Abstract
Background Recreational use of nitrous oxide (N2O) is associated with many side effects, of which neurological complications are most common. Nitrous oxide abuse is also associated with psychiatric symptoms, but these have received less attention so far. Vitamin B12 deficiency may play a role in the development of these psychiatric symptoms.
Aims To explore the relationship among the occurrence of recreational nitrous oxide-induced psychiatric symptoms, accompanying neurological symptoms, vitamin B12 status and choice of treatment.
Methods A retrospective search for case reports was conducted across multiple databases (Pubmed, Embase, Web of Science, PsycINFO and CINAHL). Keywords included variants of “nitrous oxide”, “case report” and “abuse”. No restrictions to language or publication date were applied.
Results The search retrieved 372 articles. A total of 25 case reports were included, representing 31 patients with psychiatric complications following nitrous oxide abuse. The most often reported symptoms were: hallucinations (n = 16), delusions (n = 11), and paranoia (n = 11). When neurological symptoms were present, patients were treated more frequently with vitamin B12 supplementation.
Conclusions This review highlights the need to recognize that psychiatric symptoms may appear in association with nitrous oxide use. Approximately half of the cases that presented with nitrous oxide-induced psychiatric complaints did not show neurological symptoms, and their vitamin B12 concentration was often within the hospital's reference range. Psychiatrists and emergency physicians should be aware of isolated psychiatric symptoms caused by recreational nitrous oxide abuse. We suggest asking all patients with new psychiatric symptoms about nitrous oxide use and protocolizing the management of nitrous oxide-induced psychiatric symptoms.
Introduction
Nitrous oxide (N2O) has been known as a volatile anesthetic for a long time, but has also gained popularity as a recreational drug [Citation1,Citation2]. It creates feelings of euphoria, relaxation, and dissociation, sometimes even resulting in hallucinatory experiences [Citation1]. Contrary to central nervous system depression from other inhaled drugs, nitrous oxide creates a feeling of euphoria without significantly increasing subjective sleepiness [Citation3], hence acquiring its nickname “laughing gas”.
Among adolescents, nitrous oxide is considered to be a relatively innocuous party drug. This is reflected in the amount of recreational use worldwide. The Global Drug Survey 2019 [Citation4] found that 11.9% of its respondents had used nitrous oxide in the past 12 months, as opposed to 6.3% in 2014 [Citation5]. In the UK, nitrous oxide is the third most popular recreational drug after cannabis and cocaine, with 2.3% of adults aged 16–59 reporting use in the last year. Use amongst 16–24-year olds is 8.7%, making it the second most popular drug in this age group [Citation6].
Small steel canisters of nitrous oxide are sold by most supermarkets as a food additive to generate the foaming structure of whipped cream [Citation7]. These “bulbs” or “whippets” are used to inflate a balloon from which the gas is inhaled. The gas may be exhaled directly into the open air or rebreathed into the balloon for extra effect [Citation1]. As governments have been progressively restricting the distribution of nitrous oxide over the past few years, its availability online is increasing rapidly [Citation8].
Nitrous oxide is clinically used in dentistry, ambulance services, and childbirth. It was regarded as one of the safest general anesthetics until 1956, when Lassen et al. [Citation9] reported the potential risk for bone marrow suppression with nitrous oxide anesthesia. Following several investigations, risks related to occupational exposure appear to be low with correct use. Recreational use is more hazardous due to higher dosage per day and more frequent consumption [Citation10]. The most common manifestation of nitrous oxide intoxication is subacute combined degeneration of the spinal cord, which may be marked by paresthesia, muscle weakness, and ataxia [Citation11]. Other complications of nitrous oxide abuse include megaloblastic anemia, pulmonary infiltration and emphysema, pneumomediastinum, hyperpigmentation, and frostbite to the mouth [Citation12,Citation13]. Numerous nitrous oxide-induced deaths have been reported [Citation7]. These deaths are usually the result of asphyxiation, secondary to the use of nitrous oxide in an unsafe manner.
Considerably less studied side effects of nitrous oxide abuse are psychiatric symptoms. The Dutch Poisons Information Center (DPIC) [Citation14] noted 128 cases of nitrous oxide intoxication in 2019, compared to 54 cases in 2018. Of the former, 15 patients had drug-induced psychiatric complaints such as hallucinations (n = 7) and psychosis (n = 5) [AJH Van Riel, senior toxicologist DPIC, written communication, November 2020]. Garakani et al. [Citation13] first reviewed nitrous oxide-induced psychiatric symptoms in 2016 and Sheldon et al. [Citation15] continued on that note. Since this field is rapidly progressing, multiple new case reports have been published after Sheldon’s revision, which ran until December 2018.
The aim of this review is to analyze case reports describing psychiatric manifestations of recreational nitrous oxide abuse, and to explore the pathophysiology underlying these symptoms. One persistent theory is that nitrous oxide-induced psychiatric symptoms are associated with decreased vitamin B12 concentration. Nitrous oxide inactivates vitamin B12, which plays an important role in several metabolic pathways. While we do not dispute this potential explanation, we question the assumed diagnostic value of vitamin B12 measurement in establishing nitrous oxide-induced psychiatric symptoms. We analyzed the vitamin B12-status as determined at hospitalization in case reports describing recreational nitrous-oxide induced psychiatric symptoms. We also studied what implications the presence of neurological symptoms had on choice of treatment. By doing this, we hope to raise clinical awareness about when to suspect laughing gas abuse in an emergency department patient with unexplained psychiatric symptoms.
Materials and methods
A retrospective search was conducted across several databases. The search was undertaken from December 1, 2019 to June 1, 2020. Pubmed and Embase were searched using the following search terms: “nitrous oxide”, “dinitrogen oxide”, “entonox”, “factitious air”, “hyponitrous acid anhydride”, “stickoxydul”, “laughing gas”, “nitrogen protoxide” and “whippets”. These terms were combined with: “case report”, “recreational”, “abuse” and “misuse”. Web of Science, PsycINFO and CINAHL were searched using “nitrous oxide” and “case report”. No limit was applied to the publishing year, language or study population. The authors screened the abstracts of the search results, and full-text articles were obtained for eligible case reports. Additionally, all selected case reports, as well as reviews used for background literature, were hand searched for relevant references.
Only articles meeting the following requirements were included: (1) the article is or contains a case report or case series; (2) the patient is aged >18 years; (3) it regards intoxication with inhaled nitrous oxide; (4) the use is recreational; and (5) the patient developed new psychiatric symptoms within or directly after the period of abuse. Psychiatric symptoms were defined as “some combination of abnormal thoughts, emotions, behavior and relationships with others”, corresponding to the definition given by the WHO [Citation16]. Articles were excluded if: (1) nitrous oxide addiction was the only psychiatric disorder described; or (2) the full-text article was not available, even after contacting the authors. This was the case for several congress abstracts.
Every case report was evaluated by at least two authors of this review. The presence of psychiatric symptoms was registered per category and was matched with the presence of neurological symptoms, all expressed in percentages. The choice of treatment was registered in percentages. Figures were created using GraphPad Prism 8.4.3 software (GraphPad Software, La Jolla, CA) and Microsoft Office Powerpoint 2020. This literature review was performed according to the PRISMA guidelines.
Results
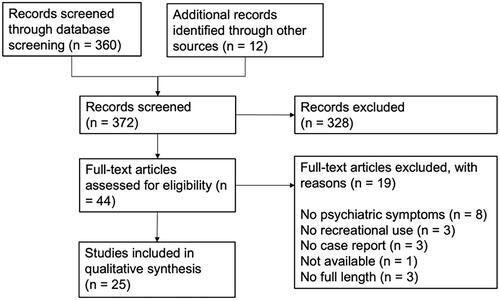
The search in Pubmed and Embase provided us with 261 results, of which 20 case reports were included (). The search on Web of Science, PsycINFO, and CINAHL yielded 99 search results in total and recovery of 1 additional case report. Examination of the reference lists of all selected case reports yielded 12 additional articles, of which 3 were included in this review. One additional case report [Citation15] was found during the search for background literature. We excluded a case series by Layzer [Citation17] from 1978, although mentioned in multiple other reviews, because the cases with psychiatric symptoms were not discussed individually. Therefore, it was not possible to distinguish the clinical course for these patients.
In total, we included 25 case reports, representing 31 patients with psychiatric complications following nitrous oxide abuse (). The cases originated from nine different countries, predominantly from North America (n = 13) and Asia (n = 10). Six patients were not staying in their country of origin at the time of use and hospitalization, e.g., immigrants and exchange students. One patient [Citation32] was staying abroad during the period of abuse but returned home prior to hospitalization. Of the 31 cases, 15 were female and 16 were male (). The median age at hospitalization was 21 years (Q1–Q3: 21–27) for the female patients and 27 years (Q1–Q3: 24–33) for the male patients. One article [Citation38] did not specify the exact age of the patient and was excluded from calculation of the median age. Most patients had abused nitrous oxide for a prolonged period of time. Only 2 cases presented with acute intoxication, and 6 cases with subacute intoxication (). In 5 cases, the period of abuse was not reported (). Simultaneous use of other recreational drugs was reported in 11 cases (). Alcohol abuse was mentioned in one case and use of alcohol was described in six other cases ().
Table 1. Overview of case reports reviewed.
Psychiatric symptoms were classified based on Kaplan & Sadock’s Comprehensive Textbook of Psychiatry into categories of disturbance: thinking, perception, consciousness, memory, cognition, mood, anxiety, and somatic [Citation39]. Neurological symptoms were found in half of the cases (16 patients). Of all the reported psychiatric symptoms (), hallucinations (n = 16), delusions (n = 11), and paranoia (n = 11) were most prevalent. The psychiatric symptoms that were most frequently accompanied with neurological symptoms were: paranoia (8 out of 11 cases with neurological symptoms), disturbances of memory (6 out of 8), disturbances in thought contents (5 out of 7), disturbances in cognition (4 out of 5), anxiety (4 out of 5), and disturbances in orientation (3 out of 4).
Table 2. Number of psychiatric symptoms (n = 31) reported and the presence of neurologic symptoms (n = 16).
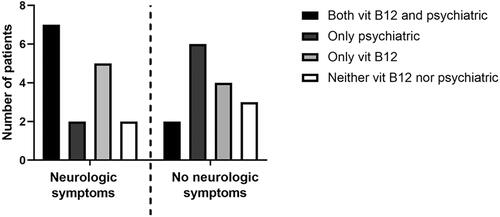
In 21 cases, total vitamin B12 concentration (either in blood or in serum) was determined. In three case reports [Citation8,Citation33,Citation36] the authors did not report an original B12 value, but reported an interpretation instead (e.g., “vitamin B12 deficiency present”). One article [Citation21] did not provide a reference range, but the B12 concentration (602 pmol/L) did not indicate a deficiency. In 14 patients, the vitamin B12 value was below the given reference range. In 7 patients, vitamin B12 was within the reference range. Treatment regimens for patients presenting with psychiatric symptoms were compared for the presence of additional neurological symptoms (). The different treatment regimens were categorized as “vitamin B12 supplementation”, “psychiatric medication” (antipsychotics, antidepressants), “both”, or “neither”. Medication for symptom relief (e.g., benzodiazepines) were excluded from our analysis as not being specific to the condition studied. A treatment schedule including vitamin B12 supplementation was chosen more frequently when neurological symptoms were present (7 cases with both treatments, 5 cases with only B12 supplementation), than if the patient presented with psychiatric symptoms only (2 cases with both treatments, 4 cases with only B12 supplementation). This may be explained by the finding that vitamin B12 concentration was determined in 15 out of 16 patients with neurological symptoms, as opposed to 7 out of 15 patients without neurological symptoms. Of the 18 patients treated with vitamin B12 supplementation, 8 made a full recovery and 5 were left with residual symptoms. In 5 cases the outcome was unknown. Of the 13 patients without vitamin B12 supplementation, 4 made a full recovery and 1 was left with residual symptoms. In 8 cases the outcome was unknown.
Pathophysiology
Nitrous oxide is a sympathomimetic gas that raises the blood concentration of circulating norepinephrine, leading to increased peripheral vascular resistance, cardiac output and blood pressure [Citation40]. In addition, nitrous oxide increases cerebral blood flow causing elevated intracranial pressure [Citation40]. Clinical neurological symptoms may affect both the central and peripheral nervous system, mostly secondary to disruption of vitamin B12 metabolism [Citation24]. For psychiatric symptoms, the pathogenic mechanism of nitrous oxide is not yet known, but studies suggest that there might also be an association with vitamin B12 metabolism [Citation13]. Hallucinations, psychosis, irritability, delirium and apathy have been described as symptoms of vitamin B12 deficiency [Citation41,Citation42].
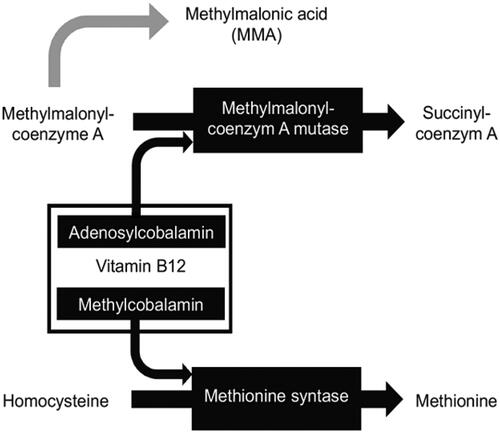
Vitamin B12, otherwise known as cobalamin, is characterized by a central cobalt ion surrounded by a corrin ring. Depending on the residual group bonded to the central cobalt ion, cobalamin is referred to as methyl-, adenosyl-, hydroxo- and cyanocobalamin. Nitrous oxide irreversibly inactivates vitamin B12 in vivo by oxidizing the cobalt ion from its Co1+ valent form to a Co2+ valent form [Citation43], transforming it from the monovalent to the divalent state [Citation44]. Cobalamin has an important metabolic role in two pathways, through two of its chemical forms adenosylcobalamin and methylcobalamin (). In the mitochondria, adenosylcobalamin serves as cofactor for methylmalonyl coenzyme A mutase, to convert methylmalonyl-coenzyme A into succinyl-coenzyme A. Without the presence of the cofactor adenosylcobalamin, methylmalonyl-coenzyme A will be converted into methylmalonic acid (MMA). In the cytosol, methylcobalamin serves as a cofactor for methionine synthase, to convert homocysteine to methionine [Citation44]. Methionine synthase is a cytosolic enzyme that plays a crucial role in the formation of methyl groups for the synthesis of DNA, RNA, and catecholamines [Citation43]. Furthermore, methionine synthase is essential for methylation of the myelin sheath phospholipids [Citation45]. Deficiency of methylcobalamin can lead to decreased activity of methionine synthase and thus decreased DNA formation and demyelination. Cobalamin deficiency is accompanied with the elevation of MMA and homocysteine due to reduced metabolism in both pathways. Accumulation of homocysteine is closely related to vascular endothelial injury and thrombosis [Citation46], and accumulation of MMA leads to demyelination of neurons [Citation47]. Both of these processes could contribute to the development of psychiatric disorders.
Figure 3. Metabolic reactions involving vitamin B12. Vitamin B12, or cobalamin, affects two metabolic pathways through its active forms adenosylcobalamin and methylcobalamin. They are cofactors for reactions in which methylmalonyl coenzyme A mutase and methionine synthase are substrates. Without vitamin B12, coenzyme A is converted to methylmalonic acid (MMA) and homocysteine is not metabolized, leading to elevated serum concentrations of MMA and homocysteine. The black arrows represent the physiological process. The grey arrow represents the process in absence of vitamin B12.
The exact pathology underlying the psychiatric symptoms is unknown. Vitamin B12 deficiency is one of the numerous proposed, and sometimes contradictory, explanations. Other potential mechanisms are an increased production of nitric oxide (NO) [Citation48] (which plays a role in various processes in the central nervous system [Citation49,Citation50]), antagonism of the N-methyl-D-aspartate (NMDA) receptor (a subtype of excitatory glutamate receptors in nerve cells [Citation24,Citation40]) and nitrous oxide induced dopamine release in the nucleus accumbens [Citation51]. Although different theories are linked to various psychiatric symptoms, the pathogenetic mechanism for many of these psychiatric symptoms cannot be exactly demonstrated.
Discussion
Of the 31 patients with nitrous oxide-induced psychiatric symptoms in the 25 case reports, only 16 had additional neurological symptoms reported. Vitamin B12 concentration was below the reference range in 14 patients, within the reference range in 7 patients and unknown in 10 patients. The choice of treatment included vitamin B12 supplementation more often when neurological symptoms were present at hospitalization. A treatment regimen containing vitamin B12 was given in 18 patients, of which 8 fully recovered. Treatment without vitamin B12 was given in 13 patients, of which 4 fully recovered.
In most cases of nitrous oxide intoxication, patients present with neurological, hematological or neuropsychiatric symptoms [Citation13]. Nevertheless, psychiatric disturbances may also be the first presenting symptoms [Citation52] and may occur in the absence of the well-known neurological and hematologic abnormalities [Citation38,Citation53]. Some reports [Citation54] suggest that psychiatric manifestations often predate the neurological symptoms, by as much as several years.
Vitamin B12 deficiency, defined according to standard reference ranges, is not found in all presented cases. This could be explained by the fact that reference ranges are often based on hematological indices only [Citation55]. Although most hospitals have a lower limit of 200 pg/mL in blood or serum, psychiatric symptoms may present before the patient’s vitamin B12 concentration drops below this limit [Citation54]. It is hypothesized that vitamin B12 levels in cerebrospinal fluid (CSF) decrease before the levels in serum, since several studies found a cobalamin deficiency in CSF without a deficiency in serum [Citation56,Citation57]. In order to prevent neuropsychiatric symptoms, some authors have proposed that the vitamin B12 threshold should be increased to at least 600 pg/mL, which is much higher than the average hospital lower limit [Citation58,Citation59]. In our search three cases presented with a vitamin B12 level in the range of 200–600 pg/mL, cases which would have been missed with the conventional hospital lower limit [Citation11,Citation34,Citation35]. Furthermore, MMA determination in serum could provide additional discriminative information about the cobalamin status [Citation60].
If present and left untreated, vitamin B12 deficiency can delay or preclude the recovery of psychiatric symptoms [Citation61]. Treatment with oral or intramuscular vitamin B12 is recommended, and short-term supplementation with daily dosages of 1000 μg cyanocobalamin ought to be sufficient [Citation24]. Complete recovery with vitamin B12 supplementation alone has been described in the literature [Citation42,Citation53], but often vitamin B12 is combined with antipsychotic or mood stabilizing medication [Citation13,Citation62]. Our results showed that measuring vitamin B12 concentration and treatment with vitamin B12 supplementation occurred more frequently when neurological symptoms were present. This results in a discordance between the treatment of patients with and without neurological symptoms, likely explained by the difference in physicians’ awareness of a possible association between psychiatric symptoms and vitamin B12. One of the case reports [Citation32] described a treatment regimen involving hyperbaric oxygen therapy (HBOT), based on the seeming effectiveness of HBOT in cases of delayed encephalopathy after acute carbon monoxide poisoning [Citation63]. The patient in this article presented with various symptoms, including tetraparesis, disturbance of orientation and memory, and irritability. He made a full recovery, upon which the authors marked HBOT as a promising treatment option for cognitive dysfunction after nitrous oxide abuse. The authors found an improvement in the patient's gyrus atrophy on MRI after hyperbaric oxygen therapy, but the patient was simultaneously being treated with vitamin B12 and quetiapine. Both these drugs can act therapeutically in nitrous oxide-induced psychiatric disease, making it difficult to judge the individual effectiveness of the HBOT.
Whilst the influence of reporting bias on case reports should not be underestimated, we noticed some interesting differences when comparing our case reports to epidemiological data from the Global Drug Survey (GDS) [Citation4]. Most of our case reports originate from the US, whilst the GDS data show that the Netherlands and the United Kingdom have the highest percentage of users. The number of case reports originating from Europe is hence relatively low compared to North America. However, the majority of the GDS respondents reside in Germany (29.0%) and other European countries (38.5%), which causes a significant selection bias. We also noticed a relative scarcity of Asian respondents in the GDS, whilst a major part of our cases originates from Asian countries (n = 9). Apart from reporting bias in case reports, this apparent discrepancy may also relate to differing attitudes towards nitrous oxide use between various countries. There is an overall underrepresentation of African and South American countries in publications about nitrous oxide-induced symptoms. It is unknown to us to what extent this results from underreporting or from less use of nitrous oxide in these countries. As the GDS data were collected through a voluntary online survey, they most likely overestimate the actual use in the world. In the US national survey of 2018 [Citation64], 0.7% of the population aged 12 years and over admitted to using inhalants that year. This is much lower than the 11.9% described by the GDS 2019 [Citation4].
This review has several limitations. First of all, the number of case reports is limited, as psychiatric symptoms are not the most common manifestation of nitrous oxide abuse. Furthermore, we only searched international databases, which carries the risk of missing articles from local journals. Because we only used case reports, recall bias may be present. Most articles could not precisely specify the amount of nitrous oxide, which inhibited further inferences about the amount of use.
We cannot rule out the dual diagnosis of a psychiatric disorder combined with a substance-use-disorder, since drugs of abuse may be utilized to self-medicate by people with psychiatric disorders and drug abuse may also unmask psychiatric disorders [Citation65,Citation66]. None of the patients in our search were subsequently diagnosed with a psychiatric disorder in their follow-up period; however, follow-up periods were short.
Two patients [Citation23,Citation28] presented with a history of vitamin B12 deficiency. These patients were not excluded, as their symptoms presumably emerged after a further decrease in vitamin B12 concentration through nitrous oxide abuse. Both patients had been using nitrous oxide for several years at the time of admission, so the possibility exists that their pre-existing vitamin B12 deficiency was the result of this earlier nitrous oxide abuse. Baseline vitamin B12 concentrations are absent in all other cases, so it is probable that some of them also had pre-existing vitamin B12 deficits, as a low or marginal vitamin B12 status is quite common [Citation67].
A study in 2019 by Yazici et al. [Citation68] found that vitamin B12 status is generally much lower in substance abusers, presumably due to poor self-care and nutrition. This may be a confounding factor in our findings, as multi-drug use is present in several case reports. Although most authors have questioned their patients about nutrition, not all authors provide information about risk factors for vitamin B12 deficiency, such as a vegan/vegetarian diet or genetic variants. Omitting animal-derived products from one’s diet and chronic alcoholism are two major risk factors for vitamin B12 deficiency [Citation67].
Since folate is another major substrate for the homocysteine pathway in the cytosol, a folate deficiency may cause similar psychiatric symptoms as a vitamin B12 deficiency [Citation54]. Most authors did not report their patients’ folate concentration, so we were not able to rule out a possible folate deficiency as a confounding factor in these cases.
Conclusion
This review highlights the need to recognize that psychiatric symptoms may appear in association with nitrous oxide abuse, especially as recreational nitrous oxide use is progressively emerging. In the reviewed case reports, approximately half of the patients did not show neurological symptoms upon hospitalization, posing a risk of nitrous oxide intoxication being missed as the cause of psychiatric symptoms. Similarly, many patients presented with a serum or blood vitamin B12 concentration that was still within the hospital’s reference range. Emergency physicians and psychiatrists should be aware of patients presenting with isolated psychiatric symptoms caused by recreational nitrous oxide abuse.
Measurement of vitamin B12 concentration and treatment with vitamin B12 supplementation occurred more frequently when neurological symptoms were present. To reduce this discordance between the treatment of patients with and without neurological symptoms, we suggest asking all patients with new psychiatric symptoms about nitrous oxide use when taking their medical history. Measuring serum or blood vitamin B12 concentration does not seem to be effective in the diagnostic process, as patients with nitrous-oxide induced psychiatric symptoms may present with seemingly normal vitamin B12 values, and serum vitamin B12 concentration may not correlate with the levels in CSF. If nitrous oxide abuse is present in the patient history, we recommend protocolized therapeutic management of the vitamin B12 concentration. This could consist of short-term supplementation with daily 1000 μg oral or intramuscular cyanocobalamin, as previously suggested in the literature [Citation24]. However, we believe that further research, such as a randomized controlled trial, is necessary to conclusively prove the effectiveness of vitamin B12 supplementation in nitrous oxide-induced symptoms.
Author contributions
All authors contributed to the study conception and design. M.C. Paulus, A.M. Wijnhoven, G.C. Maessen and S.R. Blankensteijn contributed to the data collection, the analysis of the results and to the writing of the first draft of the manuscript. All authors commented on previous versions of the manuscript, and read and approved the final manuscript. M.A.G. van der Heyden supervised the project.
Acknowledgements
The authors thank Drs. Rumia Bose, MBBS (Psychiatrist at Mondriaan, Institute of Mental Health, the Netherlands) for commenting on the draft version of our manuscript. Drs. Rumia Bose has no conflict of interest to declare.
Disclosure statement
The authors report no conflict of interest.
References
- Kaar SJ, Ferris J, Waldron J, et al. Up: the rise of nitrous oxide abuse. An international survey of contemporary nitrous oxide use. J Psychopharmacol. 2016;30(4):395–401.
- Ehirim EM, Naughton DP, Petróczi A. No laughing matter: presence, consumption trends, drug awareness, and perceptions of "Hippy Crack" (nitrous oxide) among young adults in England. Front Psychiatry. 2017;8:312.
- Howard MO, Bowen SE, Garland EL. Substance-related disorders. In: Sadock BJ, Sadock VA, Ruiz P, editors. Kaplan & Sadock's comprehensive textbook of psychiatry. 10th ed. Philadelphia, PA: Lippincott Williams And Wilkins; 2017. p. 1328–1342.
- Global Drug Survey. 2019. GDS2019 key finding report; [published 2019 May 16; cited 2020 Mar 11]. Available from: https://issuu.com/globaldrugsurvey/docs/gds2019_key_findings_report_may_16
- Global Drug Survey. 2014. Last 12 month prevalence of top 20 drugs; [published 2014 Apr; cited 2020 Mar 11]. Available from: https://www.globaldrugsurvey.com/wp-content/uploads/2014/04/last-12-months-drug-prevalence.pdf
- GOV.UK. Drug misuse: findings from the 2018 to 2019 CSEW; [published 2019 Sep 19; cited 2020 Mar 11]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832533/drug-misuse-2019-hosb2119.pdf
- Guo CJ, Kaufman BS. Inhalational Anesthetics. In: Nelson LS, Howland M, Lewin NA, Smith SW, Goldfrank LR, Hoffman RS, editors. Goldfrank's Toxicologic Emergencies [Internet]. 11th ed. New York City: McGraw-Hill; 2019 [cited 2020 May 5]. p. 1–14. Available from: https://accessemergencymedicine.mhmedical.com/content.aspx?bookid=2569§ionid=210274345
- Kwok MMK, de Lemos J, Sharaf EM. Drug-induced psychosis and neurological effects following nitrous oxide misuse: a case report. BCMJ. 2019;61(10):385–387.
- Lassen HCA, Henriksen E, Neukirch F, et al. Treatment of tetanus; severe bone-marrow depression after prolonged nitrous-oxide anaesthesia. Lancet. 1956;270(6922):527–530.
- Van Amsterdam J, Nabben T, van den Brink W. Recreational nitrous oxide use: prevalence and risks. Regul Toxicol Pharmacol. 2015;73(3):790–796.
- Sethi NK, Mullin P, Torgovnick J, et al. Nitrous oxide "whippit" abuse presenting with cobalamin responsive psychosis. J Med Toxicol. 2006;2(2):71–74.
- Chiang T, Hung C, Wang W, et al. Recreational nitrous oxide abuse-induced vitamin B12 deficiency in a patient presenting with hyperpigmentation of the skin. Case Rep Dermatol. 2013;5(2):186–191.
- Garakani A, Jaffe RJ, Savla D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. 2016;25(5):358–369.
- Dutch Poisons Information Center (DPIC), University Medical Center Utrecht, The Netherlands. Meer vergiftigingen door lachgas in 2019; [cited 2020 Nov 4]. Available from: https://www.umcutrecht.nl/nieuws/meer-vergiftigingen-met-lachgas-in-2019
- Sheldon RJG, Reid M, Schon F, et al. Just say N2O – nitrous oxide misuse: essential information for psychiatrists. BJPsych Advances. 2020;26(2):72–81.
- World Health Organization. Mental health: mental disorders; [cited 2020 June 1]. Available from: https://www.who.int/mental_health/management/en/
- Layzer RB. Myeloneuropathy after prolonged exposure to nitrous oxide. Lancet. 1978;2(8102):1227–1230.
- Alderman CP, Nyfort‐Hansen K. Nitrous oxide abuse in a community setting: case report. Aust J Hosp Pharm. 2000;30(3):109–110.
- Brett A. Myeloneuropathy from whipped cream bulbs presenting as conversion disorder. Aust N Z J Psychiatry. 1997;31(1):131–132.
- Brodsky L, Zuniga J. Nitrous oxide: a psychotogenic agent. Compr Psychiatry. 1975;16(2):185–188.
- Chen T, Zhong N, Jiang H, et al. Neuropsychiatric symptoms induced by large doses of nitrous oxide inhalation: a case report. Shanghai Arch Psychiatry. 2018;30(1):56–59.
- Chien WH, Huang MC, Chen LY. Psychiatric and other medical manifestations of nitrous oxide abuse: implications from case series. J Clin Psychopharmacol. 2020;40(1):80–83.
- Chin J, Forzani B, Chowdhury N, et al. Rehabilitation essential in the recovery of multifactorial subacute combined degeneration. Ann Phys Rehabil Med. 2015;58(3):190–192.
- Cousaert C, Heylens G, Audenaert K. Laughing gas abuse is no joke. An overview of the implications for psychiatric practice. Clin Neurol Neurosurg. 2013;115(7):859–862.
- Grigg JR. Nitrous oxide mood disorder. J Psychoactive Drugs. 1988;20(4):449–450.
- Hew A, Lai E, Radford E. Nitrous oxide abuse presenting with acute psychosis and peripheral neuropathy. Aust N Z J Psychiatry. 2018;52(4):388.
- Hughes G, Moran E, Dedicoat MJ. Encephalitis secondary to nitrous oxide and vitamin B12 deficiency. BMJ Case Rep. 2019;12(12):e229380.
- Iwata K, O'Keefe GB, Karanas A. Neurologic problems associated with chronic nitrous oxide abuse in a non-healthcare worker. Am J Med Sci. 2001;322(3):173–174.
- Johnson K, Mikhail P, Kim MG, et al. Recreational nitrous oxide-associated neurotoxicity. J Neurol Neurosurg Psychiatry. 2018;89(8):897–898.
- Kim S, Lee SH, Bang M. Double-sidedness of "laughing gas" on the N-methyl-d-aspartate receptor: a case report of acute psychosis associated with nitrous oxide-induced hyperhomocysteinemia. Schizophr Res. 2019;208:475–476.
- Lundin MS, Cherian J, Andrew MN, et al. One month of nitrous oxide abuse causing acute vitamin B 12 deficiency with severe neuropsychiatric symptoms. BMJ Case Rep. 2019;12(2):e228001.
- Luo D, Xu J, Hu L, et al. Hyperbaric oxygen therapy to improve cognitive dysfunction and encephalatrophy induced by N2O for recreational use: a case report. Neuropsychiatr Dis Treat. 2018;14:1963–1967.
- Pratt DN, Patterson KC, Quin K. Venous thrombosis after nitrous oxide abuse, a case report. J Thromb Thrombolysis. 2020;49(3):501–503.
- Roberts D, Farahmand P, Wolkin A. Nitrous oxide inhalant use disorder preceding symptoms concerning for primary psychotic illness. Am J Addict. 2020;29(6):525–527.
- Sterman AB, Coyle PK. Subacute toxic delirium following nitrous oxide abuse. Arch Neurol. 1983;40(7):446–467.
- Trivette ET, Hoedebecke K, Berry-Cabán CS, et al. Megaloblastic hematopoiesis in a 20 year old pregnant female. Am J Case Rep. 2013;14:10–12.
- Vive MG, Anguelova GV, Duim S, et al. Metabolic encephalopathy caused by nitrous oxide (‘laughing gas’) induced hyperammonaemia. BMJ Case Rep. 2019;12(11):e232163.
- Wong SL, Harrison R, Mattman A, et al. Nitrous oxide (N2O)-induced acute psychosis. Can J Neurol Sci. 2014;41(5):672–674.
- Matorin AA, Shah AA, Ruiz P. Clinical manifestations of psychiatric disorders. In: Sadock BJ, Sadock VA, Ruiz P, editors. Kaplan & Sadock's comprehensive textbook of psychiatry. 10th ed. Philadelphia, PA: Lippincott Williams And Wilkins; 2017. p. 1114–1150.
- Jevtovic-Todorovic V, Todorovic SM, Mennerick S, et al. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nat Med. 1998;4(4):460–463.
- Metzler D, Miller WH, Edwards CS. Psychiatric manifestation of vitamin B-12 deficiency: an update. JJP. 1991;9(2):43–48.
- Catalano G, Catalano MC, Rosenberg EI, et al. Catatonia. Another neuropsychiatric presentation of vitamin B12 deficiency? Psychosomatics. 1998;39(5):456–460.
- Malamed SF. Pharmacology, anatomy and physiology. In: Malamed SF, editor. Sedation: a guide to patient management. 6th ed. St. Louis, MO: Elsevier; 2017 p. 190–202.
- Chanarin I. Cobalamins and nitrous oxide: a review. J Clin Pathol. 1980;33(10):909–916.
- Pema PJ, Horak HA, Wyatt RH. Myelopathy caused by nitrous oxide toxicity. Am J Neuroradiol. 1998;19(5):894–896.
- Edirisinghe SP. Homocysteine-induced thrombosis. Br J Biomed Sci. 2004;61(1):40–47.
- Norris F, Mallia P. Lesson of the month 2: a case of nitrous oxide-induced pancytopenia. Clin Med. 2019;19(2):129–130.
- Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54(1):9–18.
- Virarkar M, Alappat L, Bradford PG, et al. L-arginine and nitric oxide in CNS function and neurodegenerative diseases. Crit Rev Food Sci Nutr. 2013;53(11):1157–1167.
- Bernstein HG, Stanarius a, Baumann B, et al. Nitric oxide synthase-containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience. 1998;83(3):867–875.
- Sakamoto S, Nakao S, Masuzawa M, et al. The differential effects of nitrous oxide and xenon on extracellular dopamine levels in the rat nucleus accumbens: a microdialysis study. Anesth Analg. 2006;103(6):1459–1463.
- Burvill PW, Jackson JM, Smith WG. Psychiatric symptoms due to vitamin B12 deficiency without anaemia. Med J Aust. 1969;2(8):388–390.
- Masalha R, Chudakov B, Morad M, et al. Cobalamin responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Assoc J. 2001;3(9):701–703.
- Jayaram N, Rao MR, Narasimha A, et al. Vitamin B12 levels and psychiatric symptomatology: a case series. J Neuropsychiatry Clin Neurosci. 2013;25(2):150–152.
- Smith ADM. Megaloblastic madness. Br Med J. 1960;2(5216):1840–1845.
- Van Tiggelen CJM, Peperkamp JPC, Tertoolen JFW. Vitamin-B12 levels of cerebrospinal fluid in patients with organic mental disorder. J Orthomol Psychiatry. 1983;12:305–311.
- Zhang Y, Hodgson NW, Trivedi MS, et al. Decreased brain levels of vitamin B12 in aging, autism and schizophrenia. PLOS One. 2016;11(1):e0146797.
- Domisse J. Subtle vitamin deficiency and psychiatry: a largely unnoticed but devastating relationship? Med Hypotheses. 1991;34:137–140.
- Berry N, Sagar R, Tripathi BM. Catatonia and other psychiatric symptoms with vitamin B12 deficiency. Acta Psychiatr Scand. 2003;108(2):156–159.
- Bolann BJ, Solli JD, Schneede J, et al. Evaluation of indicators of cobalamin deficiency defined as cobalamin-induced reduction in increased serum methylmalonic acid. Clin Chem. 2000;46(11):1744–1750.
- Bhat AS, Srinivasan K, Kurpad SS, Galgali RB. Psychiatric presentations of vitamin B12 deficiency. J Indian Med Assoc. 2007;105(7):395–396.
- Raveendranathan D, Shiva L, Venkatasubramanian G, et al. Vitamin B12 deficiency masquerading as clozapine-resistant psychotic symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2013;25(2):E34–35.
- Xiang W, Xue H, Wang B, et al. Efficacy of N-butylphthalide and hyperbaric oxygen therapy on cognitive dysfunction in patients with delayed encephalopathy after acute carbon monoxide poisoning. Med Sci Monit. 2017;23:1501–1506.
- Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health; [published 2019 Aug; cited 2020 Mar 11]. Available from: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf
- Baigent M. Managing patients with dual diagnosis in psychiatric practice. Curr Opin Psychiatry. 2012;25(3):201–205.
- Santucci K. Psychiatric disease and drug abuse. Curr Opin Pediatr. 2012;24(2):233–237.
- Green R, Allen LH, Bjørke-Monsen A, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3:17040.
- Yazici AB, Ciner OA, Yazici E, et al. Comparison of vitamin B12, vitamin D and folic acid blood levels in patients with schizophrenia, drug addiction and controls. J Clin Neurosci. 2019;65:11–16.