Abstract
Background and aims
In Mainland China and Hong Kong, health authorities utilize Agkistrodon halys antivenom in the treatment of patients who sustained bites from green pit vipers. However, the treatment benefit of Agkistrodon halys antivenom among such patients is still controversial. The purpose of this study is to evaluate the coagulation parameters normalization time of Agkistrodon halys antivenom in patients who sustained green pit viper bites and explore independent risk factors of patient prognosis.
Methods
Data were extracted from the Donghua Hospital Information System. Comparison of the two groups of patients – who used antivenom (GPUA) and who did not use antivenom (GPNUA) were performed using stratified analysis, univariate and multivariate ordered logistic regression models to evaluate the coagulation parameters normalization time. Univariate and multivariate ordered logistic regression models were used to explore independent risk factors of patient prognosis.
Results
Between the GPUA and GPNUA groups, there is no significant difference in the coagulation parameters normalization time with the treatment of Agkistrodon halys antivenom. GPNUA consumed more cryoprecipitate and platelets and had a lower cost. The patient’s severity of the bite, first coagulation profile, and dosages of fresh frozen plasma, platelet, and red cell suspension was found to be risk factors for the normalization time of coagulation parameters.
Conclusions
The therapeutic effect of Agkistrodon halys antivenom in green pit vipers bite patients is not quite satisfying. In addition, more attention should be paid to the first coagulation profile, blood clotting factors indices, platelet count (PLT), and hemoglobin when treating such patients.
Introduction
Snakebite envenomation is a category A Neglected Tropical Disease with an annual incidence of between 1.8 and 2.7 million cases worldwide [Citation1]. Among them, about 138,000 patients died annually, and around 400,000 were left with permanent disability [Citation2]. More specific to Asia, snakebite envenoming affects at least 1.2–2.0 million people per year, with estimates ranging from 57,000 to 100,000 deaths [Citation3]. At present, multiple therapeutic methods are applied in the management of snakebite poisoning patients, and antivenoms are the only recommended effective treatment [Citation4–6]. However, due to the high cost of preparation and the low economic benefits it brings, most areas, with a high incidence of snakebite envenoming, exhibit soaring costs of antivenoms as well as insufficient supply [Citation7–9].
South and Southeast Asia are the hardest hit regions in Asia, with the predominant perpetrators identified as Viperid snakes (e.g., green pit vipers, Russell’s viper, saw-scaled vipers, and mountain pit viper, etc.) and Elapidae (e.g., cobras, king cobras, kraits, and coral snakes, etc.) [Citation10–13]. Shenzhen, a city with a high incidence of venomous snake bites, is located in the south of mainland China adjacent to Hong Kong, thus it is recognized as part of Southeast Asia. Among the incidents, about 89.3% of cases were perpetrated by the green pit viper [Citation14].
Green pit viper is a venomous snake belonging to the class Reptilia, order Squamata, family Viperidae, subfamily Crotalinae, genus Trimeresurus [Citation15]. Its venom is predominantly hematotoxic which more likely to cause coagulation dysfunction and local edema or inflammation in patients but has extremely low mortality and disability [Citation16–18]. There are about 40 species of the genus Trimeresurus found globally [Citation19,Citation20], among which the main ones distributed in China are Trimeresurus stejnegeri, Trimeresurus yunnanensis, Trimeresurus gracilis, Trimeresurus albolabris, Trimeresurus medoensis, and Trimeresurus tibetanus [Citation21]. Green pit vipers are mainly found in the south of China. Based on epidemiology and species identified by admitted bitten patients [Citation22], Trimeresurus stejnegeri, and Trimeresurus albolabris are the main species found in Shenzhen and its surrounding area.
Currently, only nearby Taiwan and Thailand have routinely treated these bites with the antivenom for green pit vipers, while mainland China treats the bites of green pit vipers with antivenoms of other venomous snakes in the same subfamily, namely Agkistrodon halys and Deinagkistrodon acutus [Citation6,Citation23,Citation24]. However, the clinical benefit of antivenoms from different species of venomous snakes for the treatment of green pit viper bites is still controversial [Citation25,Citation26]. We found that the efficacy of Deinagkistrodon acutus antivenom was acceptable but it seemed unsatisfied for Agkistrodon halys antivenom in clinical practice. Due to periods of insufficient supply of Agkistrodon halys and Deinagkistrodon acutus antivenoms at the Snake Bite Treatment Center of the Shenzhen Hospital of Traditional Chinese Medicine (SZTCM), there was a resultant group of green pit viper bite patients who were treated with Agkistrodon halys antivenom and another incidental group of patients who were treated without any antivenom. In light of this, a retrospective cohort study was conducted to assess the coagulation parameters normalization time of Agkistrodon halys antivenom in the treatment of green pit viper bites.
Methods
Patient population
Inclusion criteria: Patients who were diagnosed as “bitten by green pit vipers”. There were no restrictions on age, gender, or time to presentation after snakebite.
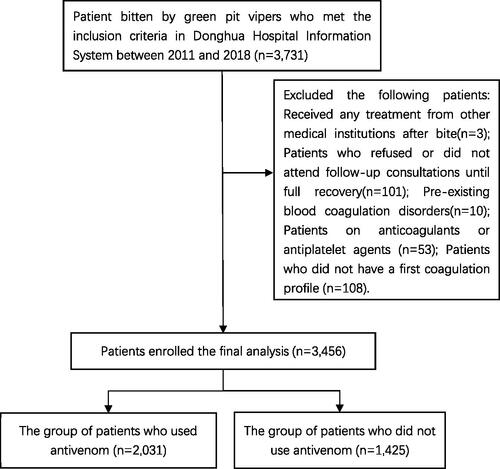
Patients who met the following criteria were excluded: (1) Received any treatment from other medical institutions after bites; (2) Patient who refused or did not attend follow-up consultations until full recovery; (3) Pre-existing blood coagulation disorders; (4) Patients on anticoagulants or antiplatelet agents; (5) Patients who did not have at least one of the following blood tests performed at first presentation (i.e., a first coagulation profile): prothrombin time (PT); activated partial thromboplastin time (APTT); platelet count (PLT); fibrinogen (Fig) levels.
In addition, according to the severity of the bite, all the patients were divided into three categories, namely mild, moderate, and severe, and they were finally assigned to outpatient, emergency observation, and inpatient ward treatment, respectively.
Data source
The clinical and laboratory information of the patients in the institution was recorded using the Donghua Hospital Information System of the SZTCM in Guangdong province, China. The Snake Bite Treatment Center within SZTCM covers a region of approximately 55,316 square kilometers containing a population of 31.42 million, which is the largest snakebite treatment agency in the province. In 2011–2018, the Snake Bite Treatment Center intermittently experienced shortages in the supply of antivenom of Agkistrodon halys and Deinagkistrodon acutus. The data on green pit viper bites that occurred between 2011 and 2018 were collected and screened based on the inclusion and exclusion criteria ().
The following data were extracted from the patient records: age, sex, severity of the bite (i.e., mild, moderate, and severe), pregnancy status (females only), geographical location of the bite incident (i.e., indoors versus outdoors), anatomical site of the snakebite, underlying comorbidities (such as respiratory diseases, cardiovascular diseases, kidney diseases, liver diseases, hematological disease (non-coagulopathy, e.g., anemia, poly-leucocythæmia, and agranulocytosis), endocrine and metabolic disease and immunodeficiency or immunosuppressive conditions), bite-to-hospital time, first coagulation profile, skin condition prior to treatment, each antivenom skin sensitivity test, length of hospital stay in days (only hospitalized patients), coagulation parameters during the recovering period (from bite to normal values of PT; APTT; Fig; PLT) and treatment costs. Additionally, the dosages of fresh frozen plasma, cryoprecipitate, platelet, and red cell suspension were also logged.
As this study was a retrospective analysis of the patient case notes that did not affect the clinical outcome and patients identifying information was anonymized, therefore after discussion with the institutional review board, individual patient consent was deemed not needed. Moreover, the study has been held according to the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Definitions of variables
The degree of first coagulation profile was measured using a modified Snakebite Severity Scale [Citation6,Citation27,Citation28]. Severity was categorized as:
Level 1 – no abnormality in hemorrhage and laboratory examination.
Level 2 – minimal coagulation anomaly: PT <20 s, APTT <50 s, PLT (100–150) × 109/L, or Fig 1–1.5 g/L.
Level 3 – mild coagulation anomaly: PT 20–<50 s, APTT 50–<75 s, PLT (50–<100) × 109/L, or Fig 0. 6–1 g/L.
Level 4 – moderate coagulation anomaly: PT 50–<100 s, APTT 75–<100 s, PLT (20–<50) × 109/L, or Fig < 0.6 g/L.
Level 5 – significant abnormalities in coagulation: PT or APTT undetectable, PLTs <20 × 109/L, Fig undetectable, unstable vital signs, cerebral hemorrhage, or other severe abnormalities in coagulation results.
Likewise, the severity of the skin condition post-snakebite but prior to treatment was categorized as:
Level 1 – without clinical symptoms and signs.
Level 2 – tenderness, swelling, or ecchymosis around the bite area (<5–7.5 cm).
Level 3 – tenderness, swelling, or ecchymosis affecting less than half of the limb.
Level 4 – tenderness, swelling, or ecchymosis affecting at least half of the limb.
Level 5 – tenderness, swelling, or ecchymosis beyond the limb.
Coagulation parameters normalization time was defined as the time from admission to the last laboratory test when all the coagulation parameters returned to within normal.
Routine treatment protocol
After the patients arrived at the hospital, diagnosis was confirmed by history taking, physical examination (include examining skin condition prior to treatment), and photographic/specimen identification of the offending snake – either through the patient/relative bringing in a photograph/specimen of the offending snake or the patient/relative identifying the snake from a photographic catalog of common snakes. Immediately afterwards, the nursing staff performed phlebotomy for laboratory examination (include PT, APTT, Fig, PLT, etc.) and set out to prepare therapeutic drugs. It should be noted that if patients showed unstable vital signs, physical examination, and phlebotomy were performed almost simultaneously during resuscitation.
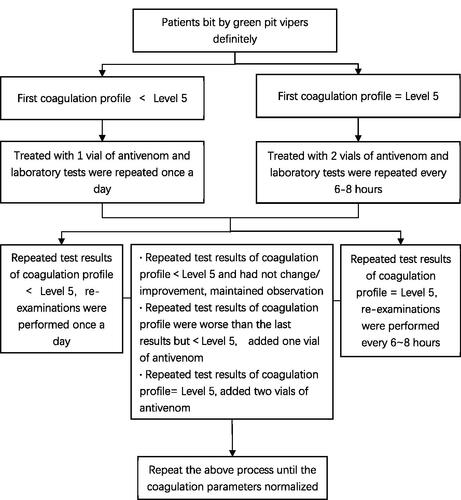
The Agkistrodon halys antivenom used in this study is manufactured by Shanghai Serum Bio-Technology Co, Shanghai, China, as a 6000 U (10 mL)/vial. When there was a normal supply of antivenom, antivenom was used for all the diagnosed individuals who were skin test‐positive and needed further desensitization therapy. For a patient with first coagulation profile below Level 5, the patient received a vial of antivenom for treatment, and the laboratory tests were repeated daily. If the results of the repeat tests found that the coagulation profile was still below Level 5, subsequent blood tests were still performed once a day or every 6–8 h until the blood parameters normalized. On the other hand, if a patient had a first coagulation profile of Level 5, two vials of the antivenom would be given for treatment, and the repeat laboratory tests would be performed every 6–8 h. If the results of the repeated blood tests found that the coagulation profile had fallen below Level 5, the frequency of further tests could be reduced to once a day or maintain the frequency of every 6–8 h for results until normalization. In addition, if the repeated test results were worsening but below Level 5, clinicians could add one vial of antivenom and another two vials of antivenom could be added later if further repeated test results still equal to Level 5. Acute allergic reactions caused by the antivenoms were treated with promethazine, dexamethasone, or adrenaline. The routine protocol of antivenom use is shown in .
As the patient received the first antivenom treatment, the clinician also performed the following treatments on the patient’s wound: 1% lidocaine 2–5 mL, dexamethasone 5–10 mg, and chymotrypsin 4000 u were used to injected intramuscularly around the wound or at the proximal joint. Then, the wound was flushed with 100–500 mL hydrogen peroxide, saline or 5‰ potassium permanganate solution, which followed by covering the surroundings of the wound with Shuanghuangsheshang powder, a Chinese medicine compound mainly consisting of Rhei Radix et Rhizoma and Phellodendri Chinensis Cortex, with the powdered dressing being changed once a day for 2–5 d.
Tetanus toxin 1500 IU/tetanus immunoglobulin 1500 IU and furosemide 20–40 mg were administered for the patients’ first admission after bite. Sheshangliangxue oral liquid (mainly contains Lobeliae Chinensis Herba, Ilex pubescens Hook. et Arn.) or Sheduqing oral liquid (mainly contains Coptidis Rhizoma, Inulae Radix), both Chinese medicine compounds, were given by 30–60 mL three times a day for 1 week.
Transfusion criteria include hemoglobin <70 g/L accompanied by hemorrhage, hemoglobin <60 g/L, or hematocrit <20% (red cell suspension), PT or APTT extension >1.5 times of normal (fresh frozen plasma), PLT <50 × 109/L with bleeding episodes or PLT <5 × 109/L (platelet), and Fig <0.8 g/L (cryoprecipitate) in any blood test reading.
Except for the antivenom treatment, the other management strategies, including treatment programs and blood test frequency, were the same for both groups.
Exposure
Patients that were bitten by green pit vipers (i.e., Trimeresurus stejnegeri and Trimeresurus albolabris) treated with or without antivenom.
Outcome measures
Coagulation parameters normalization time was defined as the primary outcome measure. Secondary outcome measures include fresh frozen plasma dosage, cryoprecipitate dosage, platelet dosage, red cell suspension dosage, length of hospital stay, and treatment costs.
Data analysis
Characteristic data and outcome data of GPUA and GPNUA were compared using the Student’s t-test for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables. Multinomial and ordinal logistic regression was used to examine the relationship between “use of antivenom or not” and “coagulation parameters normalization time” with adjustments for age, sex, severity of the bite, geographical location of the bite incident, anatomical site of the snakebite, underlying comorbidity, bite-to-hospital time, first coagulation profile, skin condition prior to treatment, and the dosages of fresh frozen plasma, cryoprecipitate, platelet, and red cell suspension. Meanwhile, we also used the model to analyze other factors that influence coagulation parameters normalization time.
Of note, in the logistic regression, coagulation parameters normalization time was classified as Level 1 (one to three days), Level 2 (four to six days), Level 3 (seven to nine days), and Level 4 (ten days or more). Furthermore, patients are stratified according to the different levels of primary coagulation profile, exploring the relationship between the “use of antivenom or not” and “coagulation parameters normalization time” for snake-bitten patients. Odds ratios (OR), including crude OR and adjusted OR, and relevant 95% confidence intervals (CI) were reported. Stata version 14.2 for Windows (StataCorp, College Station, TX) was used for all analyses. A two-tailed p value < .05 was considered statistically significant.
Result
We screened 3731 patients and enrolled 3456 patients eventually according to the inclusion and exclusion criteria, in which 2031 patients in the group of patients who used antivenom and 1425 patients in the group of patients who did not use antivenom. The process of patient selection is shown in .
The mean age of GPUA patients is 39.07 years, which is older than that of GPNUA patients (37.51 years; p = .004) (). There were statistical differences in severity of the bite between GPUA and GPNUA: GPNUA has a larger proportion of mild and moderate patients, while a smaller proportion of severe patients (p < .001). Most of the bite incidents in the two groups occurred outdoors, although there was a noticeable greater proportion of GPUA patients bitten outdoors compared to GPNUA patients (p < .001). GPUA patients were mostly bitten on the upper limb, while patients of GPNUA were bitten on the lower limb (p < .001). Patients in GPUA were more likely to have respiratory diseases (p < .001), cardiovascular diseases (p < .001), liver diseases (p < .001), and endocrine and metabolic diseases (p < .001) but less likely to have immunodeficiency or immunosuppressive diseases (p = .006).
Table 1. Baseline characteristics of GPUA and GPNUA.
Considering that there are no statistically significant differences in the first coagulation profile and pre-treatment skin condition between GPNUA and GPUA patients, we directly compared the outcomes of the two groups (). Remarkably, there is no difference between the two groups in terms of coagulation parameters normalization time (p = .066). GPNUA patients required higher dosages of cryoprecipitate (p = .027) and platelet (p < .001) transfusion, however, the costs of treatment in GPNUA patients were much lower than in GPUA patients (p < .001).
Table 2. Primary and secondary outcomes of GPUA and GPNUA.
Although univariate analysis showed that antivenom was helpful for coagulation parameters normalization time (Crude OR, 0.86; 95% CI, 0.76–0.97) (), correction for confounding factors suggests that antivenom did not play a critical role (Adjusted OR, 1.15; 95% CI, 0.99–1.34) (). Stratified analyses showed the same results in Level 3 (adjusted OR, 0.81; 95% CI, 0.60–1.09) and Level 5 (adjusted OR, 1.54; 95% CI, 0.58–4.07) of the first coagulation profile. At Level 4 of the first coagulation profile, the antivenom acts as a therapeutic agent (Adjusted OR, 0.40; 95% CI, 0.24–0.67), however, at Level 2 it conversely prolongs the normalization time of coagulation parameters (Crude OR, 1.79; 95% CI, 1.41–2.27; adjusted OR, 2.46; 95% CI, 1.85–3.27).
Table 3. Comparison of the risks of GPUA and GPNUA by multinomial and ordinal logistic regression analysis.
Independent risk factors analysis revealed that the severity of the bite and dosage of fresh frozen plasma, platelet, and red cell suspension are risk factors for the normalization time of coagulation parameters () (adjusted OR, 0.37; 95% CI, 0.32–0.44; Adjusted OR, 1.01; 95% CI, 1.01–1.02; adjusted OR, 1.24; 95% CI, 1.08–1.42; adjusted OR, 1.59; 95% CI, 1.06–2.37; respectively). Meanwhile, multinomial and ordinal logistic regression also identified that a more deranged first coagulation profile is associated with a significantly longer normalization time of coagulation parameters (adjusted OR, 1.73; 95% CI, 1.31–2.29; adjusted OR, 3.25; 95% CI, 2.46–4.29; Adjusted OR, 3.07; 95% CI, 2.27–4.16; adjusted OR, 4.32; 95% CI, 2.88–6.47; respectively).
Table 4. Independent risk factors in all patients bit by green pit vipers by multinomial and ordinal logistic regression analysis.
Discussion
In mainland China, antivenoms of Agkistrodon halys and Deinagkistrodon acutus, are recommended as the preferred treatment for green pit viper bites [Citation6]. In Hong Kong, Agkistrodon halys antivenom is also one of the options for treating green pit viper bites [Citation29–31]. It is worth noting that the antivenom of Agkistrodon halys is not made by injecting Agkistrodon halys venom into horses, but Gloydius brevicaudus (short-tailed pit viper) [Citation32,Citation33]. Both green pit viper and Gloydius brevicaudus belong to the class Reptilia, order Squamata, family Viperidae, subfamily Crotalinae while Gloydius brevicaudus is further classified into the genus Gloydius [Citation21]. Their venoms are both predominantly hematotoxic [Citation21]. The venoms of different species of snakes in the same family have some of the antigenic determinants the same [Citation34–36]. For example, the venom of snakes that belong to the Viperidae family may share common enzymes and non-enzymatic proteins, such as serine protease, phospholipase A2, L-amino acid oxidase, and disintegrin, C-type lectin-like proteins, etc. Apart from specific proteins, within the venom of different snakes, the same type of protein mostly contains common epitopes [Citation37], which leads to certain kinds of antivenoms having cross-immunological activity against venomous snakes’ venom in different genera. Agkistrodon halys antivenom falls into the category because it demonstrated immunological binding activity to the venom of green pit viper including Trimeresurus stejnegeri and Trimeresurus albolabris in vitro [Citation33]. Animal experiments further showed that among adult mice envenomed by the venom of Trimeresurus albolabris, at its median effective dose Agkistrodon halys antivenom was able to reduce the venom’s median lethal dose (LD50) much more than when using green pit viper antivenom [Citation25].
Although experiments have proved that Agkistrodon halys antivenom can neutralize Trimeresurus stejnegeri and Trimeresurus albolabris venom in vitro and Trimeresurus albolabris venom in mice, it is still uncertain whether the same effect can be demonstrated in vivo among human patients. A case report from Hong Kong described a six-year-old girl bitten by Trimeresurus albolabris and was treated with Agkistrodon halys antivenom from mainland China. However, it was reported that the therapeutic effect was not satisfactory. After receiving the antivenom injection, the girl’s coagulation indices further deteriorated [Citation26]. Although some guidelines recommended the use of Agkistrodon halys antivenom in green pit viper bites in the clinical setting [Citation6,Citation31], there is a paucity of Level 1 evidence within the current literature (such as within MEDLINE). In our study, multi-factor analysis of Agkistrodon halys antivenom for green pit viper bites showed that, overall, there was no difference in the curative and therapeutic effect of Agkistrodon halys antivenom between GPUA and GPNUA. The therapeutic effect was only observed in patients belonging to Level 4 of the first coagulation profile. However, within Level 1 and 2 patients, Agkistrodon halys antivenom even prolonged the coagulation parameters normalization time. The exact cause of this effect is unclear. Probably because the patients categorized as Level 4 from the first coagulation profile were bitten with a higher quantity of venom, Agkistrodon halys antivenom was able to neutralize more venom and thus these patients exhibited a more obvious therapeutic effect compared to other Level 4 patients who did not receive Agkistrodon halys antivenom. Conversely, Level 1 and 2 patients were bitten with less venom, meaning Agkistrodon halys antivenom could neutralize is less venom, which in turn might reduce the apparent therapeutic effect. Another possibility is that the adjuvant treatment in the treatment protocol (e.g., cleaning and sterilization of the wounds, use of glucocorticoids, blood product transfusion, herbal medicine, and acupuncture) might weaken the therapeutic effect of Agkistrodon halys antivenom in Level 1 and 2 patients. However, the exact reason for the longer normalization time of coagulation parameters in Level 1 and 2 patients is unclear and further research is needed to explore the issue. In addition, it has been reported that the dose of antivenom may also affect its efficacy [Citation5], although, in this study, our treatment protocols were adhered to [Citation6,Citation38]. Nevertheless, even so, international consensus as to the optimal dosage of antivenom still remains controversial. One vial of antivenom was recommended to treat in both child and adult patients for all snake types according to Australian guidelines [Citation39].
Apart from the relationship between antivenom and clinical efficacy, the result of this study suggests that the use of antivenom to treat snake bites is much more costly than treatment without antivenom. The economic burden of antivenom has always been a global issue that has attracted much attention [Citation7]. In snakebite-prone areas, most of which are lower-income countries and regions, the economic costs of antivenom treatment cannot be overlooked [Citation8,Citation9,Citation40]. As highlighted from a report in 2013, treatment and hospitalization costs caused by snakebite injuries could amount to up to 12 years of income for a typical farmworker or laborer in India [Citation3,Citation40]. In addition, many patients paid for medical expenses by selling their home, and even forcing their children to drop out of school prematurely in order to start work to pay for such costs [Citation40]. Although the economies of urban metropolises, such as Shenzhen and Hong Kong are highly developed, most snakebites were sustained as work-related injuries among farmers and laborers in the poorer countryside. On the other hand, due to minimal investment in antivenom research and production, as well as wasteful usage, the supply of antivenom is haphazard, which directly leads to pockets of shortage in some areas] as encountered in our study [Citation9,Citation41,Citation42]. Despite efforts by the World Health Organization and its policymakers to alleviate this situation, the economic and production challenges of antivenom persist [Citation7]. Thus, it is crucial to reduce unnecessary use of antivenom and perform precision therapy under such circumstances. As shown in other studies, Deinagkistrodon acutus antivenom may be a more effective therapeutic means for green pit viper envenoming [Citation43–45].
Bleeding episodes, complications and allergic reactions of blood products happened rarely while there was no case of clinical sequala and mortality in our study. Consequently, the recovery time of coagulation parameters was chosen in our study as the primary outcome with independent predictors including the severity of the bite, first coagulation profile, fresh frozen plasma dosage, platelet dosage, and red cell suspension dosage. We found that bite-to-hospital time is not a risk factor for the primary outcome measure of coagulation parameters normalization time. This contrasts with the study from Kalantri et al. who found that bite-to-hospital time is an independent risk factor for in-hospital mortality of snakebite through a Cox proportional-hazards regression analysis [Citation46]. However, another study on Trimeresurus stejnegeri bites showed that the time after bite to first antivenom dose did not correlate with wound necrosis [Citation23]. These differences might be related to types, strength, or amount of venom and the body’s absorption rate of venom. Further research is needed to explore the exact reasons for these observed trends. Furthermore, patients with more severe first coagulation profiles had a longer coagulation parameters normalization time. Conversely, the coagulation parameters normalization time was not related to the skin condition prior to treatment, suggesting that local injuries and systemic conditions may develop independently. This in turn suggests first coagulation profile is a key determinant factor in the recovery time of coagulation parameters.
Limitations
There are some limitations to our study. First, although we have followed the treatment protocol provided by the local guidelines, the exact amount of antivenom applied for each patient was not recorded in our study. Second, the identified types of green pit viper, amount of venom, and patient’s body mass index were not available in the Donghua Hospital Information System. Third, suspected but not confirmed cases were excluded, which to some extent weakened the representativeness of the patient population. Finally, although the number of patients included in this study is large, the species of Trimeresurus species distributed in other regions may not be consistent with ours, which might impact the generalization of our conclusions. Although these limitations might affect the inference of our data, there are clear noticeable trends and observations in our study which may aid the front-line physician in the treatment of venomous snakebites in the acute setting.
Conclusions
Based on this retrospective study in a single medical center, the administration of Agkistrodon halys antivenom did not seem to show a therapeutic effect for green pit viper bites. High-quality randomized controlled trials which should focus on the indication, dose, and frequency of antivenom administration are expected to validate our findings.
Author contributions
Study concept and design by Zhong-Yi Zeng, Pei-Ying Huang, and Yi Li. Data acquisition by Zhong-Yi Zeng, Pei-Ying Huang, Jia-Yu Du, Yu-Xiang Liu, Lin-Sheng Zeng, and Cong-Cong Zhang. Zhong-Yi Zeng and Pei-Ying Huang were involved in manuscript writing. Zhong-Yi Zeng, Pei-Ying Huang, Shi-Gong Guo, and Yi Li participated in the critical review and revision of the manuscript. Study supervision by Zhong-Yi Zeng and Yi Li.
Acknowledgments
The thank Dr. Wei Han from teaching and research office of epidemiology and statistics of the Peking Union Medical College Hospital for suggestions about statistics and Mr. Shuai Liu from teaching and research office of English of Guangzhou University of Chinese Medicine for linguistic assistance during the preparation of this manuscript.
Disclosure statement
The authors declare no potential conflict of interest.
Additional information
Funding
References
- Lancet T. Snake-bite envenoming: a priority neglected tropical disease. Lancet. 2017;390:2.
- Albulescu LO, Hale MS, Ainsworth S, et al. Preclinical validation of a repurposed metal chelator as an early-intervention therapeutic for hemotoxic snakebite. Sci Transl Med. 2020;12(542):eaay8314.
- Gutiérrez JM, Calvete JJ, Habib AG, et al. Snakebite envenoming. Nat Rev Dis Primers. 2017;3(1):1–21.
- WHO. Guidelines for the production control and regulation of snake antivenom immunoglobulins. Geneva, Switzerland: World Health Organization; 2010.
- WHO. Guidelines for the prevention and clinical management of snakebite in Africa, in, world health organization. Congo, Central Africa: WHO Regional Office for Africa; 2010.
- Consensus Expert Group of Chinese Snake Wound Treatment Experts. Expert consensus on treatment of snake wounds in China in 2018. Chin J Emerg Med. 2018;27:1315–1322.
- Minghui R, Malecela MN, Cooke E, et al. WHO's Snakebite Envenoming Strategy for prevention and control. The Lancet Global Health. 2019;7(7):e837–e838.
- Habib AG, Brown NI. The snakebite problem and antivenom crisis from a health-economic perspective. Toxicon. 2018;150:115–123.
- Brown N, Landon J. Antivenom: the most cost-effective treatment in the world? Toxicon. 2010;55(7):1405–1407.
- Jayawardana S, Arambepola C, Chang T, et al. Prevalence, vulnerability and epidemiological characteristics of snakebite in agricultural settings in rural Sri Lanka: a population-based study from South Asia. PLoS One. 2020;15(12):e0243991.
- Knudsen C, Jürgensen JA, Føns S, et al. Snakebite envenoming diagnosis and diagnostics. Front Immunol. 2021;12:661457.
- Hung DZ. Taiwan’s venomous snakebite: epidemiological, evolution and geographic differences. Trans R Soc Trop Med Hyg. 2004;98(2):96–101.
- Alirol E, Sharma SK, Bawaskar HS, et al. Snake bite in South asia: a review. PLoS Negl Trop Dis. 2010;4(1):e603.
- Liu Q, Zeng ZY, Zhang CC. Epidemiological characteristics of poisonous snakebites in Shenzhen city. Guangxi Med J. 2018;40:54–56.
- Peng G. Taxonomic and phylogenetic studies on Trimeresurus (S.L.) (Serpentes: Crotalinae). ChengDu, China: Sichuan University; 2005.
- Rathnayaka RN, Kularatne SAM, Ranathunga P. Coagulopathy and extensive local swelling following green pit viper (trimeresurus trigonocephalus) envenoming in Sri Lanka. Toxicon. 2017;129:95–99.
- Rojnuckarin P, Intragumtornchai T, Sattapiboon R, et al. The effects of green pit viper (trimeresurus albolabris and trimeresurus macrops) venom on the fibrinolytic system in human. Toxicon. 1999;37(5):743–755.
- Rathnayaka RN, Ranathunga P, Kularatne SAM. Epidemiology and clinical features of green pit viper (Trimeresurus trigonocephalus) envenoming in Sri Lanka. Toxicon. 2017;137:99–105.
- David P. Les serpents venimeux du monde: systématique et répartition. Dumerilia. 1999;3:3–499.
- McDiarmid RW, Campbell JA, Touré T. Snake species of the world: a taxonomic and geographic reference. Washington (DC): Herpetologists‘ League; 1999.
- Ermi Z. Chinese zoology reptiles volume three scaly snakes. Beijing: Science Press; 1998.
- Huang PY, Zeng ZY, Liu Q, et al. Epidemiological investigation and analysis of influencing factors before hospital on the bite of Trimeresurus stejnegeri. J Clin Emerg. 2020;21(05):393–396 + 401.
- Chiang LC, Tsai WJ, Liu PY, et al. Envenomation by Trimeresurus stejnegeri stejnegeri: clinical manifestations, treatment and associated factors for wound necrosis. J Venom Anim Toxins Incl Trop Dis. 2020;26:e20200043.
- Yee KT, Maw LZ, Kyaw AM, et al. Evaluation of the cross-neutralization capacity of Thai green pit viper antivenom against venom of Myanmar green pit viper. Toxicon. 2020;177:41–45.
- Fung HT, Yung WH, Crow P, et al. Green pit viper antivenom from Thailand and agkistrodon halys antivenom from China compared in treating cryptelytrops albolabris envenomation of mice. Hong Kong Med J. 2012;18(1):40–45.
- Yang JYK, Hui H, Lee ACW. Severe coagulopathy associated with white-lipped green pit viper bite. Hong Kong Med J. 2007;13(5):392–395.
- Lavonas EJ, Kokko J, Schaeffer TH, et al. Short-term outcomes after fab antivenom therapy for severe crotaline snakebite. Ann Emerg Med. 2011;57(2):128–137.e3.
- Rha JH, Kwon SM, Oh JR, et al. Snakebite in Korea: a guideline to primary surgical management. Yonsei Med J. 2015;56(5):1443–1448.
- Mong R, Ng VCH, Tse ML. Safety profile of snake antivenom (use) in Hong Kong - a review of 191 cases from 2008 to 2015. Clin Toxicol (Phila). 2017;55(10):1066–1071.
- Lam SK, Yip SF, Crow P, et al. Comparison of green pit viper and Agkistrodon halys antivenom in inhibition of coagulopathy due to Trimeresurus albolabris venom: an in-vitro study using human plasma. Hong Kong Med J. 2017;23(1):13–18.
- Management of Snakebite. Accident and emergency clinical guidelines number 9. Hong Kong: Central Coordinating Committee of Accident and Emergency Services, Hospital Authority; 2008.
- Oh H, Shin J, Ato M, et al. The first meeting of the national control laboratories for vaccines and biologicals in the Western Pacific in 2016. Osong Public Health Res Perspect. 2017;8(1):91–103.
- Yong MY, Tan KY, Tan CH. Potential Para-specific and geographical utility of thai green pit viper (Trimeresurus albolabris) monovalent antivenom: neutralization of procoagulant and hemorrhagic activities of diverse trimeresurus pit viper venoms. Toxicon. 2021;203:85–92.
- Ownby CL, Colberg TR. Comparison of the immunogenicity and antigenic composition of several venoms of snakes in the family crotalidae. Toxicon. 1990;28(2):189–199.
- Gao JF, Wang J, Qu YF, et al. Immunoreactivity between venoms and commercial antiserums in four Chinese snakes and venom identification by species-specific antibody. J Immunol Methods. 2013;387(1–2):211–218.
- Van Dong L, Quyen LK, Eng KH, et al. Immunogenicity of venoms from four common snakes in the South of Vietnam and development of ELISA kit for venom detection. J Immunol Methods. 2003;282(1–2):13–31.
- Gutiérrez JM, Lomonte B, León G, et al. Snake venomics and antivenomics: proteomic tools in the design and control of antivenoms for the treatment of snakebite envenoming. J Proteomics. 2009;72(2):165–182.
- Tan NH, Tan KY, Tan CH. Snakebite in Southeast Asia: envenomation and clinical management. Handbook of venoms and toxins of reptiles. Boca Raton (FL): CRC Press; 2021.
- Isbister GK, Brown SGA, Page CB, et al. Snakebite in Australia: a practical approach to diagnosis and treatment. Med J Aust. 2013;199(11):763–768.
- Vaiyapuri S, Vaiyapuri R, Ashokan R, et al. Snakebite and its socio-economic impact on the rural population of Tamil Nadu, India. PLoS One. 2013;8(11):e80090.
- Habib AG, Warrell DA. Antivenom therapy of carpet viper (Echis ocellatus) envenoming: effectiveness and strategies for delivery in west Africa. Toxicon. 2013;69:82–89.
- Visser LE, Kyei-Faried S, Belcher DW, et al. Failure of a new antivenom to treat Echis ocellatus snake bite in rural Ghana: the importance of quality surveillance. Trans R Soc Trop Med Hyg. 2008;102(5):445–450.
- Wei WL. Application of anti-Agkistrodon halys venom serum and anti-Agkistrodon acutus venom serum in the treatment of bite of Trimeresurus stejnegeri snake. J Snake. 2021;33(3):3.
- Cai TT, Li TX, Ye YQ, et al. Selection and optimization of antivenom serum in treatment of Trimeresurus stej-negeri snakebite poisoning. J Snake. 2021;33(02):121–124.
- Zhang YA. Comparative study of Chinese medicine combined with two antivenom in the treatment of green pit viper bites. Guangdong, China: Guangzhou University of Chinese Medicine; 2020.
- Kalantri S, Singh A, Joshi R, et al. Clinical predictors of in‐hospital mortality in patients with snake bite: a retrospective study from a rural hospital in Central India. Trop Med Int Health. 2006;11(1):22–30.