 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Fomepizole is the preferred antidote for treatment of methanol and ethylene glycol poisoning, acting by inhibiting the formation of the toxic metabolites. Although very effective, the price is high and the availability is limited. Its availability is further challenged in situations with mass poisonings. Therefore, a 50% reduced maintenance dose for fomepizole during continuous renal replacement therapy (CRRT) was suggested in 2016, based on pharmacokinetic data only. Our aim was to study whether this new dosing for fomepizole during CRRT gave plasma concentrations above the required 10 µmol/L. Secondly, we wanted to study the elimination kinetics of fomepizole during CRRT, which has never been studied before.
Methods
Prospective observational study of adult patients treated with fomepizole and CRRT. We collected samples from arterial line (pre-filter) = plasma concentration, post-filter and dialysate for fomepizole measurements. Fomepizole was measured using high-pressure liquid chromatography with a reverse phase column.
Results
Ten patients were included in the study. Seven were treated with continuous veno-venous hemodialysis (CVVHD) and three with continuous veno-venous hemodiafiltration (CVVHDF). Ninety-eight percent of the plasma samples were above the minimum plasma concentration of 10 µmol/L. Fomepizole was removed during CRRT with a median saturation/sieving coefficient of 0.85 and dialysis clearance of 28 mL/min.
Conclusion
Fomepizole was eliminated during CCRT. The new dosing recommendations for fomepizole and CRRT appeared safe, by maintaining the plasma concentration above the minimum value of 10 µmol/L. Based on these data, the fomepizole maintenance dose during CRRT could be reduced to half as compared to intermittent hemodialysis.
Introduction
Methanol and ethylene glycol are toxic alcohols with a potential fatal outcome when poisoned. Large outbreaks of methanol poisoning with high mortality are regularly reported [Citation1,Citation2], as are case reports of intentional or accidental ingestion of ethylene glycol [Citation3,Citation4]. Early treatment with the antidotes ethanol or fomepizole is effective and lifesaving [Citation5]. Both substances act by inhibiting the alcohol dehydrogenase enzyme, and thus preventing the formation of the toxic metabolites (formic acid from methanol and glycolic acid from ethylene glycol). Although ethanol can cause a pronounced central nervous system (CNS) depression, it also requires frequent monitoring of plasma ethanol. Fomepizole, on the other hand, has limited side effects (e.g., headache and dizziness), and does not require monitoring of plasma concentration. Treatment guidelines therefore recommend fomepizole as the antidote of choice [Citation5]. Plasma concentrations of fomepizole above 10 µmol/L are considered effective and will prevent formation of the toxic metabolites, based on studies done in non-human primates [Citation6,Citation7].
Treatment for poisonings with both of these toxic alcohols consists of bicarbonate for the metabolic acidosis, antidote and, if necessary, dialysis for removal of the toxic alcohol, their metabolites and further correction of the metabolic acidosis [Citation8]. For ethylene glycol poisoning, dialysis is sometimes also required to treat the ensuing renal failure. Intermittent hemodialysis (IHD) has been demonstrated to be superior to continuous renal replacement therapy (CRRT) in methanol poisoning regarding methanol and formate elimination [Citation9], as well as time to correction of acidosis [Citation10]. However, no difference in mortality rate or rate of long-term visual and/or CNS sequelae has been demonstrated [Citation11]. In hemodynamically unstable patients, CRRT is the preferred modality. Fomepizole is known to be removed during IHD [Citation12,Citation13], whereas data are lacking as regards to CRRT. To compensate for this loss, the dose interval for the maintenance dose is every 4 h instead of every 12 h without dialysis [Citation12,Citation13]. Alternatively, a continuous infusion of 1 mg/kg/h during dialysis has been proposed [Citation5]. Previous dosing recommendations did not distinguish between IHD and CRRT, and the same fomepizole dose has thus been recommended [Citation14]. CRRT, however, has a lower dialysate- and blood flow. Theoretically, less fomepizole will therefore be removed during CRRT as compared to IHD. In 2016, new dosing recommendations for fomepizole during CRRT were published [Citation5]. Reduced dose by increasing the interval for the maintenance dose from 4 to 8 h, or reducing the dose of continuous infusion to 0.5 mg/kg/h, as compared to IHD (1 mg/kg/h). These recommendations are based on pharmacokinetic data on fomepizole and experience from one case. There are no studies on fomepizole monitoring and kinetics during CRRT. This is of utmost importance since a plasma concentration below 10 µmol/L could potentially lead to therapy failure.
The primary aim of this study was to examine whether the proposed new dosing regimen for fomepizole during CRRT provides the desired plasma concentrations of fomepizole to inhibit the formation of toxic metabolites. Secondly, we wanted to examine the elimination kinetics of fomepizole during CRRT.
Materials and methods
Study design
This prospective observation study was conducted at the Department of Acute Medicine at Oslo University Hospital, Norway, with data collection also from Akershus University Hospital, Baerum Hospital, Ostfold Hospital Kalnes, and Levanger Hospital, all in Norway. The inclusion period was from June 2019 to November 2020. Two patients serving as pilot study patients before this study are included in the data material. The study was registered on ClinicalTrials.gov (NCT04649138).
Participants
Adults (over 18 year) with suspected or confirmed toxic alcohol poisoning treated with fomepizole and CRRT were included in this study. There were no exclusion criteria.
Treatment
Fomepizole was given as a 15 mg/kg loading dose, followed by 10 mg/kg every 8 h or 0.5 mg/kg/h as a continuous infusion. The dosing regimen was determined by the treating physician. The choice of dialysis modality and dialysis settings followed local guidelines. Observation time lasted as long as the patient received fomepizole and CRRT. Three patients received IHD before CRRT.
Data collection
We collected blood samples from the arterial line for determination of plasma concentration. In addition, blood samples after the dialysis filter (post-filter) and samples from dialysate were collected. Samples were collected every hour or every other hour. In addition, a sample was taken immediately before each fomepizole dose when dosed at timed intervals. EDTA tubes were used for blood samples and tubes without any additives for dialysate samples. Blood samples were spun at 2000 G for 10 min. All samples were stored and transported frozen (−20 °C). Fomepizole analysis was performed at Louisiana State University Health Sciences Center, USA, using high-pressure liquid chromatography with a reverse phase column (sensitivity 5 µmol/L; coefficient of variation 4.5% at 25 µmol/L) [Citation15].
Calculations
Dialysis clearance (CL) (mL/min) with continuous veno-venous hemodialysis (CVVHD) was calculated with the following formula
where D and P are dialysate and plasma concentrations, (D/P) is the saturation coefficient and QD is the dialysate flow rate [Citation16]. For continuous veno-venous hemodiafiltration (CVVHDF) with post-dilution, the following was used
where (D/P) is the sieving coefficient and QE is total effluent rate, which is the sum of ultrafiltration flow rate (QUF) and dialysate flow rate (QD) [Citation16,Citation17].
The elimination kinetics was found by plotting time versus concentration (zero-order) and as a semi-log plot (first-order), where the R2 value then could identify how close the data were to a linear decline (zero-order) or log linear decline (first-order).
Ethical considerations
The study was approved by the Regional Committee for Medical and Health Research Ethics (2017/981/REK South-East D). Consent was obtained from the patient or next of kin if the patient was unable to consent.
Results
We included 11 patients in this study. One patient died shortly after inclusion and only two plasma samples were collected. The patient was therefore excluded, leaving 10 patients for analysis. Median age was 54 years (range 31–63 years) and seven patients were men. Methanol poisoning was confirmed in three patients, and ethylene glycol poisoning was confirmed in four (). The remaining three patients were treated based on suspected toxic alcohol poisoning but were later confirmed negative. This does not affect the pharmacokinetic analysis and these patients are included. All patients were admitted to an intensive care unit (ICU) and median observation time for the study was 17 h (range 13–33 h).
Table 1. Laboratory data on admission.
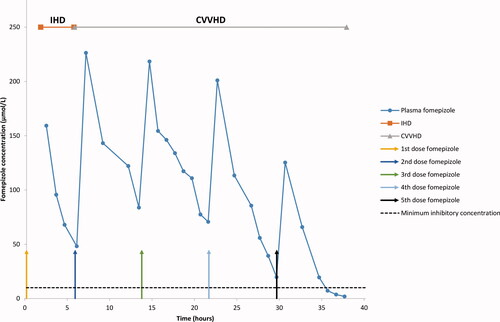
Nine patients received fixed maintenance doses of fomepizole and one patient a continuous infusion. For the fixed doses, a median of three doses were given (range two to five doses). The concentrations of fomepizole in plasma, post-filter and dialysate for patient 2 () and in plasma for patient 1 (), are presented for illustration. Note that in , fomepizole was dialyzed during CRRT and all plasma fomepizole samples were above 10 µmol/L. Both patients were treated with IHD before CRRT. In total, 123 plasma samples were drawn and analyzed for fomepizole. Of these, 120 were above the required minimum concentration of 10 µmol/L. Only three samples (2–4–7 µmol/L) from patient 1 were below this concentration, all at the end of the fifth dose (). For the fixed doses, the minimum plasma concentration (the lowest concentration observed before a new dose was given) was 108 µmol/L (median), range 2–168 µmol/L (n = 22). For continuous infusion, the lowest plasma concentration measured was 70 µmol/L.
Figure 1. Fomepizole concentration in plasma, post-filter and dialysate in patient 2. CVVHD: Continuous veno-venous hemodialysis; IHD: Intermittent hemodialysis.
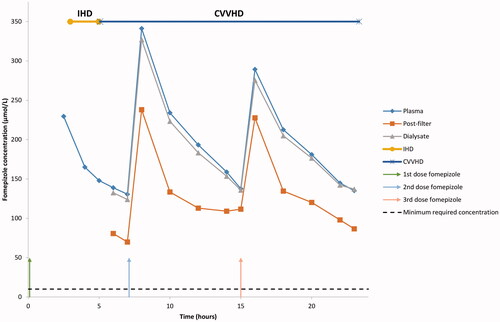
Figure 2. Fomepizole concentration in plasma in patient 1. CVVHD: continuous veno-venous hemodialysis; IHD: intermittent hemodialysis.
Seven patients were treated with CVVHD and three patients with CVVHDF (post-dilution). The elimination kinetics of fomepizole during CRRT is described in . The R2 value was approximately one for both zero- and first-order kinetics. Fomepizole was removed during CRRT treatment with a median saturation/sieving coefficient of 0.85 (range 0.46–0.96), and a dialysis clearance of 28 mL/min (median), range 8–35 mL/min (). CRRT clearance constituted 22% (median value) of total body clearance (TBC), range 9–44%. Median half-life (t1/2) calculated from first-order elimination was 5.6 h (range 1.3–10.5 h).
Table 2. Elimination kinetics of fomepizole during continuous renal replacement therapy (CRRT).
Table 3. Dialysis modality, settings and clearance for fomepizole during continuous renal replacement therapy (CRRT).
Discussion
This is the first therapeutic drug monitoring study published on fomepizole during CRRT. Our study supports that the proposed new lower dosing regimen for fomepizole during CRRT is safe, by providing a plasma concentration above the minimum concentration (10 µmol/L) recommended to inhibit the formation of toxic metabolites.
The fomepizole plasma concentrations samples were above the recommended minimum concentration of 10 µmol/L in 120/123 samples. The fact that three samples in patient 1 were below the desired concentration of fomepizole may be explained by increased dialysis clearance, increased metabolism, or auto-induction of its own metabolism. Dialysis clearance in this patient was calculated to 22 mL/min, which is lower than the median value in the group. Further, the dialysate samples were not collected until dose number four, and clearance is calculated for dose four and five. The low plasma fomepizole concentration after these doses can therefore not be explained by increased excretion by dialysis.
Without dialysis, it is shown that fomepizole is metabolized to an inactive metabolite, 4-carboxypyrazole (4-CP) [Citation15]. Metabolism will also occur in patients with dialysis, but the relationship between excretion via dialysis and metabolism is unknown. A possible mechanism for the increased elimination of fomepizole in our patient, is increased liver metabolism to 4-CP. However, the patient did not receive any enzyme inducing drugs.
According to animal data, fomepizole induces CYP 2E1 [Citation18,Citation19]. In a multiple dose study on healthy volunteers, McMartin et al. [Citation15] found that the elimination increased with repeated doses and by increasing the dose after 36 h, one could compensate for the increased elimination. The suggested mechanism for the increased elimination was auto-induction of fomepizole metabolism. Treatment guidelines therefore recommend increased maintenance dose after four doses (i.e., from the fifth dose onwards) [Citation5]. For our patient, the increased elimination occurred between dose four and five, given 21.5 and 29.5 h after the loading dose, respectively. In healthy volunteers, the auto-induction occurred after two to three days, although there was one subject where the increased elimination occurred in one day. The latter corresponds to when it appeared in our patient. More data are needed to assess how frequently the auto-induction can occur after such a short time. Since fomepizole was discontinued after the fifth dose, the concentrations below guidelines had no clinical implication for our patient.
Adverse events of the dosing regimen were not examined in this study. We found that the highest fomepizole plasma concentration measured was 440 µmol/L. A study on healthy volunteers with multiple doses of fomepizole has shown a high tolerability and with a small increase in liver transaminases, but this was not dose dependent [Citation20]. The highest plasma concentration detected in that study was around 275 µmol/L, which is lower than in our study. In a clinical trial, plasma concentrations of fomepizole were between 183 and 366 µmol/L, with few adverse events rated as possibly (bradycardia, seizure and headache) [Citation21]. This has been confirmed in a novel post-marketing study with 536 patients from 2002 to 2018, which concluded that fomepizole had minimal adverse effects [Citation22]. CRRT was given to 44 patients, and they may therefore have received both old and new dosing regimens. As the total maintenance dose becomes lower with the new dosing regimens, one should not expect increased frequency of adverse events.
We found that fomepizole was removed during CRRT, which is consistent with the theory of low-molecular weight (82 Da), low protein binding and small volume of distribution to be dialyzed. Two publications have also documented that fomepizole is removed during IHD [Citation12,Citation13]. A median value for sieving/saturation coefficient close to one indicates that fomepizole passes almost freely through the filter with CRRT. Our patient number 7 had a sieving coefficient of 0.5 and dialysis clearance of 8 mL/min, which is much lower than the median values. This can partly be explained by the low total effluent rate of 1000 mL/h (500 mL/h of dialysate flow + 500 mL/h of replacement fluid post-dilution), which is lower than recommended flow/patient weight [Citation23]. A possible explanation for the low dialysate flow in this patient was to correct the acidosis. In our study, the CRRT clearance data are from six patients with CVVHD and two patients with CVVHDF. This is not sufficient to compare the two modalities.
When comparing CRRT clearance to TBC for the individual patients it varies. In patient 7, the proportion removed by CRRT is less than in the other patients. Drug removal by dialysis is considered clinically significant when the proportion removed is more than 25–30% of TBC [Citation24]. This is not applicable in patient 7. Theoretically, in patients with low CRRT clearance, the maintenance dose could be the same as in patients not being dialyzed. However, in clinical practice, it is difficult to identify who these patients are in advance because of the heterogeneous nature of the intensive care population. Furthermore, our study measured CRRT clearance in only eight patients, and therefore more data are needed.
Our results cannot distinguish between zero- and first-order elimination of fomepizole during CRRT (). In animals, the elimination kinetics is described as zero-order [Citation6,Citation25,Citation26]. The same was found in healthy volunteers, but after four days, it changed to first-order elimination [Citation15]. Because less than 2.6% of the fomepizole dose was eliminated unchanged in the urine of healthy volunteers, elimination was thought to be predominantly by metabolism. In a study from Wallemacq et al. [Citation27], the elimination in five patients (four adult and one child) was found to follow first-order kinetics. The observation time was up to three days and three patients received concomitant treatment with ethanol and hemo- or peritoneal dialysis for a few hours. In our study, the extracorporeal clearance by the dialysis will contribute to the total body clearance. We did not measure 4-CP in urine and the relative contributions of dialysis and metabolism to the overall elimination are not known. This makes it difficult to compare with healthy volunteers. In addition, we may also have had too few data points after each dose of fomepizole to describe the elimination kinetics. The median value of t1/2 calculated from first-order kinetics was 5.6 h, whereas the half-life of patient 10 was almost twice the length. Unfortunately, dialysate samples for this patient were not collected and dialysis clearance could not be calculated. The dialysate flow rate was correct according to patient weight, but the patient had a severe liver failure. The latter could explain the reduced hepatic metabolism to 4-CP, which normally constitutes a large part of the elimination, leading to an increased t1/2.
Fomepizole has a high cost and limited availability in many parts of the world. Even in countries where this antidote is available, lack of availability or drug shortage can be a potential problem in large outbreak of methanol poisoning. By using the new dosing regimen for fomepizole and CRRT, less fomepizole is needed. This will significantly reduce the costs for antidote treatment with fomepizole.
Limitations
There are some limitations to our study. The study only includes 10 patients over a short observation time, and more intensive care patients should ideally be included. Furthermore, we have not measured the metabolite 4-carboxypyrazole in urine, which would have given additional information on the role of metabolism of fomepizole in the overall elimination during CRRT. We have also only measured plasma concentration of fomepizole and not assessed the effect of the dosing regimen on patient outcome.
Conclusion
This is the first study demonstrating that fomepizole maintenance dose given every 8 h during CRRT provides the required plasma concentration. Fomepizole was dialyzed during CRRT with a saturation/sieving coefficient of 0.85 and dialysis clearance of 28 mL/min.
Acknowledgments
The authors thank clinical pharmacist Hilde Sporsem for her help and valuable comments on this manuscript, the nurses at the Medical ICU at Oslo University Hospital Ullevaal and at the ICU at Akershus University Hospital, Baerum Hospital, Ostfold Hospital Kalnes and Levanger Hospital for their cooperation and assistance in collecting samples.
Disclosure statement
Dr. McMartin has an agreement with Mericon Investment Group from which he receives royalties from the sale of Antizol, the trade formulation of fomepizole, in North America. The other authors declare that they have no competing interests.
References
- Rostrup M, Edwards JK, Abukalish M, et al. The methanol poisoning outbreaks in Libya 2013 and Kenya 2014. PLoS One. 2016;11(3):e0152676.
- Hassanian-Moghaddam H, Zamani N, Kolahi AA, et al. Double trouble: methanol outbreak in the wake of the COVID-19 pandemic in Iran-a cross-sectional assessment. Crit Care. 2020;24(1):402.
- Karlson-Stiber C, Persson H. Ethylene glycol poisoning: experiences from an epidemic in Sweden. J Toxicol Clin Toxicol. 1992;30(4):565–574.
- Hovda KE, Julsrud J, Øvreb ØS, et al. Studies on ethylene glycol poisoning: one patient – 154 admissions. Clin Toxicol. 2011;49(6):478–484.
- McMartin K, Jacobsen D, Hovda KE. Antidotes for poisoning by alcohols that form toxic metabolites. Br J Clin Pharmacol. 2016;81(3):505–515.
- McMartin KE, Hedstrom KG, Tolf BR, et al. Studies on the metabolic interactions between 4-methylpyrazole and methanol using the monkey as an animal model. Arch Biochem Biophys. 1980;199(2):606–614.
- McMartin KE, Makar AB, Martin G, et al. Methanol poisoning. I. The role of formic acid in the development of metabolic acidosis in the monkey and the reversal by 4-methylpyrazole. Biochem Med. 1975;13(4):319–333.
- Kraut JA, Mullins ME. Toxic alcohols. N Engl J Med. 2018;378(3):270–280.
- Zakharov S, Pelclova D, Navratil T, et al. Intermittent hemodialysis is superior to continuous veno-venous hemodialysis/hemodiafiltration to eliminate methanol and formate during treatment for methanol poisoning. Kidney Int. 2014;86(1):199–207.
- Zakharov S, Pelclova D, Navratil T, et al. Efficiency of acidemia correction on intermittent versus continuous hemodialysis in acute methanol poisoning. Clin Toxicol. 2017;55(2):123–132.
- Zakharov S, Rulisek J, Nurieva O, et al. Intermittent versus continuous renal replacement therapy in acute methanol poisoning: comparison of clinical effectiveness in mass poisoning outbreaks. Ann Intensive Care. 2017;7(1):1–11.
- Jobard E, Harry P, Turcant A, et al. 4-Methylpyrazole and hemodialysis in ethylene glycol poisoning. J Toxicol Clin Toxicol. 1996;34(4):373–377.
- Faessel H, Houze P, Baud FJ, et al. 4-Methylpyrazole monitoring during haemodialysis of ethylene glycol intoxicated patients. Eur J Clin Pharmacol. 1995;49(3):211–213.
- Brent J. Fomepizole for ethylene glycol and methanol poisoning. N Engl J Med. 2009;360(21):2216–2223.
- McMartin KE, Sebastian CS, Dies D, et al. Kinetics and metabolism of fomepizole in healthy humans. Clin Toxicol. 2012;50(5):375–383.
- Mueller BA, Golper TA. Drug removal in continuous renal replacement therapy. UpToDate [Internet]. [cited 2020 Apr 29]. Available from: https://www.uptodate.com/contents/drug-removal-in-continuous-renal-replacement-therapy?csi=d48f8ef3-4790-4017-9dd5-e3879a637157&source=contentShare.
- Bugge JF. Pharmacokinetics and drug dosing adjustments during continuous venovenous hemofiltration or hemodiafiltration in critically ill patients. Acta Anaesthesiol Scand. 2001;45(8):929–934.
- Wu D, Cederbaum AI. Induction of liver cytochrome P4502E1 by pyrazole and 4-methylpyrazole in neonatal rats. J Pharmacol Exp Ther. 1993;264(3):1468–1473.
- Feierman DE, Cederbaum AI. Oxidation of the alcohol dehydrogenase inhibitor pyrazole to 4-hydroxypyrazole by microsomes. Effect of cytochrome P-450 inducing agents. Drug Metab Dispos. 1987;15(5):634–639.
- Jacobsen D, Sebastian CS, Barron SK, et al. Effects of 4-methylpyrazole, methanol/ethylene glycol antidote, in healthy humans. J Emerg Med. 1990;8(4):455–461.
- Brent J, McMartin K, Phillips S, et al. Fomepizole for the treatment of ethylene glycol poisoning. Methylpyrazole for toxic alcohols study group. N Engl J Med. 1999;340(11):832–838.
- Rasamison R, Besson H, Berleur M-P, et al. Analysis of fomepizole safety based on a 16-year post-marketing experience in France. Clin Toxicol. 2020;58(7):742–747.
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Workgroup. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(2):1–138.
- Schetz M, Ferdinande P, Van Den Berghe G, et al. Pharmacokinetics of continuous renal replacement therapy. Intensive Care Med. 1995;21(7):612–620.
- Mayersohn M, Owens SM, Anaya AL, et al. 4-Methylpyrazole disposition in the dog: evidence for saturable elimination. J Pharm Sci. 1985;74(8):895–896.
- McMartin KE, Collins TD. Distribution of oral 4-methylpyrazole in the rat: inhibition of elimination by ethanol. J Toxicol Clin Toxicol. 1988;26(7):451–466.
- Wallemacq PE, Vanbinst R, Haufroid V, et al. Plasma and tissue determination of 4-methylpyrazole for pharmacokinetic analysis in acute adult and pediatric methanol/ethylene glycol poisoning. Ther Drug Monit. 2004;26(3):258–262.
