Abstract
Introduction
Diquat-related acute kidney injury is well-known. However, neurological disorders caused by diquat are often underestimated, and changes in the imaging findings are rarely reported. We present three cases of acute diquat poisoning resulting in toxic encephalopathy.
Case report
In the first case, a 20-year-old previously healthy man ingested approximately 80–100 mL of diquat. He developed acute renal failure, neurological disorders, and respiratory failure. Central pontine myelinolysis was considered by magnetic resonance imaging (MRI), 18 days after ingestion. In the second case, a 20-year-old man ingested approximately 100 mL of diquat. Toxic encephalopathy was confirmed by MRI, 13 days after ingestion. Unfortunately, he experienced cardiac arrest and died 18 days after ingestion. In the third case, a 31-year-old previously healthy man ingested approximately 50 mL of diquat. The imaging features of toxic encephalopathy mainly involved the medulla oblongata, pons, midbrain, bilateral brachium pontis, cerebellum, and pedunculus cerebri. He demonstrated significant recovery.
Discussion
Ingestion of diquat can cause acute renal failure, neurological disorders, and respiratory failure. The pons, midbrain, pedunculus cerebri may be the most commonly impaired locations of diquat-related toxic encephalopathy.
Introduction
Diquat is a nonselective contact bipyridyl herbicide and a preharvest desiccant. As paraquat is banned in China, the use of diquat has gradually increased because of its effective herbicidal activity [Citation1], resulting in an increase in the incidence of diquat poisoning. Diquat mainly affects cell membranes through lipid peroxidation via the production of oxygen free-radicals, leading to cell death and multiple-organ failure [Citation2]. The target organs include the kidneys, liver, lungs, gastrointestinal tract, cardiovascular system, and central nervous system [Citation3,Citation4]. Acute kidney injury is common, leading to renal tubule damage. In severe cases, neurological disorders are a common clinical feature, often accompanied by a poor prognosis [Citation5]. Herein, we report three cases of acute diquat poisoning resulting in acute renal failure, neurological disorders, and respiratory failure. This report highlights the imaging features and hypothesis mechanisms of diquat-related toxic encephalopathy.
Case presentation
This study was approved by the appropriate ethics committee. Informed consent was obtained from the family members of all patients.
Case 1
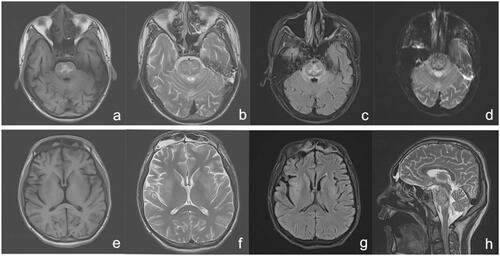
A 20-year-old previously healthy man ingested approximately 80–100 mL of diquat (20 g/100 mL). After 40 min, gastric lavage was performed and conservative medical treatment followed. After 36 h, he was transferred to our department. On admission, his blood pressure was 145/94 mmHg. Physical examination was unremarkable, except anuric and pharyngeal hyperaemia. Laboratory test results were as follows: white blood cell (WBC), 26.93 × 109/L; alanine aminotransferase (ALT), 585 IU/L; aspartate aminotransferase (AST), 533 IU/L; creatinine, 668 μmol/L; urea, 16.5 mmol/L; sodium, 139 mmol/L; and myoglobin, 2248 ng/mL (normal value 0–70 ng/mL); blood diquat concentration, 0.93 μg/mL [Citation6]. Activated charcoal (30 g) and montmorillonite powder (30 g) with mannitol (250 mL × 2) were administered orally. Betamethasone (8 mg), reduced glutathione, alanyl glutamine, polyene phosphatidyl choline, and furosemide (100 mg bid) were administered intravenously daily. Haemoperfusion was performed immediately. After 40 h, he was still anuric; haemofiltration was performed by continuous veno-venous haemodialysis (CVVHF; Prismaflex ST100). On day 2 after admission, he developed delirium, and mechanical ventilation was performed. On day 3, the results were as follows: ALT, 178 IU/L; AST, 44 IU/L; creatinine, 905 mmol/L; urea, 26.9 mmol/L; and N-terminal pro-B-type natriuretic peptide, 3538 pg/mL (reference range ≤300 pg/mL). CVVHF was performed (8–12 h/day) daily thereafter. On day 7, he still showed anuria and coma; his creatinine was 882 μmol/L, and urea was 37.4 mmol/L. On day 10, his daily urine volume was 50 mL and 1500 mL 4 days later. His brain computerized tomography (CT) scan was normal and he was weaned from the ventilator. On day 18, he could move his eyes and head on command but he had dysarthria and quadriplegia. Magnetic resonance imaging (MRI) revealed abnormal intensities in his brain () and central pontine myelinolysis was considered. After 3 months, he continued to experience dystaxia and difficulty in walking.
Figure 1. MRI 18 days after ingestion showing abnormal heterogeneous intensities on T1WI (a), T2WI (b and h), T2-FLAIR (c), and DWI (d) of the brainstem, particularly in the pons, in accordance with central pontine myelinolysis signal intensity. The bilateral caudate nucleus, putamen, and capsula externa showed abnormal low-intensity on T1WI (e), abnormal high-intensity on T2WI (f), and T2-FLAIR (g). DWI: diffusion-weighted imaging; FLAIR: fluid attenuated inversion recovery; MRI: magnetic resonance imaging.
Case 2
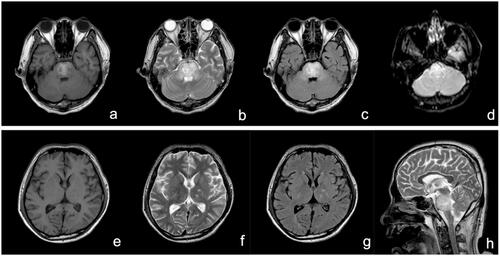
A 20-year-old previously healthy man ingested approximately 100 mL of diquat (20 g/100 mL). Gastric lavage and haemoperfusion were performed afterwards. On day 3, he displayed oliguria, agitation, convulsions, and respiratory failure; haemodialysis was performed, and he was put on mechanical ventilation was given. He was admitted to our department the next day. On admission, his heart rate was 111 beats/min, blood pressure was 145/79 mmHg. Coma, anuria, and abdominal distension were the main abnormal signs. Laboratory test results were as follows: creatinine, 882 μmol/L; urea, 37.4 mmol/L; potassium, 3.37 mmol/L; and sodium, 138 mmol/L. The pesticide ingested was confirmed as diquat by the sodium bicarbonate/dithionite test. His main therapeutic options were similar to case 1. On day 6, his urea was 38.6 mmol/L, creatinine was 989 μmol/L. His daily urine volume was 600 mL and increased gradually afterwards. On day 12, he was weaned from mechanical ventilation, but he was still semi-comatose. His brain CT showed a low density in the brainstem region. The next day, toxic encephalopathy was confirmed (). After 18 days, he experienced cardiac arrest and died.
Figure 2. MRI 13 days after ingestion showing abnormal high-intensity in the pons, bilateral brachium pontis, and pedunculus cerebri on T1WI (a), T2WI (b and h), and T2-FLAIR (c), and slight high-intensity on DWI (d); The bilateral caudate nucleus, putamen, thalamus, and corona radiata showed abnormal low-intensity on T1WI (e) and abnormal high-intensity on T2WI (f) and T2-FLAIR (g). DWI: diffusion-weighted imaging; FLAIR: fluid attenuated inversion recovery; MRI: magnetic resonance imaging.
Case 3
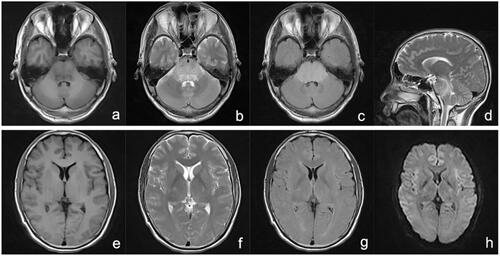
A 31-year-old previously healthy man ingested approximately 50 mL of diquat (20 g/100 mL), which was followed by nausea and vomiting. Gastric lavage and haemoperfusion (HA 330 × 2, Jafron) were performed and methylprednisolone (500 mg) was provided. After 16 h, he was transferred to our hospital. On admission, his physical examinations, except blood pressure (153/112 mmHg), were normal. Laboratory test results were as follows: creatinine, 209 μmol/L; urea, 7.6 mmol/L; potassium, 4.06 mmol/L; sodium, 138 mmol/L; and blood diquat concentration, 0.43 μg/mL. We performed haemoperfusion and comprehensive treatment. On day 3 after ingestion, he was oliguric and agitated and developed anuria and coma soon afterwards. His urea and creatine levels were 27.5 mmol/L and 600 μmol/L, respectively. His MRI indicated brain damage (). Mechanical ventilation and intermittent CVVHF (8–12 h/day) were the main supportive treatments. On day 15, he regained consciousness, and the daily urine volume, creatine level, and urea level were 150 mL, 1080 μmol/L, and 50.4 mmol/L, respectively. Mechanical ventilation was weaned. On day 17, his urine volume gradually increased, but without limb movement. MRI indicated his brain damage similar to the previous. On day 27, he developed dystaxia, especially in the upper limb. His MRI indicated that the regions of brain damage had reduced. At 57 days, his symptoms were almost completely relieved, the electroencephalogram examination was normal, and brain damage improved significantly.
Figure 3. MRI 4 days after ingestion: T1WI (a and e), T2WI (b, d, and f), T2-FLAIR (c and g), and DWI (h) depicting an abnormal medulla oblongata, pons, midbrain, cerebellum, left thalamus, surrounding posterior horn of the lateral ventricle, bilateral brachium pontis, and pedunculus cerebri. DWI: diffusion-weighted imaging; FLAIR: fluid attenuated inversion recovery; MRI: magnetic resonance imaging.
Discussion
Some cases of severe diquat poisoning may be associated with central nervous system (CNS) complications. In 1983, McCarthy et al. [Citation7] summarised 11 cases of acute diquat poisoning wherein all patients who died (6/11) presented with CNS complications. Xing et al. [Citation5] reported the case of a 21-year-old man who ingested 100 mL of diquat (20 g/100 mL) where acute pontine demyelination was confirmed by MRI. In our cases, the main clinical manifestations were acute kidney injury, neurological disorder, and respiratory failure. MRI confirmed that diquat-related brain damage mainly involved the pons, midbrain, pedunculus cerebri, and other brain areas like the basal ganglia and thalamus, meanwhile, CT excluded brain haemorrhages. The cerebral and cerebellar toxicity of diquat may be temporary, which may be due to a transient demyelination, or brain oedema, as evidenced in clinical manifestations and the serial brain MRI changes in case 3. T In all three cases, a demyelinating injury was apparent, and centropontine myelinolysis was illustrated in case 1. However, the mechanism of diquat-associated toxic encephalopathy is unclear. Although diquat is not a direct neurotoxicant, an increase in oxidative stress could occur in the brain. Reversible acute cerebellar toxicity associated with diquat is one of the possible factors for this [Citation8]. Encephalopathy can also be caused by metabolic disorders like renal failure; however, MRI features are usually normal in case of metabolic or septic encephalopathy [Citation9]. In this report, the brain MRI showing hypointensities or hyperintensities on T1WI and hyperintensities on T2WI, diffusion-weighted imaging (DWI), and fluid attenuated inversion recovery (FLAIR) image sequences in the central pons and other brain regions were consistent with osmotic demyelination syndrome (ODS) [Citation10]. Moreover, the quadriplegia and subsequent movement disorders were also in accordance with ODS [Citation11]. Hyponatremia is important; however, there are other factors considered to be involved with ODS as well [Citation11]. Neurotoxicity caused by creatinine and a sharp rise in other ureamic toxins may be considered for diquat-related brain damage. Despite the elevated blood pressure in the early stages, the MRI manifestation of reversible posterior leukoencephalopathy syndrome was inconsistent [Citation12]. For treatment, reducing absorption, enhancing elimination, mechanical ventilation, and continuous renal replacement therapy are often used.
Disclosure statement
No potential conflicts of interest were reported by the authors.
Additional information
Funding
References
- Wang D, Zhang G, Zhang W, et al. Successful extracorporeal membrane oxygenation support for severe acute diquat and glyphosate poisoning: a case report. Medicine. 2019;98(6):e14414.
- Yu G, Cui S, Jian T, et al. Diquat poisoning in a pregnant woman resulting in a miscarriage and maternal death. Clin Toxicol. ;59(12):1275–1277.
- Magalhães N, Carvalho F, Dinis-Oliveira RJ. Human and experimental toxicology of diquat poisoning: toxicokinetics, mechanisms of toxicity, clinical features, and treatment. Hum Exp Toxicol. 2018;37(11):1131–1160.
- Jones GM, Vale JA. Mechanisms of toxicity, clinical features, and management of diquat poisoning: a review. J Toxicol Clin Toxicol. 2000;38(2):123–128.
- Xing J, Chu Z, Han D, et al. Lethal diquat poisoning manifesting as Central pontine myelinolysis and acute kidney injury: a case report and literature review. J Int Med Res. 2020;48(7):300060520943824.
- Yuan G, Li R, Zhao Q, et al. Simultaneous determination of paraquat and diquat in human plasma by HPLC-DAD: its application in acute poisoning patients induced by these two herbicides. J Clin Lab Anal. 2021;35(3):e23669.
- McCarthy LG, Speth CP. Diquat intoxication. Ann Emerg Med. 1983;12(6):394–396.
- Koksel Y, McKinney AM. Potentially reversible and recognizable acute encephalopathic syndromes: disease categorization and MRI appearances. AJNR Am J Neuroradiol. 2020;41(8):1328–1338.
- Fugate JE, Kalimullah EA, Hocker SE, et al. Cefepime neurotoxicity in the intensive care unit: a cause of severe, underappreciated encephalopathy. Crit Care. 2013;17(6):R264.
- Lv X, Hong Q, Lin X, et al. Osmotic demyelination syndrome: clinical, neuroimaging characteristics, and outcomes in a series of 18 cases. Biomed Res Int. 2021; 2021:9944632.
- Bose P. Central pontine myelinolysis and the osmotic demyelination syndromes: an open and shut case? Acta Neurol Belg. 2021;121(4):849–858.
- Lee VH, Wijdicks EF, Manno EM, et al. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205–210.
