Abstract
Context
Ethylene glycol is metabolized to toxic metabolites that cause acute kidney injury, metabolic acidemia, and death. The treatment of patients with ethylene glycol poisoning includes competitively inhibiting alcohol dehydrogenase with ethanol or fomepizole to prevent the formation of toxic metabolites, and extracorporeal treatments such as hemodialysis to remove ethylene glycol and its metabolites. In the absence of significant metabolic acidemia or kidney injury, it is hypothesized that extracorporeal treatments may be obviated without adverse outcomes to the patient if alcohol dehydrogenase inhibitors are used.
Objectives
The objectives of this study are to: (1) identify indicators predicting ADH inhibitor failure in patients with ethylene glycol poisoning treated with either ethanol or fomepizole for whom extracorporeal treatment was not performed (aside from rescue therapy, see below) (prognostic study), and (2) validate if the anion gap, shown in a previous study to be the best surrogate for the glycolate concentration, is associated with acute kidney injury and mortality (anion gap study).
Methods
We conducted a systematic review to identify all reported patients with ethylene glycol poisoning treated without extracorporeal treatments but with either fomepizole (fomepizole monotherapy) or ethanol (ethanol monotherapy). Analyses were performed using both one case per patient and all cases (if multiple events were reported for a single patient). Data were compiled regarding poisoning, biochemistry, and outcomes. Treatment failure was defined as mortality, worsening of acid-base status, extracorporeal treatments used as rescue, or a worsening of kidney or neurological function after alcohol dehydrogenase inhibition was initiated. Also, we performed an analysis of previously described anion gap thresholds to determine if they were associated with outcomes such as acute kidney injury and mortality.
Results
Of 115 publications identified, 96 contained case-level data. A total of 180 cases were identified with ethanol monotherapy, and 231 with fomepizole monotherapy. Therapy failure was noted mostly when marked acidemia and/or acute kidney injury were present prior to therapy, although there were cases of failed ethanol monotherapy with minimal acidemia (suggesting that ethanol dosing and/or monitoring may not have been optimal). Ethylene glycol dose and ethylene glycol concentration were predictive of monotherapy failure for ethanol, but not for fomepizole. In the anion gap study (207 cases), death and progression of acute kidney injury were almost nonexistent when the anion gap was less than 24 mmol/L and mostly observed when the anion gap was greater than 28 mmol/L.
Conclusion
This review suggests that in patients with minimal metabolic acidemia (anion gap <28 mmol/L), fomepizole monotherapy without extracorporeal treatments is safe and effective regardless of the ethylene glycol concentration. Treatment failures were observed with ethanol monotherapy which may relate to transient subtherapeutic ethanol concentrations or very high ethylene glycol concentrations. The results are limited by the retrospective nature of the case reports and series reviewed in this study and require prospective validation.
Introduction
Ethylene glycol is commonly used in many antifreeze products and each year is responsible for thousands of toxic exposures worldwide from intentional self-harm and other reasons [Citation1,Citation2,Citation3]. Ethylene glycol itself causes little toxicity; however, its metabolites (glycolate, glyoxylate and oxalate) induce a wide anion gap metabolic acidemia and end-organ toxicity such as acute kidney injury (AKI), coma, seizures, cranial nerve defects, and death. In fact, ethylene glycol is the leading poisoning for which extracorporeal treatments are used in several countries [Citation4].
In addition to supportive care, the mainstay of treatment for patients with ethylene glycol poisoning includes two other aspects: (1) competitive inhibition of alcohol dehydrogenase (ADH) with either ethanol or fomepizole, to prevent the production of toxic metabolites, and (2) extracorporeal treatments, such as hemodialysis and continuous kidney replacement therapy, to remove both ethylene glycol and its toxic metabolites.
Ethanol has an affinity approximately 100 times greater for ADH than ethylene glycol and was first used in humans in 1965 [Citation5]. In fact, patients who co-ingest ethanol with ethylene glycol also generally experience less severe toxicity [Citation6]. Fomepizole has a greater affinity for ADH than ethanol [Citation7,Citation8] and was first used in humans for ethylene glycol poisoning in 1986 [Citation9,Citation10]. Because of its simpler dosage, lack of CNS effects, cultural concerns for ethanol use, and non-requirement of a high-dependency unit or frequent blood tests, fomepizole has largely replaced ethanol in many regions, except where it is cost-prohibitive [Citation11].
Hemodialysis is usually recommended in the presence of severe metabolic acidemia, kidney impairment, severe electrolyte imbalance, or deteriorating clinical conditions despite supportive measures [Citation12,Citation13]. If these are absent, proposed indications for hemodialysis in ethanol-treated patients include a serum ethylene glycol concentration greater than 8 mmol/L (50 mg/dL), while some authors have suggested that hemodialysis may be obviated if fomepizole is used [Citation12,Citation14,Citation15]. However, there are no prospective trials to support this approach and, to our knowledge, a comprehensive review of data supporting the withholding of extracorporeal treatments under certain clinical circumstances has not been reported.
A prior review identified the prognostic value of the glycolate concentration, demonstrating that mortality was unlikely when the glycolate concentration was less than 8.3 mmol/L (negative predictive value = 100%) whereas a glycolate concentration exceeding 12.9 mmol/L predicted AKI (positive predictive value = 86%) [Citation16]. Additionally, the anion gap was found to be the best surrogate marker for the glycolate concentration. However, extracorporeal treatment was used in 80% of cases from which these data were derived [Citation16].
Objectives
The objectives of this study are to: (1) identify indicators predicting ADH inhibitor failure in patients with ethylene glycol poisoning treated with either ethanol or fomepizole for whom extracorporeal treatment was not performed (aside from rescue therapy, see below) (prognostic study), and (2) validate if the anion gap, shown in a previous study to be the best surrogate for the glycolate concentration [Citation16], is associated with AKI and mortality (anion gap study).
Methods
Eligibility criteria
Types of studies
All study types that reported human ethylene glycol poisoning were considered eligible, including interventional trials, comparative studies, observational cohorts, and case reports. Reviews, editorials, book chapters, and commentaries were excluded if they contained no original data. In vitro and animal experiments were also excluded. Reference lists of all included and excluded articles were searched for other eligible publications. Only articles containing original case-level data were included. Cohorts without case-level data were presented in descriptive format only. For all cohorts and case series containing more than 8 patients, the authors were contacted for additional data.
Types of participants
Subjects of all ages and comorbidities with a diagnosis of ethylene glycol poisoning, confirmed from history or detectable ethylene glycol in blood, treated with ethanol and/or fomepizole without extracorporeal treatment initially were included. All types of exposures (acute, staggered, chronic) and all routes of exposure (ingestion, injection, inhalation) were eligible for inclusion. Multiple temporally separate cases in the same patient were considered as distinct cases due to variation in the amount taken, time to presentation, and treatment given. Cases were also evaluated by type of ADH inhibitor (i.e., ethanol or fomepizole) received.
Variable of interest
For the prognostic study, the following variables were analyzed to determine their influence on outcomes: dose (expressed as 100% ethylene glycol solution equivalent), time from exposure to health care presentation, ethanol co-ingestion, time from presentation to antidote administration, initial ethylene glycol concentration, kidney function, as well as acid-base parameters determined at the time ADH inhibition was initiated (anion gap, pH, bicarbonate concentration (HCO3−), base excess, glycolate concentration). For the anion gap study, the anion gap prior to the administration of ethanol or fomepizole was analyzed.
Outcomes
The outcome of interest in the prognostic portion of the analysis was “ADH inhibitor treatment failure”. This was arbitrarily defined prior to data analysis as any of the following criteria after the ADH inhibitor was started:
Increase in anion gap greater than 5 mmol/L;
Decrease in HCO3− greater than 5 mmol/L or decrease in base excess by more than 5 mmol/L, or corresponding decrease in pH;
Increase in serum creatinine concentration greater than 1.0 mg/dL (88 µmol/L) or reported new onset oliguria;
Extracorporeal treatments performed as rescue treatment (e.g., for worsening of acidemia, AKI, complications of ADH inhibition);
All-cause inpatient mortality;
New onset or worsening of neurological symptoms attributed to ethylene glycol (seizures, altered consciousness, cranial nerve palsy).
For the anion gap study, the outcomes of interest were all-cause inpatient mortality and/or KDIGO (Kidney Disease Improving Global Outcomes) stage 2 or 3 AKI, i.e., serum creatinine concentration at least 2.0 times the baseline or reference value adjusted for age and gender. If the creatinine concentration was not reported, the presence of “anuria” or “AKI” or “acute renal failure” was accepted as indicators of significant AKI.
Case-level exclusion
Cases were excluded when any of the following were met:
They involved co-exposure with other toxic alcohols (methanol, diethylene glycol or propylene glycol) [Citation17,Citation18];
Ethylene glycol exposure could not be confirmed either by history or specific assay [Citation19];
It was unclear if and when extracorporeal treatment was performed relative to ADH inhibition [Citation20,Citation21,Citation22];
No ADH inhibitor was administered, isopropanol was administered, or it was unclear which ADH inhibitor was given [Citation23,Citation24,Citation25,Citation26,Citation27];
The ethylene glycol concentration was undetectable, as there is no rationale for ADH inhibition in this context [Citation28,Citation29];
No outcomes of interest were presented [Citation30,Citation31,Citation32,Citation33,Citation34,Citation35,Citation36,Citation37];
Both ethanol and fomepizole were given as antidotes [Citation38,Citation39,Citation40].
Search strategy
The following databases were searched from their inception: Medline/PubMed, EMBASE, and Cochrane library (Review and Central). Conference proceedings/meeting abstracts of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) and North American Congress of Clinical Toxicology (NACCT) annual scientific meetings were manually searched, each from 2002 to 2020.
The following search strategy was developed for Pubmed/MEDLINE and translated for the other databases:
(ethylene glycol*).af;
(monoethylene glycol*).af;
1 or 2;
ethyl alcohol.af;
ethanol.af;
fomepizole.af;
antizol.af;
(methyl-pyrazole or methylpyrazole).af;
4-MP;
or/4–9;
3 and 10.
The search was performed on February 24th, 2021. To supplement the electronic searches, reviewers also manually searched reference lists of editorials, review articles, or similar literature for relevant research articles. No exclusions were made based on language or year of publication. Foreign language publications were all translated by native language speakers or professional translation services.
Study records
Selection process
Two reviewers (JB and MG) screened citations independently to determine eligibility for full-text assessment and subsequently screened the articles of the full text to select those meeting the inclusion criteria. Disagreement was resolved by consensus.
Data management and extraction
A standardized data extraction form was created and populated with data pertinent to the systematic review (Microsoft Excel). MG is responsible for the master copy. Two authors (JB, MG) extracted the data into Microsoft Excel software (version 2021); a methodologist (VL) reviewed all versions. Inconsistencies were resolved by consensus and data consolidated in one master flowsheet. The following data were extracted: baseline characteristics (age, gender), exposure (dose, route of exposure, percent ethylene glycol solution, time to presentation to a health care facility, ethanol co-ingestion, ethylene glycol concentration), clinical manifestations (altered mental status, seizures, hypotension, respiratory failure), laboratory values at the time ADH blockade was initiated and the worst value during hospitalization (pH, glycolate concentration, HCO3−, base excess, anion gap, creatinine concentration), treatment (ethanol, fomepizole, extracorporeal treatments, and their timing with relation to arrival at the healthcare facility), and clinical outcomes (death, AKI, seizures, altered mental status, or cranial nerve defect). The anion gap included in analyses was that incorporating potassium, i.e., (Na+ + K+) – (Cl− + HCO3−); if the anion gap was reported without potassium, 4 mmol/L (mid-range normal potassium concentration) was added to the reported number. If the method for calculating the anion gap was unknown, then 2 mmol/L was added to the reported anion gap. When parameters were described as “normal”, values were reported as age-expected creatinine concentration, HCO3− = 25 mmol/L, anion gap = 14 mmol/L, base excess = 0 mmol/L, and pH = 7.40. Fomepizole or ethanol monotherapy is defined as their use in addition to standard care which includes intravenous bicarbonate but excluded extracorporeal treatments.
Anion gap study
As mentioned, the glycolate concentration thresholds predicting clinical outcomes were found to be <8.3 mmol/L for survival and >12.9 mmol/L for AKI [Citation16]. The anion gap cut-offs correlating to these glycolate concentrations were conservatively estimated to be 24 mmol/L and 28 mmol/L, respectively [Citation16].
All cases identified from the systematic review outlined above were also used for the anion gap study if the anion gap prior to ADH blockade was reported. Three cohorts were analyzed: anion gap <24 mmol/L, anion gap 24–28 mmol/L, anion gap >28 mmol/L. Outcomes of interest were the presence of KDIGO stage 2 or 3 AKI or all-cause inpatient mortality. Cases were excluded if they reported a glycolate concentration, as these cases were already used to derive glycolate concentration cut-offs for AKI and mortality in the prior analysis [Citation16].
Quality assessment
The risk for bias of each included study was assessed using the Quality In Prognosis Studies (QUIPS) tool, as applicable [Citation41]. The quality of reports was evaluated using the CARE (CAse REport) guideline, but a systematic approach to assessing the risk of bias for the reports could not be performed due to the lack of a validated tool for case reports. Important limitations are described in the discussion.
Data analysis and synthesis
Descriptive statistical analyses were conducted, in which nonparametric continuous data were expressed as median (interquartile range (IQR) and range), and the statistical difference was determined using the Mann-Whitney U test. A p value of <0.05 was considered statistically significant. All statistics were conducted using SPSS Software (Statistical Package for the Social Sciences; IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 25 Armonk, NY: IBM Corp). No meta-analysis of prognostic studies was planned due to the expected paucity and granularity of data.
This systematic review protocol is reported in accordance with the PRISMA-P (Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols) 2015 Checklist. It was not registered to Prospero.
Results
After removal of duplicates, unrelated publications, and exclusions, 96 articles were included in the final analysis (), including 14 cohorts with case-level data (authors provided individual case data when these were not reported in the article) [Citation2,Citation6,Citation42,Citation43,Citation44,Citation45,Citation46,Citation47,Citation48,Citation49,Citation50,Citation51,Citation52,Citation53], 82 case reports/case series [Citation5,Citation9,Citation54–133]. A total of 411 cases were included, 347 of which were confirmed quantitatively by the presence of ethylene glycol in blood. The demographics, details of the poisoning, laboratory values and outcomes of included cases are presented in . When reported, all exposures were acute and all patients ingested ethylene glycol, except two who injected it subcutaneously [Citation113]. One article [Citation48] described a single patient admitted 154 times for ethylene glycol poisoning who received fomepizole monotherapy 63 times and ethanol monotherapy 16 times; case-level data were obtained. No comparative studies or randomized trials were identified. Nineteen cohorts containing no case-level data were excluded from analyses but are discussed [Citation114,Citation134–151].
Figure 1. Flow diagram (February 24th, 2021). ECTR: extracorporeal treatment; ADH: Alcohol dehydrogenase; EG: ethylene glycol.
Table 1. Characteristics of included cases with case-level data.
Prognostic study
Ethanol monotherapy
There were 180 cases reported with ethanol monotherapy, 30 of which failed (16.7%, ). There were 17 deaths, 15 of which had marked acidemia (HCO3− <8 mmol/L or pH ≤7.0) prior to ethanol therapy [Citation6,Citation42,Citation46,Citation55,Citation64,Citation65,Citation68,Citation85,Citation119,Citation124]. One patient expired as extracorporeal treatment was not available [Citation119]. Most of those who developed complications but survived also had significant kidney impairment or acidemia on arrival to the health care facility [Citation5,Citation63,Citation70,Citation81,Citation98,Citation108,Citation112,Citation127].
However, several cases with modest acidemia or kidney impairment failed ethanol therapy [Citation56,Citation67,Citation73,Citation89,Citation123,Citation132]. One patient had no AKI and pH 7.20 but died after a protracted course from AKI and cerebral edema following multiple seizures [Citation67]. Another had a pH of 7.10 but developed progressive acidemia and AKI and died 6 days later despite subsequent peritoneal dialysis [Citation91]. Failures were reported when serum ethanol monitoring was not performed [Citation91] or subtherapeutic [Citation67,Citation89]. There were only eight cases with ethylene glycol ingestions larger than 1 L or a serum ethylene glycol concentration greater than 80 mmol/L (497 mg/dL) [Citation2,Citation46,Citation48,Citation76,Citation80,Citation89,Citation111], all of which survived although one needed hemodialysis because of worsening acidemia and kidney function. [Citation89].
Compared to cases that had no treatment failure, those that failed ethanol monotherapy had a significantly greater ethylene glycol dose (207 mL vs 30 mL, p = 0.0002), a longer time to the presentation (6 h vs 1.5 h, p = 0.0002), a higher ethylene glycol concentration (9.5 mmol/L (59 mg/dL) vs 4.1 mmol/L (25.4 mg/dL), p = 0.04), and a longer time to antidote administration (10 h vs 1 h, p = 0.02). Those who failed ethanol monotherapy also had, on arrival to the healthcare facility, a significantly lower serum pH, a higher anion gap, a lower HCO3−, a lower base excess, and a higher creatinine concentration, which remained true when using 1 case per patient (). Among the cohorts with no case-level data, there were deaths reported in patients prior to receiving hemodialysis [Citation134], and in patients considered too sick to receive extracorporeal treatment [Citation137].
Table 2A. Comparison of characteristics with or without ADH inhibitor treatment failure (all included cases).
Table 2B. Comparison of characteristics with or without ADH inhibitor treatment failure (1 case per patient).
Fomepizole monotherapy
A total of 231 cases were reported with fomepizole monotherapy, 20 of which failed (8.7%, ). Three patients died [Citation52,Citation53,Citation120], all of whom had signs of late ethylene glycol toxicity and extremely elevated anion gap: one had an anion gap of 39 mmol/L [Citation53], another had a pH of 6.77 and an anion gap of 41 mmol/L, remained acidemic and developed seizures despite hemodialysis starting 8 h after presentation [Citation120], and a third had a pH of 6.88 and the family declined hemodialysis [Citation52].
Seven cases were initially treated with fomepizole and needed subsequent extracorporeal treatment; six for progression of AKI (all had a serum HCO3− ≤8.0 mmol/L when fomepizole was started [Citation72,Citation84,Citation102,Citation117,Citation126,Citation128]), and one for worsening acidemia [Citation121]. Other cases that failed fomepizole therapy had marked acidemia (serum HCO3− < 11 mmol/L or anion gap >28 mmol/L) on presentation. One patient experienced a seizure [Citation110], five patients had modest worsening of acidemia or AKI during fomepizole therapy [Citation49,Citation52], and one patient developed osmotic diuresis from an extremely elevated ethylene glycol concentration (258 mmol/L; 1601 mg/dL) and subsequently experienced electrolyte abnormalities requiring hemodialysis [Citation94]. One patient developed anaphylaxis from fomepizole that necessitated its cessation and extracorporeal treatment rescue [Citation116]. Only two patients with minimal metabolic derangements or kidney impairment failed fomepizole: one patient had a decrease in HCO3− from 17 to 11 mmol/L but had an uneventful course [Citation130] while the other developed an uncomplicated seizure but had a known seizure disorder [Citation9].
Compared to patients who had no treatment failure, those who failed fomepizole monotherapy had a significantly lower serum pH, a higher anion gap, a lower HCO3−, a lower base excess, and a higher creatinine concentration (). Neither the ethylene glycol dose, the time to presentation, nor the ethylene glycol concentration was predictive of treatment failure; thus there does not appear to be an ethylene glycol dose or ethylene glycol concentration cut-off above which toxicity was demonstrated if fomepizole is used. These include a benign evolution with a dose of ethylene glycol ≥1 L or ethylene glycol concentration >80 mmol/L (>497 mg/dL) [Citation48,Citation53,Citation75,Citation82,Citation84,Citation90,Citation96,Citation100,Citation101,Citation106,Citation107,Citation109,Citation125]. Even for cases with ethylene glycol doses exceeding 3 L or serum ethylene glycol concentration >200 mmol/L (>1241 mg/dL), the outcomes were favorable [Citation94,Citation130], except in those who were already markedly acidemic (HCO3− ≤8 mmol/L) on presentation [Citation117,Citation120,Citation128].
Published cohorts also reported good outcomes from fomepizole monotherapy (). There were, however, deaths reported, although the data regarding dose, antidote and timing of administration are lacking [Citation147,Citation150]. It is possible that in some cases, clinical markers on presentation were severe and/or extracorporeal treatment was not available. Another publication reports that no mortality or significant morbidity occurred in France in ethylene glycol-poisoned patients if treated within 24 h of exposure [Citation10].
Table 3. Cohorts of patients receiving fomepizole or ethanol.
Anion gap study
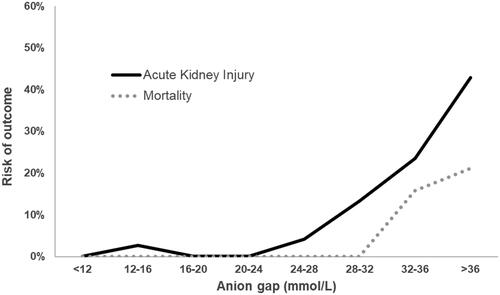
There were 207 cases fulfilling inclusion criteria and included in this analysis (), 32% of which co-ingested ethanol. Twenty-two percent were treated with ethanol alone and 78% with fomepizole alone. Among the 132 cases with an anion gap < 24 mmol/L, none died, and two patients developed stage 2 or 3 AKI: one had anion gap of 15 mmol/L but significant AKI on presentation, although extracorporeal treatment was not needed and AKI resolved spontaneously [Citation98]. One patient had an anion gap of 27 mmol/L on presentation, although the other acid-base parameters were extreme (pH 6.77, HCO3− 3 mmol/L, base excess −33 mmol/L), and later needed extracorporeal treatment for AKI [Citation81]. Comparatively, 7.8% of patients with anion gap over 28 mmol/L died, and a quarter of patients had stage 2 or 3 AKI and/or needed extracorporeal treatment.
Table 4. Baseline features, treatment and outcomes relative to the admission anion gap.
The relationship between the anion gap and the complications of AKI or death are shown in . Here, there is a low risk of AKI for anion gaps less than 24 mmol/L, and of death for anion gaps less than 28 mmol/L, but the risks increase progressively with higher anion gaps.
Discussion
This systematic review presents cases in which fomepizole or ethanol was used without extracorporeal treatment as the sole treatment of ethylene glycol poisoning. Adverse outcomes and treatment failures from ethylene glycol poisoning were mostly described when severe metabolic acidemia and or Stage 2–3 AKI was present. When these two conditions were absent on presentation, only rare, minor, and reversible complications occurred when fomepizole is used as monotherapy [Citation9,Citation49,Citation130]. This is consistent with the conclusions of other observational cohorts () [Citation114,Citation142,Citation145].
As suggested by prior reports, this study did not identify an ethylene glycol concentration over which fomepizole treatment is expected to fail, and several cases of benign outcomes were reported despite massive ethylene glycol ingestions treated with fomepizole [Citation48,Citation75,Citation82,Citation101,Citation106,Citation109,Citation130]. There was only one reported case of treatment failure with a very high ethylene glycol concentration and a low anion gap on presentation; this patient had an ethylene glycol concentration of 258 mmol/L (1,601 mg/dL) and developed osmotic diuresis with electrolyte disturbances (hypernatremia) that necessitated hemodialysis [Citation94], although the incidence of this phenomenon is unclear. One patient with repeated ethylene glycol ingestions was treated with antidote therapy alone on 81 occasions (highest ethylene glycol concentration was 112 mmol/L [695 mg/dL]) [Citation48]. The only complication that she developed was mild and reversible AKI, with a peak creatinine concentration of 133 µmol/L (1.5 mg/dL).
Although fomepizole monotherapy appears safe in selected circumstances, this likely results in higher costs and prolonged length of stay compared to when extracorporeal treatment is also used [Citation40,Citation75,Citation86,Citation152,Citation153,Citation154,Citation155,Citation156]. The study was not designed to compare cost-benefit between extracorporeal treatment with ADH inhibitors and ADH inhibitors used alone.
Ethanol also appears efficient and safe when used as monotherapy in the absence of AKI and acidemia. However, there were several failures within the cutoffs identified above [Citation56,Citation67,Citation73,Citation89,Citation123,Citation132]. A subtherapeutic ethanol concentration (from reducing the ethanol infusion rate due to CNS effects) may have contributed to some of these failures [Citation47,Citation67,Citation89,Citation91]. Further evidence of the limitations of ethanol therapy is also demonstrated by patients presenting with end-organ injury despite having co-ingested ethanol and having a therapeutic ethanol concentration on presentation. [Citation157] Additionally, the ADH inhibition from ethanol is weaker than fomepizole [Citation7,Citation8]. Ethanol also carries risks, including altered mental status, hepatitis, pancreatitis and gastritis and hypoglycemia in children [Citation45]. Contrary to fomepizole, the ethylene glycol dose and concentration appear predictive of ethanol failure (); this may reflect fomepizole’s higher affinity or better efficiency compared to ethanol [Citation158].
For these reasons, there are concerns that when the ethylene glycol concentration is >10 mmol/L (>62 mg/dL), ethanol therapy alone may be insufficient to prevent complications from ethylene glycol; this is not noted with fomepizole. There were 29 additional cases that were excluded because both ethanol and fomepizole were administered as antidotes [Citation2,Citation9,Citation38,Citation39,Citation40,Citation44,Citation45,Citation48,Citation52,Citation53,Citation84]; the only reported failure developed AKI (peak creatinine 265 µmol/L; 3.0 mg/dL) although the patient survived without extracorporeal treatment or sequelae [Citation2].
The anion gap is a good correlate for the glycolate concentration [Citation6,Citation16,Citation48,Citation159,Citation160]. A prior analysis showed that mortality did not occur if the glycolate concentration was <8 mmol/L, which is correlated to an anion gap of 24 mmol/L [Citation16]. That same study showed that AKI was expected if the glycolate concentration was >12 mmol/L, which correlated to an anion gap over 28 mmol/L.
The present study reinforces the hypothesis that in patients who receive an ADH inhibitor but not extracorporeal treatment, an anion gap <28 mmol/L is associated with no mortality and a small likelihood of AKI. This confirms earlier findings [Citation161] which were not unfortunately stratified for ADH inhibitor and extracorporeal treatment. A higher anion gap is also known to be associated with coma [Citation162] and AKI [Citation6]. These results can assist clinicians in the decision-making of the risk, costs and benefits of transferring patients to a facility with extracorporeal treatment.
This systematic review has several major limitations. The main case series and cohorts that were included [Citation2,Citation6,Citation42,Citation43,Citation44,Citation45,Citation46,Citation47,Citation48,Citation49,Citation50,Citation51,Citation52,Citation53] were considered at high risk of bias due to incomplete reporting (missing data). Publication bias is likely since most of the data consists of case reports and case series, including many conference abstracts. It is probable that good outcomes when extracorporeal treatment was not performed were preferentially reported. Better outcomes reported with fomepizole compared to ethanol could be explained by the older year of publication (and potentially inferior standard of care performed) in the latter group. One case series described a single patient with 79 presentations; however, it is unlikely that the inclusion of these cases skewed the analysis as results were very similar regardless of whether 1 case per patient or all cases was considered ().
Assumptions were required to normalize the anion gap data, although, these are unlikely to influence the analysis of patients with very elevated (>28 mmol/L) anion gaps. The retrospective nature of reports also limits the value of both analyses. There was extensive heterogeneity across the study types, treatments offered, differences in reported variables of interest and outcomes. Timing of extracorporeal treatment relative to ADH inhibition was not always reported, perhaps causing the non-inclusion of important case reports. The chosen criteria for failure of ADH inhibition were very sensitive: it is not clear that a 5 mmol/L decrease in HCO3− translates to poor clinical outcomes. We elected to take values at the time of ADH inhibition initiated rather than on arrival to the healthcare facility, to account for potential treatment delays and their impact on adverse outcomes. Finally, we acknowledge that the criterion of worsening kidney injury after ADH inhibition is controversial as kidney injury may have occurred prior to ADH inhibition but only manifested, from a serum creatinine standpoint, after ADH inhibition was initiated.
The study was not designed to compare the effectiveness of ethanol versus fomepizole; two systematic reviews failed to identify a superiority of fomepizole over ethanol [Citation163,Citation164]. Although other inhibitors of ADH have been studied in toxic alcohol poisonings, such as pyrazole [Citation165], isopropanol [Citation27], and abacavir [Citation166], this systematic review only analyzed ethanol and fomepizole. This analysis did not address situations where antidotal therapy can be avoided (e.g., marginal ethylene glycol concentration).
Conclusions
This systematic review suggests that fomepizole monotherapy, without extracorporeal treatment, is an effective and safe treatment in patients with modest AKI and/or acidemia (anion gap <28 mmol/L), regardless of the ethylene glycol concentration. These cut-offs are not applicable to ethanol monotherapy, as more failures were reported (especially at higher ethylene glycol concentrations) which may reflect challenges in dosing and/or monitoring of ethanol therapy. These findings should be confirmed prospectively. Clinicians who encounter patients who fail these cut-offs are encouraged to report them. The cost-effectiveness of a strategy combining ADH inhibitors and extracorporeal treatment should be compared to that using ADH inhibitors alone.
Acknowledgements
The authors thank Dr Stephen Curry and co-researchers for providing additional patient data from their publication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Gummin DD, Mowry JB, Beuhler MC, et al. 2019 Annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 37th annual report. Clin Toxicol. 2020;58(12):1360–1541.
- Thanacoody RH, Gilfillan C, Bradberry SM, et al. Management of poisoning with ethylene glycol and methanol in the UK: a prospective study conducted by the National Poisons Information Service (NPIS). Clin Toxicol. 2016;54(2):134–140.
- Brusin KM, Nekhoroshkov RO. A 13-year retrospective study on toxic alcohol poisoning in Middle Urals, Russia. Asia Pac J Med Toxicol. 2015;4:43–46.
- Ghannoum M, Lavergne V, Gosselin S, et al. Practice trends in the use of extracorporeal treatments for poisoning in four countries. Semin Dial. 2016;29(1):71–80.
- Wacker WE, Haynes H, Druyan R, et al. Treatment of ethylene glycol poisoning with ethyl alcohol. JAMA. 1965;194(11):1231–1233.
- Porter WH, Rutter PW, Bush BA, et al. Ethylene glycol toxicity: the role of serum glycolic acid in hemodialysis. J Toxicol Clin Toxicol. 2001;39(6):607–615.
- Lee SL, Shih HT, Chi YC, et al. Oxidation of methanol, ethylene glycol, and isopropanol with human alcohol dehydrogenases and the inhibition by ethanol and 4-methylpyrazole. Chem Biol Interact. 2011;191(1-3):26–31.
- Grauer GF, Thrall MA, Henre BA, et al. Comparison of the effects of ethanol and 4-methylpyrazole on the pharmacokinetics and toxicity of ethylene glycol in the dog. Toxicol Lett. 1987;35(2–3):307–314.
- Baud FJ, Bismuth C, Garnier R, et al. 4-Methylpyrazole may be an alternative to ethanol therapy for ethylene glycol intoxication in man. J Toxicol Clin Toxicol. 1986;24(6):463–483.
- Megarbane B, Baud F. Is there a remaining place for hemodialysis in toxic alcohol poisonings treated with fomepizole? Clin Toxicol. 2003;41:396–397.
- Ghannoum M, Hoffman RS, Mowry JB, et al. Trends in toxic alcohol exposures in the United States from 2000 to 2013: a focus on the use of antidotes and extracorporeal treatments. Semin Dial. 2014;27(4):395–401.
- Miller H, Barceloux DG, Krenzelok EP, et al. American academy of clinical toxicology practice guidelines on the treatment of ethylene glycol poisoning. Ad hoc committee. J Toxicol Clin Toxicol. 1999;37(5):537–560.
- Brent J. Current management of ethylene glycol poisoning. Drugs. 2001;61(7):979–988.
- Jacobsen D. Is there still an indication for ethanol and/or use of dialysis in ethylene glycol and methanol poisoning? Clin Toxicol. 2006;44:479–480.
- Megarbane B, Borron SW, Baud FJ. Current recommendations for treatment of severe toxic alcohol poisonings. Intensive Care Med. 2005;31(2):189–195.
- Roberts DM, Hoffman RS, Brent J, et al. The serum glycolate concentration: its prognostic value and its correlation to surrogate markers in ethylene glycol exposures. 2021.
- Arai H, Ikeda H, Ichiki M, et al. A case of poisoning by a mixture of methanol and ethylene glycol. Tohoku J Exp Med. 1983;141(4):473–480.
- Akhtar J, Abesamis M, Carlson J, et al. Do not judge a book by its cover, a bottle by its ingredients. Clin Toxicol. 2009;47:447.
- Momont SL, Dahlberg PJ. Ethylene glycol poisoning. Wis Med J. 1989;88(9):16–20.
- Maekawa N, Hoshiyama E, Suzuki K, et al. Brain magnetic resonance image changes following acute ethylene glycol poisoning. Neurol India. 2015;63(6):998–1000.
- Gilani A. Ethylene glycol toxicity masked by paracetamol overdose. Anaesthesia. 2014;69(S4):27.
- Macrae J, Sujith B. Mind the gap. Anaesthesia. 2014;69(S3):43.
- Saladino R, Shannon M. Accidental and intentional poisonings with ethylene glycol in infancy: diagnostic clues and management. Pediatr Emerg Care. 1991;7(2):93–96.
- Woolf AD, Wynshaw-Boris A, Rinaldo P, et al. Intentional infantile ethylene glycol poisoning presenting as an inherited metabolic disorder. J Pediatr. 1992;120(3):421–424.
- Bigaillon C, Thefenne H, Samy S, et al. Ethylene glycol intoxication: a case report. Ann Biol Clin. 2007;65(4):437–442.
- Samarneh MM, Shtaynberg N, Goldman M, et al. Severe oxalosis with systemic manifestations. J Clin Med Res. 2012;4(1):56–60.
- Hurley W, Chew A. Isopropanol treatment of ethylene glycol poisoning; erroneous, but successful. Clin Toxicol. 2009;47(7):709.
- Rydel JJ, Carlson A, Sharma J, et al. An approach to dialysis for ethylene glycol intoxication. Vet Hum Toxicol. 2002;44(1):36–39.
- Willis MS, Wians FH, Jr Kroft S, et al. The case of the needle-shaped urine crystals. Laboratory Med. 2002;33:637–640.
- Plackova S, Caganova B, Faltanova P, et al. Ethylene glycol poisonings associated with acute kidney injury in the Slovak Republic. Clin Toxicol. 2015;53(4):325.
- Stonys A, Kuzminskis V, Seputyte A, et al. Acute renal failure in patients with alcoholic surrogate intoxication. Medicina. 2007;43:36–39.
- White NC, Litovitz T, White MK, et al. The impact of bittering agents on suicidal ingestions of antifreeze. Clin Toxicol. 2008;46(6):507–514.
- Lepik KJ, Sobolev BG, Levy AR, et al. Medication errors associated with the use of ethanol and fomepizole as antidotes for methanol and ethylene glycol poisoning. Clin Toxicol. 2011;49(5):391–401.
- Lepik KJ, Levy AR, Sobolev BG, Purssell RA, et al. Adverse drug events associated with the antidotes for methanol and ethylene glycol poisoning: a comparison of ethanol and fomepizole. Ann Emerg Med. 2009;53(4):439–450 e10.
- Beatty L, Green R, Magee K, et al. A systematic review of ethanol and fomepizole use in toxic alcohol ingestions. Emerg Med Int. 2013;2013:638057.
- Leikin JB, Toerne T, Burda A, et al. Summertime cluster of intentional ethylene glycol ingestions. J Am Med Assoc. 1997;278(17):1406.
- Czyewska S, Winnicka R, Rzepecki J, et al. Acute ethylene glycol poisoning among patients of Nofer Institute of occupational medicine in Lodz, toxicology unit, hospitalized in the years 2000. Przegl Lek. 2013;70(8):500–505.
- Bacis G, Cantu S, Rocchi L, et al. Ethylene glycol poisoning in a child: efficact and safety of fomepizole treatment. Clin Toxicol. 2003;41:478–479.
- Baud FJ, Galliot M, Astier A, et al. Treatment of ethylene glycol poisoning with intravenous 4-methylpyrazole. N Engl J Med. 1988;319(2):97–100.
- Boyer EW, Mejia M, Woolf A, et al. Severe ethylene glycol ingestion treated without hemodialysis. Pediatrics. 2001;107(1):172–173.
- Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144(6):427–437.
- Stompor T, Szymczakiewicz-Multanowska A, Sulowicz W, et al. Ethylene glycol acute poisoning treatment results in Krakow in the years 1990-1994. Przegl Lek. 1996;53(4):360–364.
- Moreau CL, Kerns W, Tomaszewski CA, et al. Glycolate kinetics and hemodialysis clearance in ethylene glycol poisoning. META study group. J Toxicol Clin Toxicol. 1998;36(7):659–666.
- Borron SW, Megarbane B, Baud FJ. Fomepizole in treatment of uncomplicated ethylene glycol poisoning. Lancet. 1999;354(9181):831.
- Caravati EM, Heileson HL, Jones M. Treatment of severe pediatric ethylene glycol intoxication without hemodialysis. J Toxicol Clin Toxicol. 2004;42(3):255–259.
- Krenova M, Pelclova D, Navratil T, et al. Ethylene glycol poisoning in the Czech Republic (2000-2002). Blood Purif. 2006;24(2):180–184.
- Krenova M, Pelclova D. Does unintentional ingestion of ethylene glycol represent a serious risk? Hum Exp Toxicol. 2007;26:59–67.
- Hovda KE, Julsrud J, Ovrebo S, et al. Studies on ethylene glycol poisoning: one patient – 154 admissions. Clin Toxicol. 2011;49(6):478–484.
- Levine M, Curry SC, Ruha AM, et al. Ethylene glycol elimination kinetics and outcomes in patients managed without hemodialysis. Ann Emerg Med. 2012;59(6):527–531.
- Hlozek T, Bursova M, Cabala R. Fast determination of ethylene glycol, 1,2-propylene glycol and glycolic acid in blood serum and urine for emergency and clinical toxicology by GC-FID. Talanta. 2014;130:470–474.
- Tanasescu A, Macovei RA, Tudosie MS. Outcome of patients in acute poisoning with ethylene glycol–factors which may have influence on evolution. J Med Life. 2014;7 (3):81–86.
- Lung DD, Kearney TE, Brasiel JA, et al. Predictors of death and prolonged renal insufficiency in ethylene glycol poisoning. J Intensive Care Med. 2015;30(5):270–277.
- Greene HR, Krasowski MD. Data on the relationship between acetone, ethylene glycol, isopropanol, methanol, and propylene glycol serum/plasma concentrations and osmolal gaps in patients at an academic medical center. Data Brief. 2020;29:105189.
- Joly JB, Huault G, Frossard C, et al. Acute poisoning by ethylene glycol (apropos of 4 cases in young children). Bull Mem Soc Med Hop Paris. 1968;119(1):27–45.
- Gaultier M, Conso F, Rudler M, et al. Acute ethylene glycol poisoning. Eur J Toxicol Environ Hyg. 1976;9(6):373–379.
- Forycki Z, Swica P, Sniegocka-Wiśniewska W. Acute poisonings with ethylene glycol–the liquid borygo. Bull Inst Marit Trop Med Gdynia. 1979;30:97–102.
- Stivrins TJ, Moore GF. Ethylene glycol ingestion in a retarded young adult – a case report. Nebr Med J. 1982;67:181–183.
- Brown CG, Trumbull D, Klein-Schwartz W, et al. Ethylene glycol poisoning. Ann Emerg Med. 1983;12(8):501–506.
- Petit J, Viel D, Lapert D. Acute ethylene-glycol poisoning. A nine case report and review of literature. Convergences Medicales. 1983;2:487–491.
- Sarlangue J, Galperine RI, Nelson JR. Acute ethylene glycol intoxication: three familial cases. Concours Medical. 1983;105:3266–3269.
- Scherger DL, Wruk KM, Linden C, et al. Ethylene glycol intoxication. J Emerg Nurs. 1983;9(2):71–73.
- Ethylene glycol. Vet Hum Toxicol. 1985;27(6):557.
- Gabow PA, Clay K, Sullivan JB, et al. Organic acids in ethylene glycol intoxication. Ann Intern Med. 1986;105(1):16–20.
- Hewlett TP, McMartin KE, Lauro AJ, et al. Ethylene glycol poisoning. The value of glycolic acid determinations for diagnosis and treatment. J Toxicol Clin Toxicol. 1986;24(5):389–402.
- Verrilli MR, Deyling CL, Pippenger CE, et al. Fatal ethylene glycol intoxication. Report of a case and review of the literature. Cleve Clin J Med. 1987;54(4):289–295.
- Theurl A, Battista HJ, Fritzer W. Accidental glycol poisoning. Wien Med Wochenschr. 1989;139(17):390–395.
- Zeiss J, Velasco ME, McCann KM, et al. Cerebral CT of lethal ethylene glycol intoxication with pathologic correlation. AJNR Am J Neuroradiol. 1989;10(2):440–442.
- Oral U, Aribogan A, Isik G, et al. Ethylene glycol intoxication: case report. Turk Anesteziyoloji ve Reanimasyon. 1993;21:257–259.
- Harry P, Turcant A, Bouachour G, et al. Efficacy of 4-methylpyrazole in ethylene glycol poisoning: clinical and toxicokinetic aspects. Hum Exp Toxicol. 1994;13(1):61–64.
- Christiansson LK, Kaspersson KE, Kulling PE, et al. Treatment of severe ethylene glycol intoxication with continuous arteriovenous hemofiltration dialysis. J Toxicol Clin Toxicol. 1995;33(3):267–270.
- De Leacy EA, Moxon LN, Ellis VM, et al. A report of accidental ethylene glycol ingestion in 2 siblings. Pathology. 1995;27(3):273–276.
- Jobard E, Harry P, Turcant A, et al. Allain P. 4-Methylpyrazole and hemodialysis in ethylene glycol poisoning. J Toxicol Clin Toxicol. 1996;34(4):373–377.
- Niederstadt C, Lerche L, Steinhoff J. Proteinuria in ethylene glycol-induced acute renal failure. Nephron. 1996;73(2):316–317.
- Divanon F, Leroyer R, Leprince MC, et al. About an acute ethylene glycol poisoning case report. J Pharm Clin. 1997;16:177–182.
- Donovan JW, Burkhart KK, McMartin KE. A comparison of fomepizole with hemodialysis vs fomepizole alone in therapy of severe ethylene glycol toxicity. Clin Toxicol. 1998;36:451–452.
- Eder AF, McGrath CM, Dowdy YG, Tomaszewski JE, et al. Ethylene glycol poisoning: toxicokinetic and analytical factors affecting laboratory diagnosis. Clin Chem. 1998;44(1):168–177.
- Hantson P, Hassoun A, Mahieu P. Ethylene glycol poisoning treated by intravenous 4-methylpyrazole. Intensive Care Med. 1998;24(7):736–739.
- Harry P, Jobard E, Briand M, et al. Ethylene glycol poisoning in a child treated with 4-methylpyrazole. Pediatrics. 1998;102(3):E31.
- Kowalczyk M, Halvorsen S, Ovrebo S, et al. Ethanol treatment in ethylene glycol poisoned patients. Vet Hum Toxicol. 1998;40(4):225–228.
- Malone SI, Young JJ, Mazzaferri EL. An unconscious and unresponsive young man. Hosp Pract. 1999;34(10):51–62.
- Piagnerelli M, Carlier E, Lejeune P. Adult respiratory distress syndrome and medullary toxicity: two unusual complications of ethylene glycol intoxication. Intensive Care Med. 1999;25(10):1200.
- Mégarbane B, Baud F. Acute ethylene glycol intoxication: a case report. Medecine Therapeutique. 2001;7:163–167.
- Najafi CC, Hertko LJ, Leikin JB, et al. Fomepizole in ethylene glycol intoxication. Ann Emerg Med. 2001;37(3):358–359.
- Aakervik O, Svendsen J, Jacobsen D. Severe ethylene glycol poisoning treated wtih fomepizole (4-methylpyrazole). Tidsskr nor Laegeforen. 2002;122(25):2444–2446.
- Fraser AD. Clinical toxicologic implications of ethylene glycol and glycolic acid poisoning. Ther Drug Monit. 2002;24(2):232–238.
- Vasavada N, Williams C, Hellman RN. Ethylene glycol intoxication: case report and pharmacokinetic perspectives. Pharmacotherapy. 2003;23(12):1652–1658.
- Detaille T, Wallemacq P, Clement de Clety S, et al. Fomepizole alone for severe infant ethylene glycol poisoning. Pediatr Crit Care Med. 2004;5(5):490–491.
- Spivak LA, Horowitz BZ. Treatment of severe ethylene glycol poisoning without hemodialysis. Clin Toxicol. 2005;43(6):691.
- Symington L, Jackson L, Klaassen B. Toxic alcohol but not intoxicated-a case report. Scott Med J. 2005;50(3):129–130.
- Ybarra J, Donate T, Pou JM, et al. Ethylene glycol intoxication with and without simultaneous diabetic ketoacidosis: a report of nine cases and review of the literature. Int J Diabetes Metab. 2005;13:83–87.
- French S, Williams EW. Ethylene glycol poisoning highlighting the role of the Caribbean Poison Information Centre. West Indian Med J. 2006;55(6):456–457.
- Kirrane BM, Wiener SW, Hoffman RS, et al. Ethylene glycol poisoning masquerading as mesenteric ischemia because of a falsely elevated lactate. Clin Toxicol. 2006;44:558–559.
- Krenova M, Pelclova D. Complete recovery after repeated suicidal ethylene glycol ingestion. Prague Med Rep. 2006;107:130–136.
- Pizon AF, Brooks DE. Hyperosmolality: another indication for hemodialysis following acute ethylene glycol poisoning. Clin Toxicol. 2006;44(2):181–183.
- Tennant I, Crawford-Sykes A, Ward L, et al. Ethylene glycol poisoning following ingestion of brake fluid. West Indian Med J. 2006;55(4):286–287.
- Akhtar J, Petersen BA, Krenzelok E. When to stop fomepizole administration? Is 20 mg/dl too low? Clin Toxicol. 2007;45:362.
- Calello D, Osterhoudt KC, Henretig F. Fomepizole alone in a critically ill 9-month-old with ethylene glycol poisoning. Clin Toxicol. 2007;45(6):639.
- Gaines L, Waibel KH. Calcium oxalate crystalluria. Emerg Med J. 2007;24(4):310.
- Schwerk N, Desel H, Schulz M, et al. Successful therapy of paediatric ethylene glycol poisoning: a case report and annual survey by a regional poison centre. Acta Paediatr. 2007;96(3):461–463.
- Velez LI, Shepherd G, Lee YC, et al. Ethylene glycol ingestion treated only with fomepizole. J Med Toxicol. 2007;3(3):125–128.
- Beuhler MC, Kerns W. Ethylene glycol poisoning with biphasic, rapid elimination. Clin Toxicol. 2008;46(7):608.
- Chaudhry SD, Pandurangan M, Pinnell AE. Lactate gap and ethylene glycol poisoning. Eur J Anaesthesiol. 2008;25(6):511–513.
- George M, Al-Duaij N, Becker ML, et al. Re: ethylene glycol ingestion treated only with fomepizole (Journal of Medical Toxicology: volume 3, number 3, September 2007; 125-128). J Med Toxicol. 2008;4(1):67.
- Murphy N, Sonier T, Power D, et al. Treatment of severe pediatric ethylene glycol intoxication with fomepizole alone. Clin Toxicol. 2008;46(7):626.
- Holland MG, Stork CM, Hodgman MJ, et al. Elevated glycolate levels after unintentional pediatric ethylene glycol ingestion. Clin Toxicol. 2009;47:737–738.
- Buchanan JA, Alhelail M, Cetaruk EW, et al. Massive ethylene glycol ingestion treated with fomepizole alone-a viable therapeutic option. J Med Toxicol. 2010;6(2):131–134.
- Haggerty DA, Curtis J. Prolonged elimination of ethylene glycol in a patient receiving fomepizole with normal renal function. Clin Toxicol. 2010;48(6):620.
- Kostek H, Kujawa A, Szponar J, et al. Is it possible to survive metabolic acidosis with pH measure below 6.8? A study of two cases of inedible alcohol intoxication. Przeglad Lekarski. 2011;68:518–520.
- Burda A, Truitt K, DesLauries C, et al. Successful treatment of massive ethylene glycol poisoning with fomepizole only. Clin Toxicol. 2012;50(7):581.
- Hann G, Duncan D, Sudhir G, et al. Antifreeze on a freezing morning: ethylene glycol poisoning in a 2-year-old. BMJ Case Rep. 2012. DOI: https://doi.org/10.1136/bcr.07.2011.4509.
- Latus J, Kimmel M, Alscher MD, et al. Ethylene glycol poisoning: a rare but life-threatening cause of metabolic acidosis-a single-centre experience. Clin Kidney J. 2012;5(2):120–123.
- Fujita T, Nakamura N, Hitomi H, et al. Risk of sweet 'ethylene glycol' consumption. Intern Med. 2013;52(3):409.
- Murphy CM, Vitto MJ, Dulaney AR. Detectable serum ethylene glycol levels after subcutaneous injection of antifreeze. Clin Toxicol. 2013;51(9):908–909.
- Fremont D, Berleur MP, Megarbane B. Loading dose of fomepizole is safe in children with presumed toxic alcohol exposure – a case series. Basic Clin Pharmacol Toxicol. 2014;115(3):229–230.
- Harbon SCD, Thompson J. Successful use of fomepizole during second trimester of pregnancy. Clin Toxicol. 2014;52:314–315.
- Keeling T, Orozco B, Cole J, et al. Anaphylaxis following fomepizole for ethylene glycol poisoning. Clin Toxicol. 2014;52:751–752.
- Boukobza M, Baud FJ, Gourlain H, et al. Neuroimaging findings and follow-up in two cases of severe ethylene glycol intoxication with full recovery. J Neurol Sci. 2015;359(1–2):343–346.
- Hlozek T, Bursova M, Coufal P, et al. Identification and quantification of acidosis inducing metabolites in cases of alcohols intoxication by GC-MS for emergency toxicology. J Pharm Biomed Anal. 2015;114:16–21.
- Nagesh IV, Koley KC, Sen S, et al. Ethylene glycol poisoning. Med J Armed Forces India. 2015;71(Suppl 1):S36–S8.
- Nguyen V, Lucyk SN, Howland MA, et al. Too bad to be true? Osmol gap >300 mOsm/L. Clin Toxicol. 2015;53:299–300.
- Schoen JC, Cain MR, Robinson JA, et al. Adolescent presents with altered mental status and elevated anion gap after suicide attempt by ethylene glycol ingestion. Pediatr Emerg Care. 2016;32(10):688–690.
- Chétioui A, Pillant A, Rullier P, et al. Ethylene-glycol poisoning and acute drunkenness: the simultaneous intake of a poison and its antidote. Anesthesie et Reanimation. 2017;3(2):186–188.
- Nyman T, Parry MJ, Hoppu K. Cold gel packs: Harmless or not? Clin Toxicol. 2017;55(398). 2017;55(5):398.
- Rathnayaka R, Ranathunga PEAN. Acute kidney injury, myocardial infarction and death following brake fluid poisoning; a case report. Asia Pac J Med Toxicol. 2017;6:62–66.
- Toce M, Brevil A, Griswold M, et al. Acute ethylene glycol poisoning and methemoglobinemia after engine coolant ingestion treated with fomepizole and methylene blue. Clin Toxicol. 2017;55(5):391.
- Giner T, Ojinaga V, Neu N, et al. Ethylene glycol intoxication presenting with high anion gap metabolic acidosis, acute kidney injury and elevated lactate. Pediatr Int. 2018;60(2):194–195.
- Sheta HM, Al-Najami I, Christensen HD, et al. Rapid diagnosis of ethylene glycol poisoning by urine microscopy. Am J Case Rep. 2018;19:689–693.
- Thevoz L, Faundez T, Lacroix-Ducardonnoy L, et al. When to suspect ethylene glycol intoxication? Swiss Medical Weekly. 2018;147(Supplement 228):55S–56S.
- Zuckerman M, Vo T. Recurrent ethylene glycol poisoning with elevated lactate levels to obtain opioid medications. J Emerg Med. 2018;54(6):815–818.
- Spyres M, Garlich F, Peck E. Fomepizole monotherapy for massive ethylene glycol ingestion. J Med Toxicol. 2019;15(2):84–85.
- Ahmed M, Janikowski C, Huda S, et al. Ethylene glycol poisoning with a near-normal osmolal gap: a diagnostic challenge. Cureus. 2020;12(12):e11937.
- Patel R, Mistry AM, Mistry CM. Unintentional ethylene glycol poisoning in an adolescent. Cureus. 2020;12(11):e11521.
- Sasanami M, Yamada T, Obara T, et al. Oral ethanol treatment for ethylene glycol intoxication. Cureus. 2020;12(12):e12268.
- Haapanen E, Pellinen T, Partanen J. Ethylene glycol poisoning. Duodecim. 1984;100(4):214–219.
- Kidawa Z, Trznadel K. Haemodialysis in the treatment of acute ethylene glycol poisoning. Biul WAM. 1987;30:1–13.
- Puka J, Szajewski J. Acute ethylene glycol poisoning–205 cases treated at the acute poison control center. Pol Arch Med Wewn. 1988;80(2–3):88–98.
- Karlson-Stiber C, Persson H. Ethylene glycol poisoning: experiences from an epidemic in Sweden. J Toxicol Clin Toxicol. 1992;30(4):565–574.
- Etchepare YM, Nauche P, Remize J, et al. Mass acute ethylene glycol intoxication. Urgences Medicales. 1997;16(3):117–119.
- Rzepecki J, Stasiak M, Kolacinski Z. [Clinical symptomatology and laboratory diagnosis of 75 cases of ethylene glycol poisonings]. Pol Merkuriusz Lek. 1998;5:74–79.
- Jokiniemi T, Ikaheimo R. [Ethylene glycole poisonings in nothern savo 1984-98. Duodecim. 2001;117(1):23–27.
- Megarbane B, Houze P, Baud FJ. Oral fomepizole administration to treat ethylene glycol and methanol poisonings: advantages and limitations. Clin Toxicol. 2008;46(10):1097–1097.
- McKeown NJ, Hurley W, Hendrickson RG, et al. Ethylene glycol poisoning treated with fomepizole. Clin Toxicol. 2009;47:735–736.
- Wong S, King R, Molai S, et al. Medication errors occurring in fomepizole administration. Annals Emerg Med. 2009;54(3):S114.
- Kogan A, Bryant SM. Do unintentional ethylene glycol exposures require intentional management measures? Clin Toxicol. 2012;50:607.
- Pallot D, Richard A, Mougenot P, et al. Fomepizole as a toxic alcohol poisonings treatment in paediatrics: a nine-year study. Int J Clin Pharm. 2012;34(1):234–235.
- Wedge MK, Natarajan S, Johanson C, et al. The safety of ethanol infusions for the treatment of methanol or ethylene glycol intoxication: an observational study. Cjem. 2012;14(5):283–289.
- Swiderska A, Sein Anand J. [Selected data of acute poisonings with ethylene glycol and methanol in Poland in the year 2010]. Przegl Lek. 2013;70(8):479–484.
- Rahmani AH, Parsipur F. Investigating the frequency of alcohol intoxication among the patients referred to ahvaz razi hospital during 2005 to 2008. Biomed Pharmacol J. 2015;8(2):1287–1293.
- Colibao L, Kim T, DesLauriers C, et al. Unintentional ethylene glycol exposures: pediatric pearls in management. J Med Toxicol. 2018;14(1):61–62.
- Grigorasi G, Nistor I, Corlade-Andrei M, et al. Analysis of prognostic factors for survival in a large cohort of ethylene glycol intoxication cases. Nephrol Dial Transplant. 2018;33(suppl_1):i50–i1.
- Tung RC, Thornton SL. Characteristics of laboratory confirmed ethylene glycol and methanol exposures reported to a regional poison control center. Kans J Med. 2018;11(3):67–69.
- Ellsworth H, Engebretsen KM, Hlavenka LM, et al. A cost comparision of fomepizole and hemodialysis in the treatment of methanol and ethylene glycol toxicity. Clin Toxicol. 2011;49:590–591.
- Cannarozzi AA, Mullins ME. A cost analysis of treating patients with ethylene glycol poisoning with fomepizole alone versus hemodialysis and fomepizole. Clin Toxicol. 2010;48:299–300.
- Darracq MA, Rentmeester LL, Clark RF, et al. Cost of hemodialysis versus fomepizole-only for treatment of ethylene glycol intoxication. Clin Toxicol. 2013;51(3):188.
- Wiles D, Tzeng J, Russell J, et al. Comment on treatment methods for ethylene glycol intoxication. Netherlands J Med. 2014;72:383–384.
- Roberts D. Cost-assessment of the use of fomepizole for the treatment of toxic alcohol poisonings in Australian practice. Clin Toxicol. 2018;57(12):1202–1203.
- Davis DP, Bramwell KJ, Hamilton RS, et al. Ethylene glycol poisoning: case report of a record-high level and a review. J Emerg Med. 1997;15(5):653–667.
- McMartin K, Jacobsen D, Hovda KE. Antidotes for poisoning by alcohols that form toxic metabolites. Br J Clin Pharmacol. 2016;81(3):505–515.
- Hovda KE, Hunderi OH, Ovrebo S, et al. Diagnosis in metabolic acidosis of unknown origin. Tidsskr nor Laegeforen. 2004;124(24):3203–3205.
- Brent J, McMartin K, Phillips S, et al. Fomepizole for the treatment of ethylene glycol poisoning. Methylpyrazole for toxic alcohols study group. N Engl J Med. 1999;340(11):832–838.
- Coulter CV, Farquhar SE, McSherry CM, et al. Methanol and ethylene glycol acute poisonings – predictors of mortality. Clin Toxicol. 2011;49(10):900–906.
- Iliuta IA, Lachance P, Ghannoum M, et al. Prediction and validation of the duration of hemodialysis sessions for the treatment of acute ethylene glycol poisoning. Kidney Int. 2017;92(2):453–460.
- Druteika DP, Zed PJ, Ensom MH. Role of fomepizole in the management of ethylene glycol toxicity. Pharmacotherapy. 2002;22(3):365–372.
- Wallman P, Hogg K. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Management of acute ethylene glycol poisoning. Emerg Med J. 2002;19(5):431–432.
- Mundy RL, Hall LM, Teague RS. Pyrazole as an antidote for ethylene glycol poisoning. Toxicol Appl Pharmacol. 1974;28(2):320–322.
- Ghannoum M, Haddad HK, Lavergne V, et al. Lack of toxic effects of methanol in a patient with HIV. Am J Kidney Dis. 2010;55(5):957–961.
- Hylander B, Kjellstrand CM. Prognostic factors and treatment of severe ethylene glycol intoxication. Intensive Care Med. 1996;22(6):546–552.