.e., whether it resulted in rapid resolution of systemic features and/or cessation in spread of local oedema and whether any early adverse reactions had occurred.
Box 1. UK national guidelines for antivenom administration for Vipera berus envenoming (TOXBASE, NPIS).
Antivenom should be given if the patient has any of the following features:
Early anaphylaxis-like reactions to the venom.
Hypotension persisting for more than 10 minutes, with or without features of shock.
Systemic features including abdominal pain or diarrhoea.
Definite leucocytosis (especially if over 20 × 109/l)
ECG abnormalities (e.g., bradycardia or widespread ischaemia)
Metabolic acidosis.
Elevated creatine kinase.
Severe local envenoming (even in the absence of systemic features) i.e., swelling spreading beyond the next major joint.
Any other evidence of systemic envenoming e.g., spontaneous haemorrhage, pulmonary oedema
Anonymous data were extracted from data recorded in UKPID. Statistical analysis was performed on Graphpad Prism, Version 8. Data were described using median and interquartile range (IQR). Categorical data were compared using non-parametric statistical tests with a p value of <0.05 considered significant.
This audit did not require approval by a UK Research Ethics Committee as it used information collected routinely as part of usual clinical care, with information provided for analysis in a fully anonymized format.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
One hundred and seventy patients were identified as having received ViperaTAb for snake envenoming during the study period (average of 34 cases/year; range 31–40).
A further 57 cases (seven children) of envenoming who presented to hospital (2 in 2016, 9 in 2017, 14 in 2018, 17 in 2019, 15 in 2020) and were discussed with NPIS did not receive antivenom (because they had only mild local effects not requiring antivenom [36/57, 63.2%], presented too late [usually after 48 h] [17/57, 29.8%], had an unclear diagnosis on initial presentation [3/57, 5.3%]), or because antivenom was not available [1/57. 1.8%]). Of note, five of the patients presenting late, and one of the patients presenting with mild envenoming, were adults who had had early systemic features but had gone to bed rather than coming to hospital. They presented after 1–3 days when their features of their systemic envenoming had settled, and local signs did not require antivenom. These cases are not further discussed here.
Bite occurrence
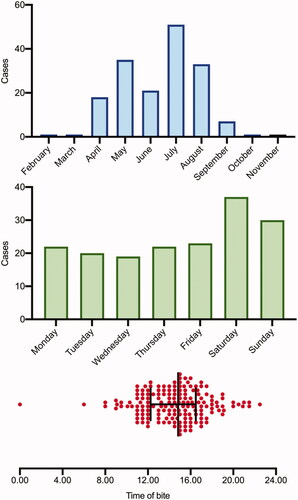
Envenoming occurred predominantly over the summer months with the first case on 26 February and the last case on 06 November (). Envenoming occurred most frequently on weekends between the hours of 12:00 and 18:00 h ().
Figure 1. Time of day (median and IQR), day of the week, and monthly distribution of 170 bites requiring antivenom.
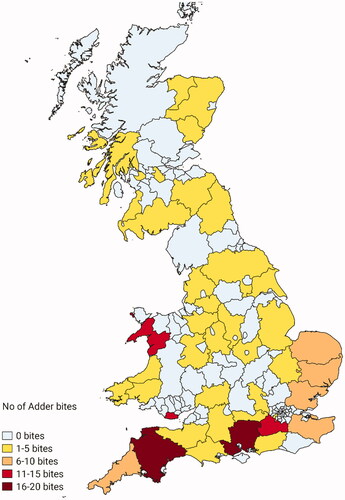
Cases had a predominantly coastal distribution with clusters occurring in West Wales and in South-West and East England. Bites often occurred on coastal paths or beaches, or in forests/heaths. The hospitals that treated most patients with antivenom were located in Bangor (case n = 11), Bournemouth/Poole (n = 11), Truro (n = 10), Swansea (n = 9) and Exeter (n = 8) (). Two patients were envenomed in Sweden but travelled to the UK before seeking medical attention. The snakes were not always seen, sometimes delaying the presentation to hospital and/or the diagnosis.
Patients and clinical features on presentation
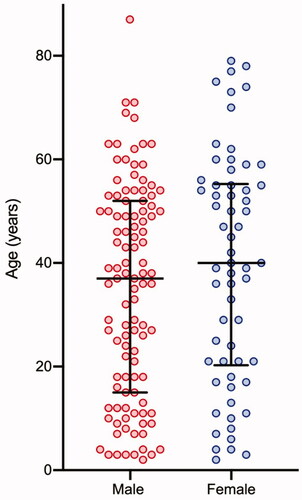
The median age of patients was 38.0 years (IQR 17.5–53.5) with a male predominance (108 male, 62 female) (). There were 38 children (range 2–15 years), 13 of whom were less than five years old.
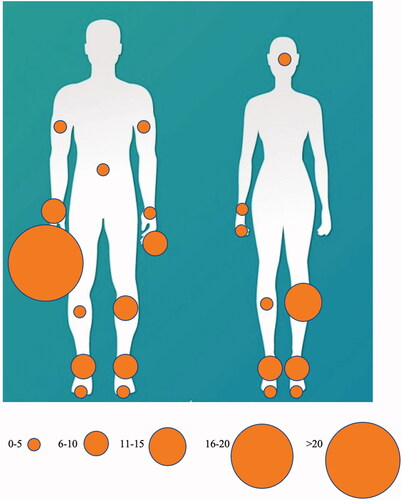
There was a marked difference in bite site for women and men. In men, 74 bites (68.5%) were on upper limbs, particularly the right hand, while 33 (30.6%) occurred on the lower limbs. By contrast, only ten (16.1%) occurred on the upper limbs of women, while 51 (82.3%) occurred on the lower limbs (p < 0.0001) (). There was one bite on the face and one bite on the abdomen.
Figure 4. Location of bites for women and men. Circle size is proportional to the number of bites at that site.
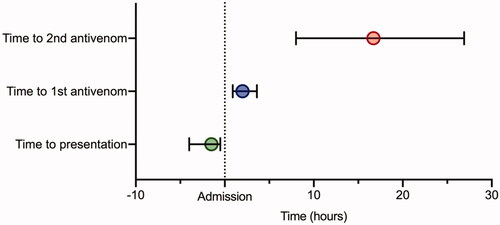
Patients generally arrived quickly at hospital – median time to presentation was 2.1 (IQR 1.5–4.0) h, although 19 cases (11.2%) presented to hospital more than 12 h after the bite. At least four patients were transferred by air ambulance, either from rural areas or from the west Scottish islands to the mainland.
All but two patients presented with localized swelling, with or without pain. Twenty-three (13.5%, including two children) presented with a non-immune anaphylactic reaction, attributable directly to V. berus venom characterized by angioedema, urticaria, and wheeze (). Twenty-six (15.3%, four children) had hypotension, nine with other features of anaphylaxis and 17 without. Of the 41 patients with anaphylaxis and/or hypotension, 11 required bolus intramuscular (IM) or IV adrenaline, usually during transfer, before antivenom could be accessed. Other features of systemic envenoming included vomiting (32) and diarrhoea (14) (). A total of 64 patients (37.6%, 14 children) presented with systemic features. The incidence of systemic features including anaphylaxis and hypotension was similar for children, adults aged 16–64 years old and adults aged >64 years old ().
Table 1. Systemic features related to age in patients envenomed by V berus subsequently treated with antivenom.
Two patients were intubated and ventilated: due to bronchospasm and hypoxia in an asthmatic adult while antivenom was being located and prepared, and due to airway compromise following a facial bite to a child. Six patients were noted to have transient ECG abnormalities attributed to the envenoming (ST changes [2], left axis deviation [1], prolonged PR interval [1], prolonged QT interval [1] and unspecified abnormality [1]). Although laboratory values were not systematically documented, leucocytosis and elevated D-Dimer were commonly reported.
Antivenom administration
Median time to antivenom administration from admission was 2.0 (IQR 0.9–3.6) h – a median of 4.6 [2.8–9.4) h after the bite (). However, seventeen cases (10.0%) received their first dose of antivenom more than 12 h after presenting to hospital. In six patients, antivenom was either delayed or staggered due to insufficient antivenom stock at the initial hospital/health care centre presentation. Envenoming progressed while in the hospital for other patients, leading to the need for antivenom.
The appropriate initial dose of two vials (200 mg) was given to 168 patients. One patient, an 8-year-old girl, received half a dose (1 vial) with the second vial administered 5 h later; the second patient, a 45-year-old woman, received half a dose (1 vial), responded sufficiently and did not require further dosing. Of the 169 (99.4%) patients for whom follow up was completed, 43 (25.4%) received one additional dose and 12 (7.1%) received two additional doses. Overall, 55/169 (32.5%) received additional antivenom dosing, giving a total of 235 administrations.
The reason for repeated dosing of antivenom was identified for 51/55 patients. Persistent systemic features were responsible in five patients (coagulopathy (n = 2), arrythmia and cardiovascular instability (1), thrombocytopenia (1) and hypotension (1)). Forty-six patients received additional dose(s) due to worsening or persistent local effects (swelling, less often haematoma, bite site necrosis). For just two patients, the repeated dose was not clinically indicated according to NPIS consultant review.
Median time to the second dose from the first dose was 15.2 (IQR 6.1–24.5, range 0.8–70.2) h and from presentation was 16.7 (8.0–26.9, range 1.5–70.2) h (). Patients administered a second dose of antivenom for persistent or worsening local envenoming <6 h after the first dose (n = 16) had a shorter length of hospital stay (IQR 52.6 (47.1–69.0)) h than those who received a second dose >6 h after the first dose (n = 29) (71.0 (46.9–98.4)) h (p = 0.08).
The latest a patient received antivenom was 67.0 h after the bite and 47.5 h after presentation. The reasons for the very late antivenom administrations (>36 h after the bite) were delays to presentation to hospital (n = 3), misdiagnosis as infection (1), initial discharge followed by evolution of cytotoxicity (1) and self-discharge and re-presentation (1).
Assessment of effectiveness was attempted by asking clinicians whether they considered the antivenom to have been associated with improvement in their patients. They deemed the initial antivenom to have been effective in 122/169 patients (72.2%), generally with resolution of systemic features and/or a rapid halt in the spread of swelling up the limb. Nineteen of the 122 cases (15.6%) in which an initial response was judged to have been favourable subsequently required at least one additional dose of antivenom. One patient required redosing of antivenom for systemic envenoming (hypotension), 6 h after the initial dose. The remaining eighteen patients received an additional dose of antivenom due to worsening or persistent local effects at a median of time of 22.8 (IQR 7.1–29.1) h after the initial dose.
Safety of ViperaTAb
There were three cases of probable early adverse reactions to antivenom: two of hypotension and one of urticaria tracking up the line of the arm vein receiving the antivenom. The hypotension resolved without treatment while the patient with urticaria was given adrenaline and hydrocortisone. Each of these patients received the dose of antivenom in full and at least one further dose of antivenom without reaction.
There were three possible early reactions to antivenom: one case of worsening facial oedema (already present before antivenom therapy), one case of profuse diarrhoea, and one case of abdominal pain and retching. Each resolved without specific therapy.
Three patients were noted to develop thrombocytopenia (nadir platelet counts of 145, 88 and 5 × 109/L). The lowest count occurred in a child with a platelet count of 35 on admission, before antivenom administration. The first two cases were managed conservatively without platelet infusion; the final case was treated with 1 unit of platelets and two further doses of antivenom as a decision was made that the thrombocytopenia was likely due to the venom, and not the antivenom.
Complications of envenoming
No deaths occurred during the study. Complications of envenoming are listed in . Four patients underwent surgery (necrotic digital ulcers debridement [n = 2], incision and drainage of a haematoma in a patient on an anticoagulant when envenomed [1], and fasciotomy for suspected compartment syndrome (healthy muscle was found at operation) [1]). Five developed metabolic acidosis, three acute kidney injury, three mildly deranged clotting function, and two mild transaminitis. Two patients were noted to have microscopic haematuria on dipstick urinalysis, while three (noted above) had thrombocytopenia that may have been due to venom. All laboratory abnormalities improved prior to discharge from hospital.
Table 2. Effects and complications of envenoming in patients treated with antivenom.
Length of hospital stay
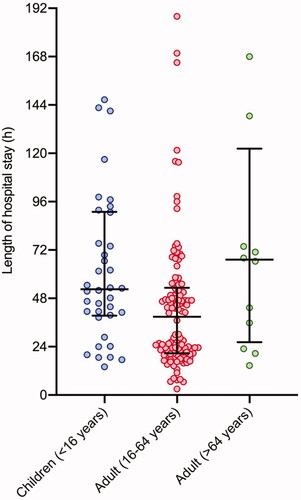
Median duration of hospital stay (n = 169, 99.4%) was 43.7 (IQR 22.5–66.5) h. It was longer for patients aged >64 yrs (67.2 [IQR 26.2–122.3] h), compared to children aged <16 yrs (52.5 [IQR 39.4–91.0] h), and adults aged 16–64 years old (38.8 [IQR 20.7–53.3] h) (Kruskal–Wallis p = 0.004) ().
Eight patients remained in hospital for seven or more days (). The reasons for these prolonged admissions were rehabilitation due to extensive local effects (n = 4), suspected compartment syndrome managed conservatively (2), incision and drainage of haematoma formed at the bite site in a patient on rivaroxaban (1), and fasciotomy for suspected compartment syndrome (1).
Figure 6. Length of hospital stay for children (<16 years old), adults (16–64 years old) and adults (>64years old). Five values lie outside of the limit of the Y-axis (Children <16 years = 212h, Adults (16-64 years) = 242 h, 254 h and 261 h and Adult (>64 years) = 406 h)
presents five cases of envenoming that illustrate a spectrum of severe local and systemic envenoming, the importance of early antivenom and importance of compartmental pressure assessment. The inappropriate prioritisation of surgical assessment over antivenom therapy was observed in several patients.
Table 3. Illustrative cases of severe envenoming and learning points.
Discussion
This prospective study of ViperaTAb supports Scandinavian experience that the antivenom is safe and appears effective for V. berus envenoming. The median length of hospital stay (a useful marker of antivenom efficacy given the predominant local effects of envenoming) of 43.7 h was similar to that in a study of V. berus and Vipera aspis bites treated with a French viper antivenom, ViperFAV, and shorter than reported for cases receiving the Zagreb antivenom [Citation5,Citation19,Citation20].
Patients requiring antivenom typically presented to hospital, were assessed, and received antivenom in a timely manner, often within 4 h of the bite. However, there were delays in presentation and antivenom administration in a few cases. Delays to antivenom after arrival at hospital were sometimes explained by evolving clinical features. However, on other occasions antivenom was delayed due to the limited experience of the clinicians involved, with a sense that orthopaedic review rather than antivenom was the priority. There were delays to antivenom administration in six patients due to limited stock at the presenting hospital. Delays to antivenom therapy for V. berus and V. aspis envenoming has been found to be associated with increased risk of haematoma formation and prolonged functional impairment [Citation21,Citation22].
Seventeen patients delayed their presentation to hospital until it was considered too late (after 48 h) for benefit from antivenom. Scanning of newspaper articles revealed other patients who chose to not attend hospital, with clear consequences for the functional status over the coming weeks and months [Citation23]. Unfortunately, a radio and newspaper campaign in 2017 [Citation24,Citation25] failed to reduce the number of people presenting late to hospital.
The frequency of repeated dosing of ViperaTAb in this study (33.1%) is greater than previously reported in two large case series in Sweden (15.7% and 20.0%) [Citation11,Citation17] and a systematic review of all European antivenom (15.8%) [Citation5]. This may be because of frequent contact between poison centre staff and clinicians looking after the patients, with careful discussion about need for additional antivenom. The common indication for repeated doses in this study was persistence or worsening of local envenoming, in particular swelling and increasing pain. On several occasions, clear benefit became apparent shortly after antivenom administration. This is supported by the shorter length of hospital duration for patients administered early (<6 h) repeated dosing of antivenom for persistent or increasing local envenoming and the illustrative cases 1 and 5 in . However, several French papers on V. berus and V. aspis envenoming report limited beneficial effect on local oedema and no reduction in length of hospital stay for patients administered multiple doses of ViperFAV for persisting oedema [Citation20–22]. As a result, the practice of repeated dosing is reserved for patients with increasing Audebert envenoming severity score [Citation26] or recrudescence of clinical features, following advice from French Poison Control Centres [Citation20].
The need for repeated antivenom dosing because of persisting/recurrent systemic envenoming was uncommon in this study (only 2.9%), similar to that for patients treated with ViperaTAb by the Swedish Poisons information Service (13/415, 3.1%) [Citation17]. This contrasts with a French study which showed that 4/23 (17.4%) and 7/64 (10.9%) of patients with V. berus and V. aspis envenoming, respectively, treated with ViperaTAb developed persisting systemic envenoming after the initial dose of antivenom [Citation20]. This apparent difference in effectiveness between UK/Scandinavia and France warrants further investigation. A comparative randomized control trial would help determine comparative effectiveness and the clinical implications of Fab and F(ab′)2 pharmacokinetics in Vipera genera envenoming [Citation9,Citation27].
Despite promising in vitro cross-reactivity of ViperaTAb across the Vipera genus [Citation10], further evidence is required to demonstrate clinical effectiveness, especially in light of its relatively poor performance against Vipera ammodytes envenoming [Citation23]. Both ViperaTAb and ViperFAV antivenoms are manufactured using V. berus venom sourced from Russia (Ian Cameron, personal communication). Consequently, the possibility of intraspecies geographical variation in venom composition should be considered as this might cause variable effectiveness of antivenom in different geographical locations [Citation28,Citation29].
ViperaTAb was well tolerated, with only three probable and three possible adverse reactions detected. With the exception of one patient who was treated cautiously with adrenaline and corticosteroids, the reported adverse reactions resolved spontaneously. Four of the six patients went on to receive a further dose of antivenom without any additional reaction. The lack of recurrent adverse reactions in repeatedly dosed individuals is suggestive of an alternative mechanism to classical Type-1 IgE mediated hypersensitivity [Citation30]. In addition, it supports the advice that intradermal testing should not be used to predict patients susceptible to adverse reactions to antivenom [Citation31]. It seems likely that the three observed cases of thrombocytopenia, in one case before antivenom treatment and in two cases after antivenom treatment, represent a manifestation of envenoming as commonly described in literature rather than an adverse reaction to antivenom [Citation3,Citation12,Citation22].
Although rare, the pronounced early anaphylactic reaction to venom is a medical emergency and necessitates immediate management with adrenaline while antivenom is prepared. In this cohort, all 23 patients with early anaphylactic reactions – some of whom required multiple doses of adrenaline – responded to antivenom with rapid resolution of symptoms. In one patient, angioedema returned following antivenom administration and a further dose of antivenom was given with good effect. The frequency of systemic features of envenoming was similar for children, adults, and older adults aged >64 yrs, consistent with previous reports of V. berus and V. aspis envenoming [Citation21,Citation22]. Despite this, children more frequently received multiple dosing of antivenom and had modestly longer durations of hospital stay. One possible explanation of this discrepancy is that children experience greater bite site cytotoxicity with more pronounced oedema and haematoma formation due to their higher venom dose per body weight [Citation21].
Four patients underwent surgery during their hospital admission resulting in an increased length of hospital stay (median of 6 days). One case underwent a fasciotomy without prior assessment of compartmental pressure (). The resulting surgery revealed healthy muscle. A further case was due to undergo imminent fasciotomy before advice to reconsider altered the plan and a conservative approach was taken. The patient with severe local envenoming with marked oedema of the thorax, after a bite on the hand, was effectively treated with antivenom only, after measurement of compartmental pressures showed normal pressures (). Local cytotoxicity from V. berus envenoming can result in marked soft tissue oedema but seldom if ever results in compartment syndrome. Both ultrasound and compartmental pressure assessment have been strongly advocated to distinguish extra fascial from subfascial oedema, thus limiting surgical intervention where possible [Citation32–34].
There was marked gender difference in bite site location, with male subjects more likely to be bitten on the upper limbs, especially on their hands (), and females more likely to be bitten on the lower limbs. This tendency was also observed in snakebites in the USA, where 56% of bites in males were on the upper extremity, and 67% resulted from handling the snake [Citation35]. Unlike many tropical countries where envenoming is associated with agricultural work, envenoming in the UK typically occurs during leisure activities. As a result, a significant number of upper limbs bites resulted from deliberate interaction with the snake and demonstrates the need for educational messaging, especially to men, to prevent such envenoming in future.
Limitations
The rarity of adder bite and envenoming in the UK necessitates the use of national poison centre records for prospective data collection. Efforts were made to overcome the inherent limitations of this method by using single caller follow up of cases and objective assessments of outcome (duration of hospital stay, adverse events and need for recurrent antivenom administration) in addition to an independent subjective assessment of effectiveness by the attending clinician. However, limitations are acknowledged with incomplete data collection for three patients, a lack of systematic collection of laboratory findings, a lack of comprehensive ancillary medications used, and no routine follow up arranged after discharge.
Conclusion
V. berus bites necessitating antivenom use are rare but remain a potentially life-threatening emergency in some circumstances as shown by the occasional need for adrenaline or intubation. ViperaTAb antivenom appears to be effective and safe and should be administered as soon as possible for patients meeting clinical criteria outlined in Box 1. Patients require close observation following antivenom for adverse reactions and recrudescence of swelling and pain in the limbs. Close collaboration with expert consultant advice from the NPIS can help optimise antivenom timing, ensure repeated dosing is given appropriately, and avoid unnecessary surgical intervention. All hospitals, particularly those located in areas of relatively high incidence, should stock sufficient antivenom available at short notice, 24 h a day. Increased community awareness of the dangers of snake handling may help avoid some unnecessary upper limb bites.
Acknowledgements
We thank the directors, managers and staff of the UK’s National Poisons Information Service for their help in collecting information for the audit. We thank Annie Watt for her assistance with data collection and management.
Disclosure statement
ME has received non-specific research funding from Micropharm Ltd. The other authors report no conflict of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Coulson JM, Cooper G, Krishna C, et al. Snakebite enquiries to the UK National Poisons Information Service: 2004-2010. Emerg Med J. 2013;30:932–934.
- Jackson G, Eagling VA, Lupton DJ, et al. Native British snake bites in the UK: an estimation of occurence and antivenom usage. Abstract. Clin Toxicol. 2016;54:461–462.
- Warrell D. Treatment of bites by adders and exotic venomous snakes (vol 331, pg 1244, 2005). BMJ. 2006;332:1244–1247.
- Reid HA. Adder bites in Britain. Br Med J. 1976;2:153–156.
- Lamb T, de Haro L, Lonati D, et al. Antivenom for European Vipera species envenoming. Clin Toxicol. 2017;55:557–568.
- Kurtović T, Brvar M, Karabuva S, et al. Pharmacokinetic evaluation of Vipera ammodytes snakebites treated with currently available antivenoms. Abstract. Clin Toxicol. 2019;57:505.
- World Health Organization. Annex 5, WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins. WHO Expert Committee on Biological Standardization, Sixty-seventh report; 2017. p. 197–388.
- Meyer WP, Habib AG, Onayade AA, et al. First clinical experiences with a new ovine Fab Echis ocellatus snake bite antivenom in Nigeria: randomized comparative trial with Institute Pasteur Serum (Ipser) Africa antivenom. Am J Trop Med Hyg. 1997;56:291–300.
- Bush SP, Ruha A-M, Seifert SA, et al. Comparison of F (ab’) 2 versus Fab antivenom for pit viper envenomation: a prospective, blinded, multicenter, randomized clinical trial. Clin Toxicol. 2015;53:37–45.
- Casewell NR, Al-Abdulla I, Smith D, et al. Immunological cross-reactivity and neutralisation of European viper venoms with the monospecific Vipera berus antivenom ViperaTAb. Toxins. 2014;6:2471–2482.
- Karlson-Stiber C, Salmondson H, Persson H. Antivenom treatment in Vipera berus bites – repeated administration in 66 cases treated during the period 1995 to 2008. Abstract. Clin Toxicol. 2009;47:436–510.
- Karlson-Stiber C, Salmonson H, Persson H. A nationwide study of Vipera berus bites during one year-epidemiology and morbidity of 231 cases. Clin Toxicol. 2006;44:25–30.
- Karlson‐Stiber C, Persson H, Heath A, et al. First clinical experiences with specific sheep Fab fragments in snake bite. Report of a multicentre study of Vipera berus envenoming. J Intern Med. 1997;241:53–58.
- Persson H. Envenoming by European vipers antivenom treatment–influence on morbidity. Przegl Lek. 2001;58:223–225.
- Grönlund J, Vuori A, Nieminen S. Adder bites. A report of 68 cases. Scand J Surg. 2003;92:171–174.
- Hønge BL, Hedegaard SK, Cederstrøm S, et al. Hospital contacts after bite by the European adder (Vipera berus). Dan Med J. 2015;62:A5022.
- Personne M, Hultén P. The need of a second antivenom dose after snake bites by Vipera berus. Abstract. Clin Toxicol. 2017;55:488.
- Lapatto-Reiniluoto O, Grönlund J. Severity of Vipera berus bites in Finland. Abstract. Clin Toxicol. 2013;51:274–275.
- Karlson-Stiber C, Persson H. Antivenom treatment in Vipera berus envenoming-report of 30 cases. J Intern Med. 1994;235:57–61.
- Boels D, Hamel JF, Le Roux G, et al. Snake bites by European vipers in Mainland France in 2017–2018: comparison of two antivenoms Viperfav® and Viperatab®. Clin Toxicol. 2020;58:1050–1058.
- Boels D, Hamel JF, Deguigne MB, et al. European viper envenomings: assessment of Viperfav™ and other symptomatic treatments. Clin Toxicol. 2012;50:189–196.
- Jollivet V, Hamel JF, de Haro L, et al. European viper envenomation recorded by French poison control centers: a clinical assessment and management study. Toxicon. 2015;108:97–103.
- Brvar M, Kurtović T, Grenc D, et al. Vipera ammodytes bites treated with antivenom ViperaTAb: a case series with pharmacokinetic evaluation. Clin Toxicol. 2017;55:241–248.
- The Newsroom. Snake bite victims in UK wait too long for treatment. The Scotsman; 2017.
- Usbourne S. How to avoid being bitten by a snake – and what to do if you are. The Guardian; 2017.
- Audebert F, Sorkine M, Robbe-Vincent A, et al. Viper bites in France: clinical and biological evaluation; kinetics of envenomations. Hum Exp Toxicol. 1994;13:683–688.
- Boyer LV, Chase PB, Degan JA, et al. Subacute coagulopathy in a randomized, comparative trial of Fab and F(ab')2 antivenoms. Toxicon. 2013;74:101–108.
- Casewell NR, Jackson TN, Laustsen AH, et al. Causes and consequences of snake venom variation. Trends Pharmacol Sci. 2020;41:570–581.
- Zanetti G, Duregotti E, Locatelli CA, et al. Variability in venom composition of European viper subspecies limits the cross-effectiveness of antivenoms. Sci Rep. 2018;8:9818.
- León G, Herrera M, Segura Á, et al. Pathogenic mechanisms underlying adverse reactions induced by intravenous administration of snake antivenoms. Toxicon. 2013;76:63–76.
- Malasit P, Warrell DA, Chanthavanich P, et al. Prediction, prevention, and mechanism of early (anaphylactic) antivenom reactions in victims of snake bites. Br Med J (Clin Res Ed). 1986;292:17–20.
- Cawrse N, Palmer J, Hayes C, et al. A snake in the clinical grass: late compartment syndrome in a child bitten by an adder. Br J Plast Surg. 2002;55:434–435.
- Türkmen A, Temel M. Algorithmic approach to the prevention of unnecessary fasciotomy in extremity snake bite. Injury. 2016;47:2822–2827.
- Darracq MA, Cantrell FL, Klauk B, et al. A chance to cut is not always a chance to cure- fasciotomy in the treatment of rattlesnake envenomation: a retrospective poison center study. Toxicon. 2015;101:23–26.
- Curry SC, Horning D, Brady P, et al. The legitimacy of rattlesnake bites in central Arizona. Ann Emerg Med. 1989;18:658–663.