Abstract
Introduction
Non-medical use of novel benzodiazepines has recently become common. Here, we describe the recent frequent detection of flubromazolam in patients attending United Kingdom emergency departments.
Methods
Adults presenting to participating hospitals with toxicity after suspected drug misuse were studied between March 2015 and January 2021. Clinical features were recorded using consistent methodology and biological samples analysed using liquid chromatography–tandem mass-spectrometry.
Results
Flubromazolam and/or its mono-hydroxylated metabolite were detected in samples from 14 of 957 patients, all presenting since July 2020. Reported clinical features included reduced level of consciousness (10), confusion/agitation (6) and acidosis (5) but multiple other substances were detected in all patients. All patients survived to discharge (length of hospital stay 3.0 to 213 h, median 24.1 h). There was no correlation between admission blood/serum flubromazolam concentrations (range 1.7–480.5 ng/ml, median 7.4 ng/ml) and Glasgow Coma Scale or length of hospital stay. In one patient who needed intubation and ventilation for five days, there was an exponential decline in flubromazolam concentrations with time (calculated half-life 39.8 h). Hydroxyl-flubromazolam was also identified at all time points.
Conclusions
Flubromazolam has been detected frequently in drug users presenting to UK emergency departments since July 2020. Prolonged toxicity may occur as a result of the long half-life of flubromazolam and the production of metabolites likely to be active.
Introduction
Non-medical use of novel benzodiazepines has become common over the last decade [Citation1–3]. These compounds constituted 13% of all new psychoactive substance (NPS) seizures reported in Europe in 2019 and the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) are currently monitoring 30 benzodiazepine NPS, including 21 that have been identified in Europe since 2015 [Citation4]. They may be supplied by dealers or purchased via the internet, and may masquerade as licensed medicinal benzodiazepines in counterfeit medicines [Citation1,Citation4,Citation5]. Novel benzodiazepines have been associated with toxicity which may be severe, especially if used in combination with opioids [Citation1].
Flubromazolam (8-bromo-6-(2-fluorophenyl)-1-methyl-4H-[1, 2, 4]triazolo-[4,3a] [1, 4]benzodiazepine) is a triazole analogue of flubromazepam structurally related to midazolam and alprazolam (). It was patented in 1978 [Citation1], but is not licensed as a medicine anywhere in the world. Non-medical use was first reported in Europe (Sweden) in 2014 and flubromazolam is now a controlled drug in many European countries including the UK [Citation6]. The main feature of flubromazolam toxicity is sedation, which occurs at oral doses less than 0.5 mg and may be protracted [Citation6–8] as a result of prolonged elimination and/or the presence of active metabolites. Hypotonia, amnesia, disorientation, miosis, mydriasis, tachycardia, and bradycardia have also been reported [Citation7,Citation9–12].
Here, we report recent increases in those attending emergency departments with suspected drug toxicity in the UK where flubromazolam was detected analytically. Elimination half-life is estimated in one case where multiple blood samples were available.
Methods
The UK Identification Of Novel psychoActive substances (IONA) study, launched in March 2015, is collecting clinical information and analysing biological samples from adults (aged at least 16 years) presenting to participating emergency departments with toxicity suspected to result from drug use. Between March 2015 and Jan 2017, inclusion required the presence of severe toxicity (except in Scotland) and suspected use of NPS. This was broadened in January 2017 to include suspected exposure to non-pharmaceutical opioids (e.g., heroin) as part of UK surveillance for the emergence of use of fentanyl and its analogues. In January 2020, criteria were further broadened to include non-medical use of any drug and any severity of toxicity. Inclusion and exclusion criteria were assessed by local research teams under the supervision of the principle investigator at that site (an emergency department physician or clinical toxicologist), in consultation with the treating clinical team.
The study has ethical approval and participant consent is required for inclusion, although this can be deferred for those initially lacking capacity. Anonymised demographic data (age, sex, limited post code) clinical details (admission observations, clinical features recorded during hospital stay, laboratory results, clinical outcome) and linked biological samples (blood or urine with dates and times of collection) are recorded using a standardised data collection sheet and consistent methodology [Citation13]. Further clinical details can subsequently be requested from the clinical notes when necessary, without compromising anonymity. Up to September 2019, samples from England and Wales were analysed in Newcastle University and from Scotland by the Scottish Police Authority Forensic Services. Subsequently, all samples have been analysed by LGC Ltd. For this report, flubromazolam was detected and quantified by mass spectrometry and high-pressure liquid chromatography. Details of the methodology are provided in the Supplementary Material.
Results
Flubromazolam and/or a mono-hydroxylated metabolite, tentatively identified as alpha hydroxy flubromazolam based on accurate mass MS2 data, were identified in samples from 14 of the 957 IONA participants recruited since March 2015 who had analytical data available by 31 January 2021. Flubromazolam and hydroxyl flubromazolam were found in seven, flubromazolam alone in six and the mono-hydroxylated metabolite alone in one. All these 14 participants (age range 20–42 y, median 30 y, 11 males) presented between July 2020 and January 2021, during which period a total of 109 participants were recruited. The first eight flubromazolam cases presented in the south of England including London () but presentations in Northern England and Scotland were also subsequently documented.
Table 1. Details of cases with analytically confirmed exposure to flubromazolam.
Changes in inclusion criteria or laboratory analysis arrangements might have affected the detection of flubromazolam, however, flubromazolam was not detected in any of the 100 participants recruited between October 2019 and June 2020 (all LGC analysed) or the subset of 52 recruited in the first half of 2020 using the broader inclusion criteria. There were also 47 detections of other NPS benzodiazepines between March 2015 and December 2019.
Of those with analytically confirmed exposure to flubromazolam or its mono-hydroxylated metabolite, seven reported use of at least one benzodiazepine, specifically diazepam (6), alprazolam (2) or flubromazolam (1). Other substances reported more than once were cannabis (3), heroin (3), ketamine (3) and “spice” (3). Larger numbers of substances were identified analytically in samples from these participants including other benzodiazepines (12), opioids (10), cocaine (9), gabapentinoids (7), synthetic cannabinoid receptor agonists (“spice”, 5) and ketamine (4, ).
A reduced level of consciousness was reported in 11 patients, with Glasgow Coma Scales (GCS) ranging from 3 to 14. Confusion or agitation (7) and acidosis (5) were also commonly documented. All patients survived to discharge with lengths of hospital stay ranging from 3.0 to 213 h (Median 24.1 h).
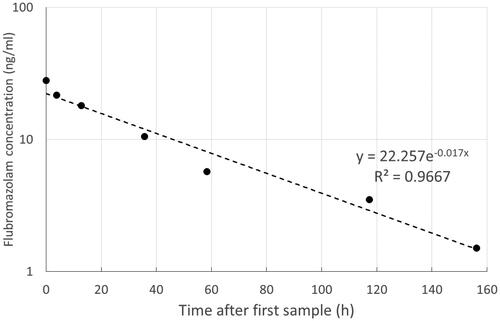
In eight patients where quantification was available, plasma flubromazolam concentrations at presentation ranged from 1.7 to 480.5 ng/ml (median 7.4 ng/ml) and did not correlate with GCS or length of hospital stay. Multiple blood samples were available for analysis from one participant (case 7), who had a history of regular high dose benzodiazepine and gamma hydroxybutyrate use and whose sample analysis demonstrated that he had been exposed to other sedative substances (). He was admitted to the intensive therapy unit (ITU) because of reduced GCS and acidosis, was intubated and received mechanical ventilation for five days. There was an exponential decline in flubromazolam concentrations with time, with a calculated half-life of 39.8 h (). Hydroxyl-flubromazolam was also identified at all time points. This patient was not obese and did not have an elevated plasma creatinine or liver function test abnormalities during this hospital admission.
Discussion
Although detected in Europe since 2014, especially Sweden [Citation3], flubromazolam was not identified in samples from IONA study participants until the second half of 2020, suggesting a recent increase in use and toxicity requiring emergency department attendance in the UK, initially in the South of England. We considered the possibility that the recent detection of flubromazolam in IONA participants reflected changes in inclusion criteria or laboratory analysis procedures, but while other NPS benzodiazepines have previously been detected, flubromazolam was not detected in any participants recruited using the same inclusion criteria and whose samples were analysed in the same laboratory up to July 2020.
This recent apparent increase in use of flubromazolam is consistent with results of analysis of purchased drug product submitted voluntarily by UK users. Flubromazolam was occasionally identified in submitted drug samples during 2015 and 2016 but was not found in any samples in 2017 and 2018. There has subsequently been a steep rise in samples containing flubromazolam in 2019 and especially 2020 [Citation5,Citation8].
Recorded clinical features were similar to those of other benzodiazepines and included reduced level of consciousness, confusion and agitation, but the potential contribution of the large numbers of co-used drugs also detected complicated interpretation and may also explain the lack of correlation between flubromazolam concentrations and the reduction in conscious level or length of hospital stay. The frequent use of benzodiazepines with other sedative substances, especially opioids, is of particular concern because of the potential for enhanced toxicity, especially in view of their high potency [Citation5].
Limited information has been published on flubromazolam concentrations after non-medical use. In a single healthy volunteer ingesting 0.5 mg flubromazolam, the peak plasma concentration was 8.8 ng/ml and estimated elimination half-life 10–20 h. This volunteer experienced somnolence and amnesia for 10 h, although the drug and its mono-hydroxylated metabolite could be detected in urine for 6.5 days [Citation10]. Flubromazolam was detected in concentrations ranging from 0.48 to 100 ng/ml (median 12 ng/ml) in 25 people in Norway suspected of drugged driving or other criminal offences [Citation14]. Serum and urine concentrations of 59 ng/ml and 105 ng/ml were found 19 h after the reported ingestion of 3 mg flubromazolam in one Polish case, who required mechanical ventilation for four days [Citation11]. In two post mortem samples from Denmark, flubromazolam concentrations in femoral blood were 8 and 4.4 ng/mg [Citation9]. In four British post mortem cases presenting between August 2019 and January 2020, femoral blood flubromazolam concentrations ranged from 1 to 70 ng/ml [Citation15]. Other substances, were also found in all six post mortem cases (as in our clinical series) and flubromazolam was not considered the primary cause of death an any of these. Taken together, these data suggest a poor correlation between flubromazolam concentrations and clinical effects and limited utility of blood concentrations for determining flubromazolam as the cause of death. This may be explained by the frequent co-use of other substances that contribute to toxicity as well as variable benzodiazepine tolerance between individuals, depending on prior patterns of use.
Conclusions
During the second half of 2020, there has been an increase in analytically confirmed flubromazolam exposure in people attending UK emergency departments with drug toxicity, mirroring an increase in detections in submitted samples and suggesting recent re-emergence of non-medical use. Other substances were also identified in all cases, with multiple drug exposures common. As a result of its long half-life and the production of metabolites likely to be active, especially hydroxyl-flubromazolam [Citation9,Citation10], prolonged toxicity may be anticipated in severe cases of flubromazolam exposure.
Supplemental Material
Download MS Word (21.2 KB)Acknowledgements
The authors are grateful to the clinicians and research nurses in hospitals enrolled in the IONA study for their essential contribution in recruiting patients and collecting samples and clinical data.
Disclosure statement
The authors report no declarations of interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Manchester KR, Lomas EC, Waters L, et al. The emergence of new psychoactive substance (NPS) benzodiazepines: a review. Drug Test Anal. 2018;10(2):392–393.
- Moosmann B, Auwärter V. Designer benzodiazepines: another class of new psychoactive substances. Handb Exp Pharmacol. 2018;252:383–410.
- Bäckberg M, Pettersson Bergstrand M, Beck O, et al. Occurrence and time course of NPS benzodiazepines in Sweden – results from intoxication cases in the STRIDA project. Clin Toxicol (Phila). 2019;57(3):203–212.
- European Monitoring Centre for Drugs and Drug Addiction. New psychoactive substances: global markets, global threats and the COVID-19 pandemic; 2021; [cited 2021 Feb 17]. Available from: https://www.emcdda.europa.eu/system/files/publications/13464/20205648_TD0320796ENN_PDF_rev.pdf
- Welsh Emerging Drugs & Identification of Novel Substance Project (WEDINOS). Sample results – keyword search 'flubromazolam'; 2020; [cited 2021 Feb 17]. Available from: https://wedinos.org/db/samples/search
- Andersson M, Kjellgren A. The slippery slope of flubromazolam: experiences of a novel psychoactive benzodiazepine as discussed on a Swedish online forum. Nordisk Alkohol Nark. 2017;34(3):217–229.
- El Balkhi S, Monchaud C, Herault F, et al. Designer benzodiazepines' pharmacological effects and potencies: how to find the information. J Psychopharmacol. 2020;34(9):1021–1029.
- World Health Organisation. Critical review report: flubromazolam; 2020; [cited 2021 Feb 17]. Available from: https://www.who.int/docs/default-source/controlled-substances/43rd-ecdd/final-flubromazolam-a.pdf?sfvrsn=887bdf43_4
- Noble C, Mardal M, Bjerre Holm N, et al. In vitro studies on flubromazolam metabolism and detection of its metabolites in authentic forensic samples. Drug Test Anal. 2017;9(8):1182–1191.
- Huppertz LM, Moosmann B, Auwärter V. Flubromazolam – basic pharmacokinetic evaluation of a highly potent designer benzodiazepine. Drug Test Anal. 2018;10(1):206–211.
- Łukasik-Głębocka M, Sommerfeld K, Teżyk A, et al. Flubromazolam—a new life-threatening designer benzodiazepine. Clin Toxicol (Phila). 2016;54(1):66–68.
- Bohnenberge K, Liu MT. Flubromazolam overdose: a review of a new designer benzodiazepine and the role of flumazenil. Ment Health Clin. 2019;9(3):133–137.
- Hill SL, Najafi J, Dunn M, et al. Clinical toxicity following analytically confirmed use of the synthetic cannabinoid receptor agonist MDMB-CHMICA. A report from the Identification of Novel Psychoactive Substances (IONA) study. Clin Toxicol (Phila). 2016;54(8):638–643.
- Høiseth G, Tuv SS, Karinen R. Blood concentrations of new designer benzodiazepines in forensic cases. Forensic Sci Int. 2016;268:35–38.
- Abdul K, Hikin L, Smith P, et al. Flubromazolam: detection in five post-mortem cases. Med Sci Law. 2020;60(4):266–269.