Abstract
Background and aims
Amanita phalloides poisoning causes severe liver damage which may be potentially fatal. Several treatments are available, but their effectiveness has not been systematically evaluated. We performed a systematic review to investigate the effect of the most commonly used therapies: N-acetylcysteine (NAC), benzylpenicillin (PEN), and silibinin (SIL) on patient outcomes. In addition, other factors contributing to patient outcomes are identified.
Methods
We searched MEDLINE and Embase for case series and case reports that described patient outcomes after poisoning with amanitin-containing Amanita mushrooms. We extracted clinical characteristics, treatment details, and outcomes. We used the liver item from the Poisoning Severity Score (PSS) to categorize intoxication severity.
Results
We included 131 publications describing a total of 877 unique cases. The overall survival rate of all patients was 84%. Patients receiving only supportive care had a survival rate of 59%. The use of SIL or PEN was associated with a 90% (OR 6.40 [3.14–13.04]) and 89% (OR 5.24 [2.87–9.56]) survival rate, respectively. NAC/SIL combination therapy was associated with 85% survival rate (OR 3.85 [2.04, 7.25]). NAC/PEN/SIL treatment group had a survival rate of 76% (OR 2.11 [1.25, 3.57]). Due to the limited number of cases, the use of NAC alone could not be evaluated. Additional analyses in ‘proven cases’ (amanitin detected), ‘probable cases’ (mushroom identified by mycologist), and ‘possible cases’ (neither amanitin detected nor mushroom identified) showed comparable results, but the results did not reach statistical significance. Transplantation-free survivors had significantly lower peak values of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total serum bilirubin (TSB), and international normalized ratio (INR) compared to liver transplantation survivors and patients with fatal outcomes. Higher peak PSS was associated with increased mortality.
Conclusion
Based on data available, no statistical differences could be observed for the effects of NAC, PEN or SIL in proven poisonings with amanitin-containing mushrooms. However, monotherapy with SIL or PEN and combination therapy with NAC/SIL appear to be associated with higher survival rates compared to supportive care alone. AST, ALT, TSB, and INR values are possible predictors of potentially fatal outcomes.
Introduction
Poisoning with the highly toxic amanitin-containing mushroom has been a long-standing problem and has a wide range of reported overall mortality with rates between 1.8% and 22% in patients receiving either supportive care or treatment [Citation1–5]. Amanita species that contain amanitin are hepatotoxic, and Amanita phalloides is responsible for most of the fatal intoxications with Amanita species [Citation6–8].
The first phase of intoxication with an amanitin-containing mushroom is asymptomatic [Citation7]. Approximately 6 to 8 h post-ingestion, the second phase starts which is characterized by gastrointestinal symptoms, such as nausea, vomiting, diarrhea, abdominal pain, and dehydration [Citation9]. The third phase, which starts 36 to 48 h post-ingestion, is characterized by progressive deterioration of liver function [Citation8,Citation10]. Hepatocellular injury causes the release of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) into the blood circulation. Total serum bilirubin (TSB) increases due to the disturbed bilirubin conjugation and excretion process [Citation11]. Concurrently, international normalized ratio (INR) is prolonged as the hepatocellular production of clotting factors is disrupted [Citation11,Citation12]. Nephrotoxicity may occur later in the course of the intoxication with elevated levels of serum creatinine (Cr) and blood urea nitrogen (BUN) [Citation13–15]. Depending on the severity of the intoxication, liver and renal function may deteriorate further, leading to death if no treatment is started [Citation13].
Despite the severity and potentially fatal outcome of poisoning with amanitin-containing mushrooms, no standardized treatment regimens are currently available. Devising a standardized treatment is complicated, not only because controlled studies are difficult to perform for ethical reasons, but also due to the lack of solid and confirmed data on the mechanism of action (toxicodynamics) of amatoxins [Citation16]. Several therapeutic regimens have been used over the years, but none of these has a proven clear advantage over the other, making it difficult to choose the best treatment [Citation2,Citation8,Citation14]. The three most commonly used drugs are N-acetylcysteine (NAC), benzylpenicillin (PEN), and silibinin (SIL). They are administered either alone or in combination [Citation6,Citation7,Citation14]. In addition to these therapies, supportive care such as fluid resuscitation and fresh-frozen plasma are often administered [Citation8,Citation10,Citation17]. Various detoxification procedures (activated charcoal, hemodialysis, plasmapheresis, etc.) are used either to prevent intestinal absorption or to improve amatoxin elimination [Citation6].
To better guide treatment choices, we performed a systematic review to investigate the effect of the most commonly used drugs (NAC, PEN, SIL) on patient outcomes. We also analyzed patient outcomes separately in proven, probable, and possible amanitin cases. In addition, we identified factors contributing to the outcome of the intoxication.
Materials and methods
Search strategy
We performed a systematic review of published cases of amanitin-containing Amanita species poisonings. The cases were dated from 21 July 1975 to 31 July 2020, focusing on the treatment of poisoning with an amanitin-containing Amanita mushroom in humans.
We searched the online databases MEDLINE (through PubMed), Embase, and Google Scholar with the keywords: ‘Amanita intoxication’, ‘Amanita poisoning’, and ‘amatoxin’ to identify amanitin poisoning case reports and case series. We included only amanitin-containing Amanita species were in this study, other amanitin-containing mushrooms (Lepiota and Galerina species) and non-hepatotoxic Amanita mushrooms were excluded. We checked the primary references of the collected studies and included these if relevant.
We pooled the studies retrieved from the online databases and removed the duplicates. The preliminary exclusion encompassed removing papers with any language other than English, Dutch, or Chinese. We included the papers with inaccessible full-text versions if the abstracts provided sufficient information that met our inclusion criteria (20 abstracts were included, which accounts for 154 patients [18% of all 877 patients]). On the second screening, the primary investigators (JLT and JS) checked the papers if inclusion criteria were met. The secondary investigators (DT and BD) checked the laboratory data and unit conversions. See for the flowchart of the selection process.
Figure 1. Flowchart of the selection process for the case reports and case series included in this study (n indicates the number of publications). A total of 20 abstracts and 112 published manuscripts were included. The whole dataset was used for the analysis of patient characteristics and clinical laboratory values. Subgroup analyses were performed on patients treated with NAC, PEN, SIL, or combinations in proven, probable, and possible cases.
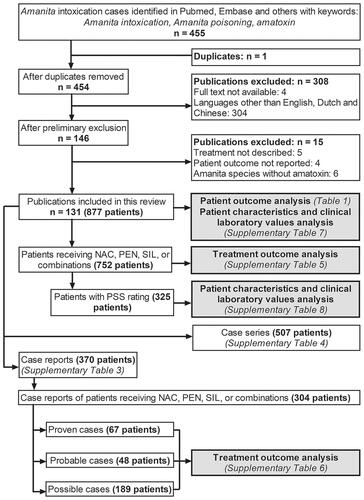
Inclusion and exclusion criteria
We included patients who consumed amanitin-containing Amanita mushrooms determined either by clinical evaluation with presenting symptoms, amatoxin measurement, or identification by a mycologist. All included patients received supportive care and/or therapies and had known survival outcomes. We excluded patients with no gastrointestinal symptoms, patients with normal liver and kidney function by laboratory analysis, patients who were not hospitalized, and patients with recorded history of prior liver disease (in total two asymptomatic cases were excluded). For the analysis of treatment outcome, patients receiving other treatments than PEN, SIL, NAC and combinations thereof (thioctic acid, cimetidine, ceftazidime and others) were excluded.
Data extraction
The primary investigators performed data extraction. The secondary investigators independently checked the extracted data. We recorded demographic information, including patient age (years) and gender. The patients were grouped into three age groups: children (<18 years), adults (18–65 years), and elderly (>65 years). If reported, we noted the time from ingestion to the onset of symptoms (hours), the time from ingestion to clinical care (hours), and the length of hospital stay (days). In the event of death, the length of hospital stay indicates the days from ingestion to the day the patient died. We recorded the patient outcomes as survived or died with or without (w/o) liver transplantation (LTx).
We recorded peak clinical laboratory values related to the liver [AST, ALT, INR, total bilirubin] and kidney [creatinine, BUN] from reports including any of these. We defined hepatotoxicity as an elevation in either AST, ALT, or INR values past the normal ranges; nephrotoxicity as an elevation in either Cr or BUN values past the normal ranges. See Supplementary Table 1 for normal ranges and unit conversions if the laboratory parameters were provided in other units [Citation18–21].
Data classification
We classified patient outcomes among four different outcomes: ‘Survived w/o LTx’, ‘Survived with LTx’, ‘Died w/o LTx’, and ‘Died with LTx’. We rated intoxication severity using the liver item from the Poisoning Severity Score (PSS) by Persson et al. using peak ALT and AST values as described in Supplementary Table 2 [Citation22]. For consistency, we only rated PSS if ALT and/or AST values of the patient were provided, other factors such as biochemical laboratory values (ammonia, clotting factors) or signs of liver failure were not considered.
For the purpose of treatment outcome analysis, we classified the patients into supportive care group and treatment groups. The patients either received only supportive care (supportive care group), or were treated with NAC, PEN, SIL or their combinations (treatment groups). We defined supportive care as standard hospital care that is routinely given to patients admitted to the hospital, which includes fluid replacement, electrolyte replacement, symptomatic treatment (antiemetics, anti-diarrheal), corticosteroids or any treatment not specific to the treatment of amanitin poisoning, such as activated charcoal and dialysis. We considered ‘Survived w/o LTx’ as treatment success, whereas ‘Survived with LTx’, ‘Died w/o LTx’, and ‘Died with LTx’ were collectively classified as ‘Treatment failure’.
We carried out additional treatment outcome analyses with only cases that outlined detailed data of individual patients in the papers by further subdividing the patients into subgroups with proven, probable, and possible amanitin poisoning. We defined ‘proven cases’ as cases with laboratory amanitin measurement in body fluids; ‘probable cases’ as cases in which mushroom samples were identified by a mycologist, but without laboratory amanitin determination; and ‘possible cases’ as cases where there were neither of these, but the patients showed clinical symptoms that resemble a typical amanitin poisoning. In addition, if one person in a group met the criteria for “proven” or “probable”, they were all classified in the same category as those who shared the same meal and became ill.
Statistical analysis
We performed statistical analyses using SPSS Statistics software (version 26.0.0.1). We used descriptive statistics for the patient characteristics, expressed as median and range. We carried out independent samples t-tests to determine the potential confounders for patient outcomes. We used the Kolmogorov-Smirnov test for normality for the continuous variables, and p > 0.05 indicates the data followed a normal distribution. We used the Kruskal-Wallis H test and the Dunn-Bonferroni post-hoc test to test the correlations between patient outcomes and PSS groups, as the majority of the data were not normally distributed. We compared the survival rates of patients treated with different therapeutic regimens using Logistic Regression to calculate the adjusted odds ratio (OR) at 95% confidence interval (CI) against the supportive care group. Using a similar method, we compared treatment groups against each other. The differences between groups were considered statistically significant if p < 0.05.
Results
Descriptive analysis
We included a total of 131 publications, identifying 877 unique patients (). summarizes the patient characteristics and the clinical laboratory values of the patients in this study [Citation9,Citation10,Citation15,Citation16,Citation23–150]. An overview of the individual cases can be found in Supplementary Table 3 and case series in Supplementary Table 4.
Table 1. Patient characteristics and clinical laboratory values for the complete data set.
The overall survival of the patients included in this analysis was 84% (739 out of 877 patients). The survival rate in patients who underwent LTx (with or without therapy) was 75% (57 out of 76 patients), whereas patients who only received supportive care had a treatment success rate of 59% (79 out of 133 patients). The difference in survival rate between LTx patients and supportive care was not significant (OR = 1.28, p = 0.47, 95% CI [0.66, 2.46]).
The treatment analysis consisted of 752 patients that received either supportive care only, or in addition with NAC, PEN, SIL, or combinations of NAC, PEN and SIL. Patients receiving supportive care consist of patients receiving only standard hospital care (n = 343), activated charcoal (n = 203), dialysis (n = 41), gastric lavage (n = 21), charcoal and dialysis (n = 98), charcoal and gastric lavage (n = 14), dialysis and gastric lavage (n = 11), combination of charcoal, dialysis, and gastric lavage (n = 74), or other combinations of nonspecific measures such as induced emesis and forced diuresis (n = 72). Patients receiving other treatments (thioctic acid, cimetidine, ceftazidime and others) were excluded. The clinical characteristics and blood test results of included patients can be found in Supplementary Table 5 and were comparable to the characteristics of the group with all patients. Among these patients, 82% of the patients (619 out of 752 patients) received treatment in addition to supportive care. The remaining 18% (n = 133) of the patients received only supportive care. The frequencies of therapeutic regimen use were as follows: NAC 1% (n = 7), PEN 21% (n = 156), SIL 15% (n = 114), NAC/PEN 6% (n = 42), NAC/SIL 14% (n = 106), PEN/SIL 8% (n = 59), and NAC/PEN/SIL 18% (n = 135).
The survival rate in the supportive care group was 59% (). The survival rate in the SIL, PEN, NAC/SIL, and NAC/PEN/SIL treatment groups in our study was significantly higher compared to the group that received only supportive care. The patients treated with SIL had the highest survival rate of 90% (OR 6.40, p < 0.001, 95% CI [3.14, 13.04]). In the patient group treated with PEN, survival was 89% (OR 5.24, p < 0.001, 95% CI [2.87, 9.56]), while NAC/SIL treatment had led to survival in 85% of the patients (OR 3.85, p < 0.001, 95% CI [2.04, 7.25]). When treated with the NAC/PEN/SIL combination, 76% of the patients survived (OR 2.11, p < 0.01, 95% CI [1.25, 3.57]). Although the survival rate for the combination of PEN/SIL was 68% (OR 1.44, p = 0.27, 95% CI [0.75, 2.75]), slightly higher than the control group, this did not reach statistical significance. The NAC and NAC/PEN treatment groups had a survival rate of 43% (OR 0.513, p = 0.39, 95% CI [0.11, 2.38]) and 45% (OR 0.57, p = 0.11, 95% CI [0.28, 1.14]), respectively, which was not significantly different from the supportive care group. (Part 1) shows the statistical comparison of survival rates between the different treatment groups in all patients. We observed no significant differences between SIL, PEN, and NAC/SIL treatment groups. The survival rates of SIL monotherapy and PEN monotherapy were significantly higher than the remaining treatment groups. NAC/PEN combination therapy in particular, had a significantly lower survival rate compared to all treatment groups except the control group and NAC monotherapy.
Figure 2. Survival outcomes of different therapeutic regimens in 752 patients treated with selected therapeutic regimens. The percentage of patients for the patient group that survived without liver transplantation (white) and patients with treatment failure (grey). Supportive care (Control, n = 133), silibinin (SIL, n = 114), benzylpenicillin (PEN, n = 156), N-acetylcysteine/silibinin (NAC/SIL, n = 106), N-acetylcysteine/benzylpenicillin/silibinin (NAC/PEN/SIL, n = 135), benzylpenicillin/silibinin (PEN/SIL, n = 59), N-acetylcysteine/benzylpenicillin (NAC/PEN, n = 42), N-acetylcysteine (NAC, n = 7). *p < 0.05, **p < 0.01 and ***p < 0.001 compared to the supportive care group.
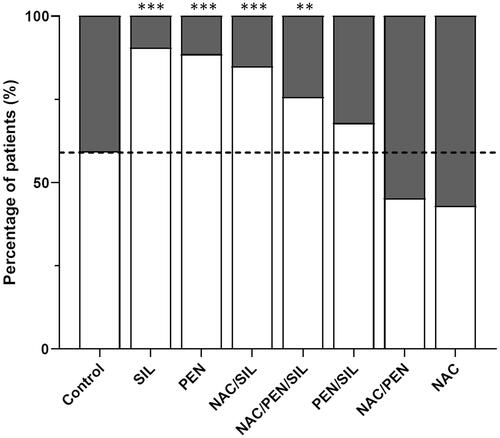
Table 2. Logistic regression of survival rates in rows (in Odds Ratio, bolded cell indicates significant comparison) relative to the different treatment groups in columns for all cases (n = 752), proven cases (n = 67), probable cases (n = 48), and possible cases (n = 189). No adjustments were made for multiple comparisons.
Subgroup analysis: treatment outcomes in patients treated with selected therapeutic regimens
For this subgroup analysis of treatment outcome, only cases that outlined detailed data of individual patients in the papers were included. In total 304 cases were identified. These cases were further divided into three case subgroups: ‘Proven cases’ (laboratory proof of amanitin), ‘Probable cases’ (mushroom visually identified by a mycologist), and ‘Possible cases’ (clinical symptoms after eating suspected meal). The patient characteristics and clinical laboratory values of these case groups can be found in Supplementary Table 6 for comparison.
The distribution of patient survival in these subgroups is illustrated in . The survival rate of proven cases was 78%, slightly higher than probable cases (71%), but comparable to possible cases (78%). The differences in patient outcomes between these case groups were not statistically significant. The results of the outcome analysis can be found in . The results of Logistic Regression (in Odds Ratio) that compares different therapeutic regimens can be found in (Part 2–4).
Figure 3. The distribution of survival outcomes in the subgroup treated with selected therapeutic regimens in all cases (n = 752), proven (n = 67), probable (n = 48), and possible cases (n = 189). White: patients that survived without liver transplantation (LTx), light grey: patients that survived with LTx, dark grey: patients that died without LTx, black: patients that died with LTx.
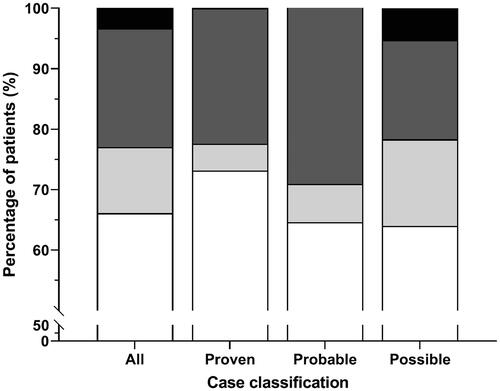
Figure 4. The effects of different therapeutic regimens on patient outcomes. The percentage of patients for the patient group that survived without liver transplantation (white) and patients with treatment failure (grey). (A) Survival outcomes in proven cases (determined amanitin concentration) treated with different therapeutic regimens (total n = 67). Supportive care (control, n = 19), SIL (n = 1), PEN (n = 126), NAC/SIL (n = 49), NAC/PEN/SIL (n = 8), PEN/SIL (n = 13), NAC/PEN (n = 1), and NAC (n = 0). (B) Survival outcomes in probable cases (identified by mycologist, but no amanitin determination) treated with different therapeutic regimens (total n = 48). Supportive care (n = 67), SIL (n = 6), PEN (n = 7), NAC/SIL (n = 3), NAC/PEN/SIL (n = 4), PEN/SIL (n = 8), NAC/PEN (n = 4), and NAC (n = 3). (C) Survival outcomes in possible cases (clinical symptoms presentation without mycologist identification or amanitin determination) treated with different therapeutic regimens (total n = 189). Supportive care (n = 47), SIL (n = 107), PEN (n = 23), NAC/SIL (n = 54), NAC/PEN/SIL (n = 123), PEN/SIL (n = 38), NAC/PEN (n = 37), and NAC (n = 4). (D) Survival outcomes of the different therapeutic regimens in the patients with severe intoxication (total n = 160). Supportive care (n = 53), SIL (n = 17), PEN (n = 22), NAC/SIL (n = 19), NAC/PEN/SIL (n = 14), PEN/SIL (n = 14), NAC/PEN (n = 17), and NAC (n = 4). *p < 0.05, **p < 0.01 and ***p < 0.001 compared to the supportive care group.
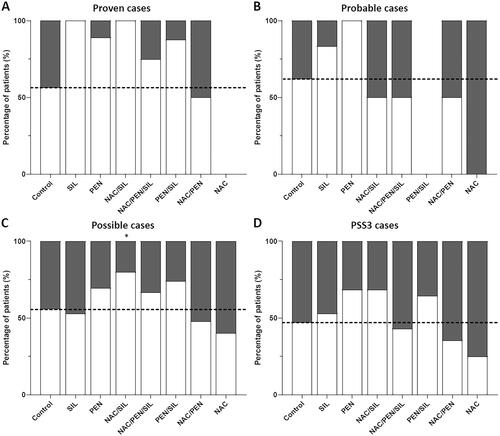
shows treatment outcomes of proven cases (), probable cases (), and possible cases (). Due to a low number of cases, only a limited number of comparisons were statistically significant, but a similar trend was observed for the overall group (). Only for the combination of NAC/SIL a significant increase in survival rate was observed (of 80%, OR 3.17, p < 0.05, 95% CI [1.14, 8.82]) for the possible cases subgroup. Surprisingly, no effects were observed for combination therapies in the probable subgroup analysis, whereas in the possible subgroup both the effect of SIL and PEN appear to be less effective as observed in the overall group.
In a subgroup analysis of patients with the most severe intoxications, patients with a PSS score of 3 appeared to have a worse outcome in general, as expected (). Although the data were not significant due to the low number of samples, PEN, NAC/SIL, and PEN/SIL appeared to improve outcomes in PSS3 patients. Also, in this analysis NAC and NAC/PEN appeared to not improve outcomes.
Correlating patient characteristics and clinical laboratory values with patient outcomes
Patient characteristics and their blood test results in different outcome groups were analyzed and compared. The data can be found in Supplementary Table 7.
Hepatotoxicity was reported in 72% (n = 634) of the patients. shows the liver clinical laboratory values of the outcome groups. Kruskal-Wallis test showed significant differences in AST [χ2(3) = 34.88, p < 0.001], ALT [χ2(3) = 46.68, p < 0.001], INR [χ2(3)=95.52, p < 0.001], and TSB [χ2(3) = 20.02, p < 0.001] values among different patient outcomes. Pairwise comparisons using Dunn-Bonferroni post-hoc test indicated that peak AST and ALT values of the group ‘Survived without LTx’ were significantly lower compared to group ‘Survived with LTx’ (p < 0.001) and group ‘Died w/o LTx’ (p < 0.001). The INR value of the group ‘Survived w/o LTx’ was significantly lower compared to all other groups (p < 0.001 compared to ‘Survived with LTx’; p < 0.001 compared to ‘Died w/o LTx’; P0.05 compared to ‘Died with LTx’). The patients who died without LTx had a higher peak TSB than other outcome groups, with a significant difference compared to the group ‘Survived w/o LTx’ (p < 0.001).
Figure 5. The peak values of liver clinical laboratory values in 877 patients that survived without liver transplantation (LTx), patients that survived with LTx, patients that died without LTx and that died with LTx. (A) aspartate aminotransferase (AST), (B) alanine aminotransferase (ALT), (C) total serum bilirubin (TSB), and (D) international normalized ratio (INR). *p < 0.05, **p < 0.01, and ***p < 0.001 compared to patients that survived without LTx.
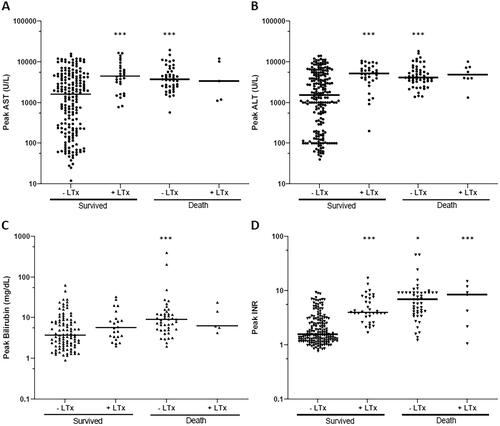
Nephrotoxicity was reported in a relatively low percentage of the patients (15%, n = 131). In the rest of cases, either no nephrotoxicity was observed (23%, n = 199) or no kidney function test results were reported (62%, n = 547). Nephrotoxicity was reported in 41% (n = 54) of the patients with fatal outcomes, about five times more prevalent than the patients who survived [8%, p < 0.001, 95% CI (0.365, 0.605)]. The peak Cr values were significantly different among outcome groups [χ2(3)=8.74, p < 0.05], with a significant pairwise comparison between group ‘Survived with LTx’ and group ‘Died w/o LTx’ (p < 0.05). There were no significant differences in the peak BUN values between all outcome groups [χ2(2)=1.71, p = 0.43].
The age of patients among the outcome groups were significantly different [χ2(3)=12.54, p < 0.01]. The 32 patients that survived with LTx were the youngest group (median age of 21). These differences were significant compared to the groups ‘Survived w/o LTx’ (median = 36, p < 0.05) and ‘Died w/o LTx’ (median = 37, p < 0.01).
Patient characteristics and clinical laboratory values in PSS groups
Intoxication severity was grouped based on liver PSS score as well. Based on hepatic enzyme values reported the PSS score could be calculated for 325 patients, for the remaining 552 patients, hepatic enzyme values were not reported. The patient characteristics and clinical laboratory values of the patients for which the PSS score could be calculated are summarized in Supplementary Table 8. Between the PSS groups, there were no significant differences in age, onset of gastrointestinal symptoms, and length of hospital stay (p > 0.05).
In terms of patient outcome, survival rates were 100% in the PSS0 (25 patients) and PSS1 groups (44 patients), 90% (64 out of 71 patients) in the PSS2 group, and 70% (130 out of 185 patients) in the PSS3 group (). In line with the expectations from the PSS score, peak AST, ALT, and INR values simultaneously increase with the PSS score (Supplementary Figures 1(A–C)). Peak AST, ALT, and INR values of patients with a PSS score of 3 were significantly higher than all other PSS groups (p < 0.001). The pairwise comparisons between PSS0 and PSS2 were also significant in AST (p < 0.01), ALT (p < 0.05), and INR (p < 0.05). TSB values were significantly different between PSS2 and PSS3 (p < 0.05, Supplementary Figure 1(D)).
Figure 6. The relationship between patient outcome and PSS scores. White: patients that survived without liver transplantation (LTx), light grey: patients that survived with LTx, dark grey: patients that died without LTx, black: patients that died with LTx.
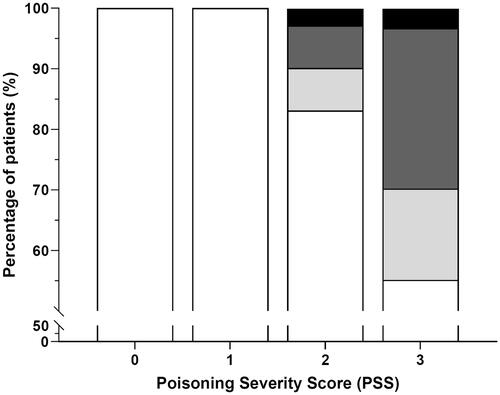
The occurrence of nephrotoxicity increased with PSS scoring, but there was no correlation between the degree of nephrotoxicity and PSS rating. The Cr and BUN values were not significantly different across PSS groups [χ2(3) = 3.05, p = 0.39 and χ2(3) = 3.21, p = 0.36].
Potential confounders
Various potential confounders (age, gastrointestinal onset, and time to clinical care) were tested for their impact on survival rate (Supplementary Table 9) and treatment success rate of the therapies (Supplementary Table 10).
The time from ingestion to receiving hospital care was tested to be a potential confounder for survival rate without LTx (p < 0.05, 95% CI [-18.80, −0.63]). This variable was further tested in treatment groups, and the differences were significant in NAC/SIL (t = 23.8 h, p < 0.01, 95% CI [8.32, 30.90]) compared to the supportive care group (t = 43.4 h).
The survival rates in different age groups were examined, children (<18 years) and elderly (>65 years) were comparable (76% and 70% respectively), and 84% in adults. Additionally, to rule out the possibility of improved hospital care over the years as a potential confounder, we compared the survival rates for every decade (1981–1990 [87%], 1991–2000 [73%], 2001–2010 [88%], and 2011–2020 [83%]). There was no correlation found between outcome and average survival rate per decade.
None of the other tested variables were potential confounders for survival rate and treatment survival rate of the therapies in this study (p > 0.05).
Discussion
No statistically significant differences were observed between the different treatment groups when only cases with a proven amanitin ingestion were analyzed. Based on the analysis of all Amanita cases, however, we found that SIL or PEN monotherapy and NAC/SIL combination therapy appear to be associated with the best outcomes for patients. Hepatic enzymes appear to be essential predictors for patient outcomes. Supportive care alone was not sufficient, and the use of most therapies (except NAC and NAC/PEN) improved patient outcomes. The regular use of the combination of NAC/PEN/SIL in patients may need to be reevaluated, as combination regimens in our analysis did not provide additional benefits compared to monotherapy.
The overall survival rate of intoxications with amanitin-containing mushrooms in our study is 84%, similar as reported by Schenk-Jaeger et al. (84.4%) [Citation3], but lower compared to Ganzert et al. (88.4%) [Citation151], De Olano et al. (91.2%) [Citation5], and Giannini et al. (98.2%) [Citation23]. This could be due to the inclusion of only amanitin-containing Amanita mushrooms in our study, whereas the other papers included all amanitin-containing mushrooms (Amanita, Galerina, and Lepiota species). The review paper from Escudié et al. may be the closest comparison, with a slightly lower survival rate of 78% since only cases with the highly toxic Amanita phalloides were included [Citation2]. The supportive care group in our study, receiving only supportive care, had a low survival rate of 59%, which is comparable to Enjalbert et al. (52.7%) [Citation14]. While there are possibilities of the lack of therapy usage in older publications, our data showed that the papers that used only supportive care ranged from 1982 to 2018, with no noticeable difference in the trend of survival rates per decade. In our study, time from ingestion to receiving hospital care was tested as a potential confounder for survival rate without LTx, since a longer waiting time to receive clinical care may result in a lower survival rate. Time from ingestion to receiving hospital care was significantly longer in the supportive care groups compared to the NAC/SIL group. This might have contributed to the lower survival rate in the supportive care group.
Several molecular mechanisms may be involved in amatoxin toxicity. One of the well-known mechanisms is the interaction of amatoxins with the RNA polymerase II enzyme. After gastrointestinal absorption, amatoxin is taken up by hepatocytes via organic anion-transporting octapeptide transporters [Citation152]. Intracellularly, amatoxins bind to the RNA polymerase II enzyme, inhibiting messenger RNA production, eventually causing cell death [Citation8,Citation152,Citation153]. Other possible mechanisms include the induction of apoptosis via p53- and caspase-3-dependent pathways, tumor necrosis factor-α upregulation in liver cells, and induction of oxidative stress [Citation8,Citation154]. In theory, the commonly used therapies PEN, SIL, and NAC treat intoxications with amanitin-containing mushrooms by tackling one of the aforementioned mechanisms [Citation8,Citation14,Citation17,Citation152,Citation153,Citation155–157].
PEN is a β-lactam antibiotic widely used in intoxications with amanitin-containing mushrooms [Citation8]. In vitro, it is an inhibitor of the human hepatocyte organic anion-transporting octapeptide transporter, thus preventing the uptake of amatoxins by hepatocytes [Citation152]. It also exhibits weak antioxidant properties in vitro and reduces the expression of apoptotic markers [Citation17,Citation157]. Among the investigated therapeutic regimens, PEN monotherapy was associated with a survival rate of 89% in both overall and proven cases, and 100% in probable amanitin cases, although the subgroup analyses did not reach statistical significance. Previous studies have reported positive results with PEN treatment. In a retrospective analysis of 2108 intoxication cases with various amanitin-containing mushrooms, Enjalbert et al. found that PEN monotherapy yielded a mortality rate of approximately 11.4% in reported cases [Citation14]. Another clinical study showed a complete recovery of all 109 patients after receiving PEN treatment, except for two patients who died after late admission to the hospital [Citation23]. While there are concerns on the possible high sodium exposure and increased risk of adverse effects due to the required high dose to achieve its therapeutic effects, there are no studies reporting hypernatremia in patients treated with PEN [Citation6].
SIL is a flavonolignan isolated from the milk thistle (Silybum marianum) extract used to treat various liver diseases [Citation158]. Like PEN, SIL inhibits organic anion-transporting octapeptide transporters in human hepatocytes [Citation152,Citation153,Citation155]. In vitro data have suggested that SIL is a more potent and non-competitive inhibitor of the organic anion-transporting octapeptide transporter (IC50 = 0.4 µM) compared to PEN (IC50 = 25 µM), which could explain the higher dose requirement of PEN [Citation152]. In addition, SIL is proposed to be able to scavenge free radicals, inhibit lipid peroxidation, and protect glutathione from oxidation by amatoxins [Citation153,Citation156]. Also, SIL significantly attenuates apoptotic activity in vivo [Citation155]. SIL stimulates RNA polymerase I activity to increase ribosomal protein synthesis, which may compensate for the loss in RNA polymerase II activity [Citation155,Citation156]. In the overall analysis, SIL monotherapy appears to be associated with a survival rate of 90% in reported cases. Unfortunately, the number of proven cases using SIL in our study was too low to be analyzed statistically. However, it showed positive results in this group and the probable subgroup. In the possible subgroup, however, no statistical effect of SIL was observed. The positive effect in the overall groups is in agreement with studies that reported positive effects of this therapy compared to supportive treatment [Citation14,Citation159,Citation160]. Conversely, a recent study by De Olano et al. on suspected cyclopeptide mushroom poisoning reported no improvement in SIL-treated patients (90.5% survival rate) compared to supportive care patients (91.5%) [Citation5]. The survival rate of the supportive care group from this study is vastly different from ours, which could be due to the regional difference in hospital care and treatment protocols. In addition to that, SIL is less easily available in the US which may directly or indirectly impact treatment outcome compared to Europe, where SIL is readily available as the drug of choice for mushroom poisoning. All things considered, SIL could be a potential amanitin treatment choice, but more evidence is required.
NAC is a hepatoprotective agent used as an antidote for acetaminophen poisoning [Citation161]. It acts as a precursor to replenish the intracellular glutathione content in the liver, while it also scavenges free radicals generated due to oxidative stress [Citation8,Citation14]. NAC may improve liver blood flow by vasodilation in vivo and decreases inflammation via indirect NF-κB inhibition [Citation153,Citation162,Citation163]. We identified seven cases using NAC monotherapy, and four of these cases resulted in treatment failure. Unfortunately, it is not possible to evaluate NAC monotherapy as a treatment choice. Similarly, Trakulsrichai et al. reported seven cases of NAC treatment that resulted in four deaths [Citation24]. On the other hand, two papers that studied both Amanita and non-Amanita amanitin poisoning reported a reduction in mortality rate compared to control when NAC was used (from 10.7% to 6.7%) [Citation14,Citation164]. Recently, a systematic review by Liu et al. showed that NAC is beneficial for poisoning with amanitin-containing mushrooms. However, this study was not limited to only NAC monotherapy and several combination therapies were included as well [Citation1]. Nevertheless, the evidence of NAC monotherapy in treating poisoning with amanitin-containing mushrooms remains limited [Citation165].
Some combined therapeutic regimens generally showed additional beneficial effects in our study. NAC/SIL combination therapy achieved significant results in the overall group and possible cases. Although NAC/PEN/SIL combination therapy resulted in a significant benefit in the overall cases, this combination was not significantly different from the supportive care group in the other subgroups. Additionally, no beneficial effects were found when patients were treated with NAC/PEN and PEN/SIL combination therapies. When combining all three drugs (NAC/PEN/SIL), the survival rate was 76% of overall cases, which is significantly less favorable than SIL or PEN monotherapies in the overall group. A similar trend was observed for the subgroup with proven cases. These differences cannot be explained by the severity of the intoxications as the numbers of severely intoxicated patients (PSS3) were comparable between these groups.
Contradictory to our results, other studies have reported favorable results for the use of combination therapy in amanitin poisoning, including NAC/PEN and PEN/SIL combination therapies. A retrospective study showed that the combination of NAC/PEN performed better than supportive care with an 8.2% mortality rate [Citation14]. According to their results, PEN/SIL had a 6.0% mortality rate, statistically significant compared to PEN (11.0%) [Citation14]. Another retrospective cohort study also reported a beneficial effect of NAC/PEN combination therapy, although their study only had a small sample size of 55 [Citation24]. However, it is important to point out that these studies with positive results included both Amanita and non-Amanita amanitin-containing mushrooms, which may have vastly different amatoxin compositions. In general, combination therapy appeared not to provide a clear additional benefit compared to monotherapy in our study.
Liver PSS rating increases with the mortality rate of the patients, meaning the peak ALT and AST values could predict patient outcome. On a related note, peak INR values of the patients that survived without LTx were significantly lower than other outcome groups, which is in agreement with the Model for End-Stage Liver Disease (MELD) criteria used to estimate mortality and allocate donor livers [Citation166]. Indeed, several other studies have also reported ALT, AST, TSB, and INR as potential prognostic markers for outcome in poisonings with amanitin-containing mushrooms [Citation23,Citation24,Citation167,Citation168]. Our results also showed significantly higher TSB value in the patients with fatal outcomes, but only when the LTx cases were excluded.
The survival rates of patients receiving LTx were not significantly different from the patients that received supportive care. The comparison could be biased as the LTx patients were most likely in life-threatening situations, whereas the patients in the supportive care group likely had milder conditions. If we looked at PSS3 patients, the differences were also not significant (p = 0.09, OR = 2.24, 95% CI [0.88, 5.74]). Alas, the number of LTx cases were not enough and the results generated from these data were limited in our study.
Nephrotoxicity was reported in about 15% of the patients, about eight times more prevalent than reported by Vesconi et al., where one out of 53 patients developed renal failure [Citation16]. Amatoxins were reported to cause either direct injury in renal tubules during renal excretion or indirect effects of hepatorenal syndrome in cases of late-onset renal toxicity [Citation25,Citation169]. In our study, nephrotoxicity was five times more likely to be reported in patients with fatal outcomes. Despite a study reporting significantly higher Cr values in patients with fatal outcomes, no significant differences were found in our study, possibly due to the lack of reported kidney function test results [Citation24,Citation151].
Limitations
The main limitation of this study is that only published case reports and detailed case series were included, probably leading to bias. There may be publication bias where papers that presented statistically significant results are more likely to be accepted for publication. Case reports often focus on notable or severe cases depending on the direction or goal of the report. Cases with supportive care may favor more serious or fatal cases, whereas cases with treatment may have a bias towards reporting cases with positive outcomes. This is a common problem in medical research that could lead to overestimation of treatment effects or exaggeration of the negative effects of supportive care [Citation170].
Secondly, data inaccuracy is highly possible, especially on missing or unreported data. It is impossible to confirm whether the unreported values were within the normal range or not [Citation171]. This is especially true for the papers reported in the 1970–1980s, where data are likely no longer available for request. In addition, a total of 304 papers in foreign languages and all amanitin-containing non-Amanita mushrooms (Lepiota and Galerina) were excluded, further limiting the available cases for our analysis.
Furthermore, our study does not account for the detoxification procedures such as activated charcoal and dialysis as a potential confounder [Citation6]. These procedures may have contributed to the patient outcome and produced false-positive results as positive outcomes are reported in patients treated by detoxification procedures [Citation14,Citation26,Citation27].
In addition, clinical laboratory values were recorded in peak values observed during hospital admission, hence the true peak values may have been missed in cases of late admissions. In the context of kidney function test, the peak values were likely inclusive of BUN and Cr elevation early in the course due to gastrointestinal fluid loss from vomiting and diarrhea, instead of only amanitin-induced nephrotoxicity.
In a clinical setting, treatment is often initiated before a positive confirmation of amanitin ingestion by laboratory analysis is made, which could be one of the limitations of our study. This is evident in our study where possible cases are the majority. There was only 29% of cases with laboratory proof of amanitin and 14% with mushroom samples visually identified by mycologists. On a related note, laboratory data of the patients were limited, adding to the challenge of rating PSS, where the scarce amount of data could skew the relationship between PSS score and patient outcome. Additionally, the quantities of mushrooms consumed were underreported (only in 13% or 116 out of 877 cases, data not shown). The number of mushrooms ingested could be an important prognostic indicator and a potential confounder that influences treatment outcomes [Citation28–30].
Another limitation and also a common challenge of this study is that the mechanisms of actions of the therapies are not entirely clear, as well as the interactions between treatment regimens. An example of this knowledge gap is the significant difference in survival rate between combining NAC/PEN and NAC/SIL as is found in our study. Further understanding of the mechanisms will aid greatly in the selection of treatment choices for amanitin poisoning.
Lastly, while it will be interesting to determine the effectiveness of the therapies based on the time delay from mushroom ingestion to treatment administration, this information is unfortunately not readily available in most case reports. Although not a focus of this study, the changes in the clinical laboratory values before and after treatment may also give more insights into the clinical effectiveness of the therapies.
Conclusions
In this systematic review, no statistical differences between different treatment regimens were observed when only those cases with proven ingestion of amanitin-containing mushrooms were analyzed. However, SIL or PEN monotherapy, and NAC/SIL combination therapy showed positive results in improving patient outcome compared to the supportive care group in treatment of all Amanita cases. When taken in aggregate these treatments had statistically significant better survival rates compared with supportive care alone.
Although NAC was widely used in various therapeutic regimens, there were not enough NAC monotherapy cases to evaluate its effectiveness in treating poisoning with amanitin-containing mushrooms. In line, NAC/PEN and PEN/SIL combination therapies did not show significant improvement in survival compared to the supportive care group.
Liver markers blood tests in patients intoxicated with amanitin-containing mushrooms are essential to monitor and predict patient outcomes. Peak ALT, AST, INR, and TSB values are more elevated in patients with fatal outcomes, making them possible prognostic markers for survival outcomes after poisoning with an amanitin-containing mushroom.
| Abbreviations | ||
| ALT | = | alanine aminotransferase |
| AST | = | aspartate aminotransferase |
| BUN | = | blood urea nitrogen |
| Cr | = | serum creatinine |
| INR | = | international normalized ratio |
| LTx | = | liver transplantation |
| NAC | = | N-acetylcysteine |
| PEN | = | benzylpenicillin |
| PSS | = | Poisoning Severity Score |
| SIL | = | silibinin |
| TSB | = | total serum bilirubin |
Supplemental Material
Download MS Word (241.4 KB)Supplemental Material
Download MS Word (227.7 KB)Disclosure statement
The authors have no conflicts of interest regarding this study.
Data availability statement
Upon reasonable request, and subject to review, the authors will provide the data that support the findings of this study.
Additional information
Funding
References
- Liu J, Chen Y, Gao Y, et al. N-acetylcysteine as a treatment for amatoxin poisoning: a systematic review. Clin Toxicol. 2020;58(11):1015–1022.
- Escudié L, Francoz C, Vinel JP, et al. Amanita phalloides poisoning: Reassessment of prognostic factors and indications for emergency liver transplantation. J Hepatol. 2007;46(3):466–473.
- Schenk-Jaeger KM, Rauber-Lüthy C, Bodmer M, et al. Mushroom poisoning: a study on circumstances of exposure and patterns of toxicity. Eur J Intern Med. 2012;23(4):e85–91–e91.
- Aveline J. The death of claudius. Hist - Zeitschrift Fur Alte Geschichte. 2004;53(4):453–475.
- De Olano J, Wang JJ, Villeneuve E, et al. Current fatality rate of suspected cyclopeptide mushroom poisoning in the United States. Clin Toxicol. 2021;59(1):24–27.
- Karlson-Stiber C, Persson H. Cytotoxic fungi - An overview. Toxicon. 2003;42(4):339–349.
- Diaz JH. Amatoxin-Containing mushroom poisonings: species, toxidromes, treatments, and outcomes. Wilderness Environ Med. 2018;29(1):111–118.
- Garcia J, Costa VM, Carvalho A, et al. Amanita phalloides poisoning: mechanisms of toxicity and treatment. Food Chem Toxicol. 2015;86:41–55.
- Faybik P, Hetz H, Baker A, et al. Extracorporeal albumin dialysis in patients with amanita phalloides poisoning. Liver Int. 2003;23(Suppl. 3):28–33.
- Alves A, Gouveia Ferreira M, Paulo J, et al. Mushroom poisoning with amanita phalloides - A report of four cases. Eur J Intern Med. 2001;12(1):64–66.
- Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367–1384.
- Shikdar S, Vashisht R, Bhattacharya P. International Normalized Ratio (INR) [Internet]. StatPearls Publishing. 2020. https://www.ncbi.nlm.nih.gov/books/NBK507707/
- Santi L, Maggioli C, Mastroroberto M, et al. Acute liver failure caused by amanita phalloides poisoning. Int J Hepatol. 2012;2012:487480.
- Enjalbert F, Rapior S, Nouguier-Soulé J, et al. Treatment of amatoxin poisoning: 20-Year retrospective analysis. J Toxicol Clin Toxicol. 2002;40(6):715–757.
- Klein AS, Hart J, Brems JJ, et al. Amanita poisoning: Treatment and the role of liver transplantation. Am J Med. 1989;86(2):187–193.
- Vesconi S, Langer M, Iapichino G, et al. Therapy of cytotoxic mushroom intoxication. Crit Care Med. 1985;13(5):402–406.
- Ye Y, Liu Z. Management of amanita phalloides poisoning: a literature review and update. J Crit Care. 2018;46(36):17–22.
- Ng VL. Liver disease, coagulation testing, and hemostasis. Clin Lab Med. 2009;29(2):265–282.
- DePond W. The relationship of the international normalized ratio (INR) to the prothrombin time (PT). Medlab. 2011;6:1–6.
- Lindahl TL, Egberg N, Hillarp A, et al. INR calibration of owren-type prothrombin time based on the relationship between PT% and INR utilizing normal plasma samples. Thromb Haemost. 2004;91(6):1223–1231.
- Harrison MF. The misunderstood coagulopathy of liver disease: a review for the acute setting. West J Emerg Med. 2018;19(5):863–871.
- Persson HE, Sjöberg GK, Haines JA, et al. Poisoning severity score. Grading of acute poisoning. J Toxicol Clin Toxicol. 1998;36(3):205–213.
- Giannini L, Vannacci A, Missanelli A, et al. Amatoxin poisoning: a 15-year retrospective analysis and follow-up evaluation of 105 patients. Clin Toxicol. 2007;45(5):539–542.
- Trakulsrichai S, Sriapha C, Tongpoo A, et al. Clinical characteristics and outcome of toxicity from amanita mushroom poisoning. Int J Gen Med. 2017;10:395–400.
- Langer M, Gridelli B, Piccolo G, et al. A liver transplant candidate (fulminant hepatic failure from amanita phalloides poisoning) as a multiorgan donor. Transplant Proc. 1997;29(8):3343–3344.
- Lionte C, Sorodoc L, Simionescu V. Successful treatment of an adult with amanita phalloides-induced fulminant liver failure with molecular adsorbent recirculating system (MARS). Rom J Gastroenterol. 2005;14(3):267–271.
- Shi Y, He J, Chen S, et al. MARS: Optimistic therapy method in fulminant hepatic failure secondary to cytotoxic mushroom poisoning - A case report. Liver. 2002;22(SUPPL. 2):78–80.
- Erguven M, Yilmaz O, Deveci M, et al. Mushroom poisoning. Indian J Pediatr. 2007;74(9):847–852.
- Olesen LL. Amatoxin intoxication. Scand J Urol Nephrol. 1990;24(3):231–234.
- Olson KR, Pond SM, Seward J, et al. Amanita phalloides-type mushroom poisoning. West J Med. 1982;137(4):282–289.
- Teutsch C, Brennan RW. Amanita mushroom poisoning with recovery from coma: a case report. Ann Neurol. 1978;3(2):177–179.
- Dumont AM, Chennebault JM, Alquier P, et al. Management of amanita phalloides poisoning by bastien’s regimen. Lancet. 1981;1(8222):722.
- Plotzker R, Jensen DM, Payne JA. Amanita virosa acute hepatic necrosis: Treatment with thioctic acid. Am J Med Sci. 1982;283(2):79–82.
- Hazani E, Taitelman U, Shasha SM. Amanita verna poisoning in Israel - Report of a rare case of time and place. Arch Toxicol. 1983;53:186–189. p.
- Hruby K, Csomos G, Fuhrmann M, et al. Chemotherapy of amanita phalloides poisoning with intravenous silibinin. Hum Toxicol. 1983;2(2):183–195.
- Belliardo F, Massano G, Accomo S. Amatoxins do not cross the placental barrier. Lancet. 1983;1(8338):1381.
- Kendrick B, Shimizu A. Mushroom poisoning: Analysis of two cases, and a possible new treatment. Plasmapheresis. Mycologia. 1984;76(3):448–453.
- Woodle ES, Moody RR, Cox KL, et al. Orthotopic liver transplantation in a patient with amanita poisoning. JAMA. 1985;253(1):69–70.
- Pond SM, Olson KR, Woo OF, et al. Amatoxin poisoning in Northern California, 1982-1983. West J Med. 1986;145(2):204–209.
- Sanz P, Reig R, Piqueras J, et al. Fatal mushroom poisoning in barcelona, 1986-1988. Mycopathologia. 1989;108(3):207–209.
- Piering WF, Bratanow N. Role of the clinical laboratory in guiding treatment of amanita virosa mushroom poisoning: Report of two cases. Clin Chem. 1990;36(3):571–574.
- Pinson CW, Daya MR, Benner KG, et al. Liver transplantation for severe amanita phalloides mushroom poisoning. Am J Surg. 1990;159(5):493–499.
- Lawrence RA, Pehlivanoglu E, Canpolat C. Treatment of amanita phalloides intoxication: a report of 3 cases and review of the literature. Marmara Med J. 1990;(3):42–45.
- Cappell MS, Hassan T. Gastrointestinal and hepatic effects of amanita phalloides ingestion. J Clin Gastroenterol. 1992;15(3):225–228.
- Galler GW, Weisenberg E, Brasitus TA. Mushroom poisoning: the role of orthotopic liver transplantation. J Clin Gastroenterol. 1992;15(3):229–232.
- Christen Y, Minazio P, De Moerloose P. Monitoring of haemostatic parameters in five cases of amanita phalloides poisoning. Blood Coagul Fibrinolysis. 1993;4(4):627–630.
- Pérez-Moreno J, Pérez-Moreno A, Ferrera-Cerrato R. Multiple fatal mycetism caused by amanita virosa in Mexico. Mycopathologia. 1994;125(1):3–5.
- Nagy I, Pogatsa-Murray G, Zalanyi S, et al. Amanita poisoning during the second trimester of pregnancy. Clin Investig. 1994;72(10):794–798.
- Yacyshyn B, Cheung CM, Malkin DG, et al. A potential prognostic indicator of fulminant hepatic failure: a valuable adjunct in preliver transplant assessment? Can J Gastroenterol. 1994;8(5):308–312.
- Aji DY, Çalişkan S, Nayir A, et al. Haemoperfusion in amanita phalloides poisoning. J Trop Pediatr. 1995;41(6):371–374.
- Gonzalez J, Lacomis D, Kramer DJ. Mushroom myopathy. Muscle Nerve. 1996;19(6):790–792.
- Serné EH, Toorians AWFT, Gietema JA, et al. Amanita phalloides, a potentially lethal mushroom: Its clinical presentation and therapeutic options. Neth J Med. 1996;49(1):19–23.
- Rosenthal P, Roberts JP, Ascher NL, et al. Auxiliary liver transplant in fulminant failure. Pediatrics. 1997;100(2):E10.
- Korman MG, Nicholson FB, Korman MG. Death from amanita poisoning. Aust N Z J Med. 1997;27(4):448–449.
- Zevin S, Dempsey D, Olson K. Amanita phalloides mushroom poisoning-Northern California. Clin Toxicol. January 1997;35(5):461–463. 1997
- Scocco P, Rupolo G, Leo DD. Failed suicide by amanita phalloides (mycetismus) and subsequent liver transplant: Case report. Arch Suicide Res. 1998;4(2):201–206.
- Chaiear K, Limpaiboon R, Meechai C, et al. Fatal mushroom poisoning caused by amanita virosa in Thailand. Southeast Asian J Trop Med Public Health. 1999;30(1):157–160. p.
- Horn KD, Wax P, Schneider SM, et al. Biomarkers of liver regeneration allow early prediction of hepatic recovery after acute necrosis. Am J Clin Pathol. 1999;112(3):351–357.
- Trim GM, Lepp H, Hall MJ, et al. Poisoning by amanita phalloides ('deathcap’) mushrooms in the Australian Capital Territory. Med J Aust. 1999;171(5):247–249.
- Splendiani G, Zazzaro D, Di Pietrantonio P, et al. Continuous renal replacement therapy and charcoal plasmaperfusion in treatment of amanita mushroom poisoning. Artif Organs. 2000;24(4):305–308.
- Lim JG, Kim JH, Lee CY, et al. Amanita virosa induced toxic hepatitis: report of three cases. Yonsei Med J. 2000;41(3):416–421.
- Broussard CN, Aggarwal A, Lacey SR, et al. Mushroom poisoning-from diarrhea to liver transplantation. Am J Gastroenterol. 2001;96(11):3195–3198.
- Boyer JC, Hernandez F, Estorc J, et al. Management of maternal amanita phalloïdes poisoning during the first trimester of pregnancy: a case report and review of the literature. Clin Chem. 2001;47(5):971–974.
- Himmelmann A, Mang G, Schnorf-Huber S. Lethal ingestion of stored amanita phalloides mushrooms. Swiss Med Wkly. 2001;131(41-42):616–617.
- Jander S, Bischoff J. Treatment of amanita phalloides poisoning: I. Retrospective evaluation of plasmapheresis in 21 patients. Ther Apher. 2000;4(4):303–307.
- Hallik M, Tamme K, Väli T, et al. Successful liver transplantation after 21 days of hepatic coma. Asaio J. 2011;57(6):545–546.
- Covic A, Goldsmith DJA, Gusbeth-Tatomir P, et al. Successful use of molecular absorbent regenerating system (MARS) dialysis for the treatment of fulminant hepatic failure in children accidentally poisoned by toxic mushroom ingestion. Liver Int. 2003;23(SUPPL. 3):21–27.
- Wu BF, Wang MM. Molecular adsorbent recirculating system in dealing with maternal amanita poisoning during the second pregnancy trimester: a case report. Hepatobiliary Pancreat Dis Int. 2004;3(1):152–154.
- Kucuk HF, Karasu Z, Kilic M, et al. Liver failure in transplanted liver due to amanita falloides (sic). Transplant Proc. 2005;37(5):2224–2226.
- Hydzik P, Gawlikowski T, Ciszowski K, et al. Liver albumin dialysis (MARS)–treatment of choice in amanita phalloides poisoning? Przegl Lek. 2005;62(6):475–479.
- Madhok M, Scalzo AJ, Blume CM, et al. Amanita bisporigera ingestion. Pediatr Emerg Care. 2006;22(3):177–180.
- Araz C, Karaaslan P, Esen A, et al. Successful treatment of a child with fulminant liver failure and coma due to amanita phalloides poisoning using urgent liver transplantation. Transplant Proc. 2006;38(2):596–597.
- Soysal D, Çevik Ç, Saklamaz A, et al. Coagulation disorders secondary to acute liver failure in amanita phalloides poisoning: a case report. Turkish J Gastroenterol. 2006;17(3):198–202.
- Xiao GL, Zhang CH, Liu FY, et al. Clinical experience in treatment of amanita mushroom poisoning with glossy ganoderma decoction and routine Western medicines. Chin J Integ Med. 2007;13(2):145–147.
- Krenová M, Pelclová D, Navrátil T. Survey of amanita phalloides poisoning: Clinical findings and follow-up evaluation. Hum Exp Toxicol. 2007;26(12):955–961.
- Unverir P, Soner BC, Dedeoglu E, et al. Renal and hepatic injury with elevated cardiac enzymes in amanita phalloides poisoning: a case report. Hum Exp Toxicol. 2007;26(9):757–761.
- Ennecker-Jans SAM, Van Daele PLA, Blonk MI, et al. Amatoxin poisoning due to soup from personally picked deathcap mushrooms (amanita phalloides. Ned Tijdschr Geneeskd. 2007;151(13):764–768.
- Thaler T, Aceto L, Kupferschmidt H, et al. First intoxication with freshly picked amanita phalloides in winter time in Central Europe. J Gastrointest Liver Dis. 2008;17(1):111–117.
- Yildiz BD, Abbasoglu O, Saglam A, et al. Urgent liver transplantation for amanita phalloides poisoning. Pediatr Transplant. 2008;12(1):105–108.
- Wacker A, Riethmüller J, Zilker T, et al. Fetal risk through maternal amanita phalloides poisoning at the end of pregnancy. Am J Perinatol. 2009;26(3):211–214.
- Garrouste C, Hémery M, Boudat AM, et al. Amanita phalloides poisoning-induced end-stage renal failure. Clin Nephrol. 2009;71(5):571–574.
- Jiranantakan T, Olson KR, Magge HB. Acute pancreatitis in amanita phalloides poisoning. Clin Toxicol. 2009;47(7):702–765.
- Matthews A, Kleiman R, Thomas J, et al. Abstracts of the 2009 North American congress of clinical toxicology annual meeting, september 21–26, 2009, san antonio, Texas, USA (abstract 208. Survival of amanita virosa poisoning treated with plasmapheresis). Clin Toxicol. 2009;47(7):702–765.
- Aygul N, Duzenli MA, Ozdemir K, et al. A case report of an unusual complication of amanita phalloides poisoning: Development of cardiogenic shock and its successful treatment with intra-aortic balloon counterpulsation. Toxicon. 2010;55(2-3):630–632.
- Põld K, Oder M, Paasma R. Abstracts of the 2010 international congress of the european association of poisons centres and clinical toxicologists, 11-14 may 2010, bordeaux, France (abstract 314. Poisoning by amanita phalloides). Clin Toxicol. 2010;48(3):240–318.
- Mrzljak A, Knotek M, Gustin D, et al. End-stage kidney disease after mushroom poisoning and ABO-incompatible liver transplantation. Nephrology (Carlton). 2010;15(6):660–661.
- Evrenoglou D, Michelidou S, Sakagianni K. 23rd ESICM annual congress – barcelona, Spain – 9–13 october 2010 (abstract 1299. Mushroom poisoning: Successful treatment with silibinin and N-acetylcysteine). Intensive Care Med. 2010;36(S2):326–433.
- Sorodoc L, Lionte C, Sorodoc V, et al. Is MARS system enough for A.phalloides-induced liver failure treatment? Hum Exp Toxicol. 2010;29(10):823–832.
- Lu F, Li G, Lu G, et al. Acute liver failure resulting from toadstool poison in three patients. ADRJ. 2010;12(4):273–275.
- Cassidy N, Duggan E, Tracey JA. Mushroom poisoning in Ireland: the collaboration between the national poisons information Centre and expert mycologists. Clin Toxicol (Phila). 2011;49(3):171–176.
- Freeman RJ, Elko CJ, Martin TG, et al. Abstracts (abstract 194. An amanita phalloides poisoning in Washington state confirmed by poison center field collection. Clin Toxicol. 2011;49(6):515–627. )
- Garcia De La Fuente I, McLin VA, Rimensberger PC, et al. Amanita poisoning and liver transplantation: Do we have the right decision criteria? J Pediatr Gastroenterol Nutr. 2011;53(4):459–462.
- Méndez-Navarro J, Ortiz-Olvera NX, Villegas-Ríos M, et al. Hepatotoxicity from ingestion of wild mushrooms of the genus amanita section phalloideae collected in Mexico city: two case reports. Ann Hepatol. 2011;10(4):568–574.
- Cress CM, Malliah A, Herrine SK. Fulminant hepatic failure caused by amanita phalloides toxicity. Clin Gastroenterol Hepatol. 2011;9(2):A26.
- French LK, Hendrickson RG, Horowitz BZ. Amanita phalloides poisoning. Clin Toxicol (Phila). 2011;49(2):128–129.
- Bergis D, Friedrich-Rust M, Zeuzem S, et al. Treatment of amanita phalloides intoxication by fractionated plasma separation and adsorption (prometheus®). J Gastrointest Liver Dis. 2012;21(2):171–176.
- Oghabian Z. Baniasad N, darvish moghadam S. Successful management of severe hepatotoxicity after fungal poisoning with amanita sp. of a patient: a case report. J Gastroenterol Hepatol. 2012;27(Suppl. 5):59–438.
- Hays H, Casavant M, Jolliff H. Annual meeting of the North American congress of clinical toxicology (NACCT) october 1–6, 2012 las vegas, NV, USA (abstract 191. Not just for elementary school: a field trip and a new antidote solidify trainees’ knowledge of amatoxin poisoning). Clin Toxicol. 2012. 2012;50(7):574–720.
- Chen WC, Kassi M, Saeed U, et al. A rare case of amatoxin poisoning in the state of Texas. Case Rep Gastroenterol. 2012;6(2):350–357.
- Lawton LD, Bhraonain SN. Accidental poisoning by death cap mushrooms: Be careful what you eat. Wilderness Environ Med. 2013;24(2):168–170.
- Roberts DM, Hall MJ, Falkland MM, et al. Amanita phalloides poisoning and treatment with silibinin in the Australian Capital Territory and New South Wales. Med J Aust. 2013;198(1):43–47.
- Ward J, Kapadia K, Brush E, et al. Amatoxin poisoning: Case reports and review of current therapies. J Emerg Med. 2013;44(1):116–121.
- Erden A, Esmeray K, Karagöz H, et al. Acute liver failure caused by mushroom poisoning: a case report and review of the literature. Int Med Case Rep J. 2013;6(1):85–90.
- Grabhorn E, Nielsen D, Hillebrand G, et al. Successful outcome of severe amanita phalloides poisoning in children. Pediatr Transplant. 2013;17(6):550–555.
- Vanooteghem S, Decock S, Arts J, et al. Four patients with amanita phalloides poisoning. Gut. 2013;62(Suppl 1):A187.1–A187.
- Altintepe L, Yazici R, Yazici M, et al. Temporary left ventricular dysfunction in mushroom poisoning: Report of three cases. Ren Fail. 2014;36(8):1337–1339.
- Baniasad N, Oghabian Z, Mehrpour O. Hepatotoxicity due to mushroom poisoning: a case report. Int J Med Toxicol Forensic Med. 2014;4(2):68–73.
- Purcell MM, Marraffa JM. XXXIV international congress of the european association of poisons centres and clinical toxicologists (EAPCCT) 27–30 may 2014, brussels, Belgium (abstract 219. Aggressive treatment results in complete resolution of amanita bisporigera toxicity). Clin Toxicol. 2014;52(4):295–443.
- Dirican A, Yilmaz M, Baskiran A, et al. Urgent liver transplantation for amanita phalloides caused acute liver failure. Eur Univ Inst. 2014;(2):2–5.
- Chan YC, Tse ML, Lau FL. Hong Kong poison information Centre: Annual report 2013. Hong Kong J Emerg Med. 2014;21(4):249–259.
- Sodavarapu S, Singh S, Hosea S. Fulminant hepatic failure after consumption of wild mushrooms. Am J Gastroenterol. 2014;109:S389.
- Frass M, Zagorchev P, Yurukova V, et al. Two cases of fulminant hepatic failure from amanita phalloides poisoning treated additively by homeopathy. Ochsner J. 2014;14(2):252–258.
- Zhang J, Zhang Y, Peng Z, et al. Experience of treatments of amanita phalloides–induced fulminant liver failure with molecular adsorbent recirculating system and therapeutic plasma exchange. Asaio J. 2014;60(4):407–412.
- Gores KM, Hamieh TS, Schmidt GA. Survival following investigational treatment of amanita mushroom poisoning: Thistle or shamrock? Chest. 2014;146(4):e126–9.
- Verma N, Bhalla A, Kumar S, et al. Wild mushroom poisoning in North India: Case series with review of literature. J Clin Exp Hepatol. 2014;4(4):361–365.
- Ros JJW, Semplonius G, Mulder-Spijkerboer HN, et al. Intoxicatie met groene knolamaniet (amanita phalloides). Pharm Weekbl. 2015;150(22):117–120.
- Yilmaz I, Ermis F, Akata I, et al. A case study: What doses of amanita phalloides and amatoxins are lethal to humans? Wilderness Environ Med. 2015;26(4):491–496.
- Rahmani F, Bakhtavar HE, Ghavidel A. Acute hepatorenal failure in a patient following consumption of mushrooms: a case report. Iran Red Crescent Med J. 2015;17(3):1–4.
- Garcia J, Costa VM, Costa AE, et al. Co-ingestion of amatoxins and isoxazoles-containing mushrooms and successful treatment: a case report. Toxicon. 2015;103:55–59.
- Chibishev A, Perevska Z, Simonovska N, et al. Severe mushroom poisoning in one macedonian family. Int J Artif Organs. 2015;38(8):425–432.
- Hendrik Pillukat M, Schomacher T, Baier P, et al. Early initiation of MARS® dialysis in amanita phalloides-induced acute liver injury prevents liver transplantation. Ann Hepatol. 2016;15(5):775–787.
- Olsson EK, Petersson E. 36th international congress of the european association of poisons centres and clinical toxicologists (EAPCCT) 24-27 may, 2016, Madrid, Spain (abstract 312. Amatoxin poisoning during pregnancy: a case report and review of the literature). Clin Toxicol. 2016;54(4):344–519.
- Arif T, Schiel H, Bartecka-Mino K. 36th international congress of the european association of poisons centres and clinical toxicologists (EAPCCT) 24-27 may, 2016, Madrid, Spain (abstract 300. Amanita phalloides ingestion in children in Austria, 1996 to 2014). Clin Toxicol. 2016;54(4):344–519.
- Stankiewicz R, Lewandowski Z, Kotulski M, et al. Effectiveness of fractionated plasma separation and absorption as a treatment for amanita phalloides poisoning. Ann Transplant. 2016;21:428–432.
- Vondran FWR, Schumacher C, Johanning K, et al. Application of the liver maximum function capacity test in acute liver failure: a helpful tool for Decision-Making in liver transplantation? Case Rep Transplant. 2016;2016:7074636–7074635.
- Zhu S, Chen ZH, Zhou P, et al. 起因食用淡红鹅膏菌引起的中毒事件报告[a case report of intoxication caused by amanita pallidorosea]. Prev Med. 2016;28(7):719–720. 一 Chinese.
- Austin E, Tirona R, Thompson M, et al. North American congress of clinical toxicology (NACCT) abstracts 2017 (abstract 35. Cyclosporine as a novel treatment for amatoxin-containing mushroom poisoning). Clin Toxicol. 2017;55(7):689–868.
- Vendramin A, Jamsek M, Brvar M. 37th international congress of the european association of poisons centres and clinical toxicologists (EAPCCT) 16-19 may 2017, basel, Switzerland (abstract 295. Amanita phalloides poisoning in Slovenia, 1999–2015). Clin Toxicol. 2017;55(5):371–544.
- Pantoflicek T, Fronek J. The 52nd congress of the european society for surgical research [abstract O112 AB0 – incompatible split liver transplantation in a married couple with fulminant hepatic failure due to mushroom poisoning (amanita phalloides) – a case report]. Eur Surg Res. 2017;58:1–69.
- Surmaitis R, Pietrowski M, Kolecki P, et al. Abstracts from the 2017 American college of medical toxicology (ACMT) annual scientific meeting (abstract 57. Thistle while you work: a case of mushroom poisoning with liver recovery evident before silibinin administration). J Med Toxicol. 2017;13(1):3–46.
- Vo KT, Montgomery ME, Mitchell ST, et al. Amanita phalloides mushroom poisonings — Northern California, december 2016. MMWR Morb Mortal Wkly Rep. 2017;66(21):549–553.
- Ma KW, Chok KSH, Chan CK, et al. Liver transplantation: a life-saving procedure following amatoxin mushroom poisoning. Hong Kong Med J. 2017;23(1):93–96.
- Hongo T, Zheng M, Malamood M, et al. A case of amanita phalloides poisoning that avoided liver transplantation. Med Forum. 2017;18(1):12–13.
- Dutta A, Kumar Dutta P, Kar S. Mushroom poisoning induced acute liver failure with deranged coagulation profile. jemds. 2017;6(66):4783–4786.
- Li Y, Mu M, Yuan L, et al. Challenges in the early diagnosis of patients with acute liver failure induced by amatoxin poisoning. Medicine. 2018;97(27):e11288.
- Barman B, Warjri S, Lynrah KG, et al. Amanita nephrotoxic syndrome: Presumptive first case report on the indian subcontinent. Indian J Nephrol. 2018;28(2):170–172.
- Foutris A, Kalostou A, Basanou E. 38th international congress of the european association of poisons centres and clinical toxicologists (EAPCCT) 22-25 may 2018, bucharest, Romania (abstract 278. Amanita phalloides poisoning: effectiveness of timely administration of antidotes). Clin Toxicol. 2018;56(6):453–608.
- Kieslichova E, Frankova S, Protus M, et al. Acute liver failure due to amanita phalloides poisoning: Therapeutic approach and outcome. Transplant Proc. 2018;50(1):192–197.
- Schmutz M, Carron PN, Yersin B, et al. Mushroom poisoning: a retrospective study concerning 11-years of admissions in a swiss emergency department. Intern Emerg Med. 2018;13(1):59–67.
- Pakala T, Siu M, Bunim A, et al. Amanita phalloides induced hepatitis: Watch out for renal failure? Am J Gastroenterol. 2018;113(Supplement):S1259.
- Lu Z, Chen YB, Huang B, et al. Mixed amanita phalloides poisoning with rhabdomyolysis: analysis of 4 cases. J South Med Univ. 2018;38(5):635–638.
- Sun J, Li HJ, Zhang HS, et al. Investigating and analyzing three cohorts of mushroom poisoning caused by amanita exitialis in Yunnan, China. Hum Exp Toxicol. 2018;37(7):665–678.
- Baskiran A, Dirican A, Ozgor D, et al. Significance and outcome of living-donor liver transplantation in acute mushroom intoxication. Niger J Clin Pract. 2018;21(7):888–893.
- Olaru C, Burlea LS, Rosu TS. Gastritis masking late symptoms of mushroom poisoning in a teenager. a case report. Rom J Oral Rehabil. 2018;10(1):160–165.
- Pillay Mauree A, de Maayer T, Botes A, et al. Amanita phalloides poisoning: One harvest, three outcomes. S Afr j Surg. 2019;57(1):61–64.
- Beaumier M, Rioult JP, Georges M, et al. Mushroom poisoning presenting with acute kidney injury and elevated transaminases. Kidney Int Rep. 2019;4(6):877–881.
- Karakoç E, Demirtaş K, Ekemen S, et al. Mushroom that breaks hearts: a case report. Turkish J Intensive Care. 2019;18(1):43–46.
- Mărginean CO, Meliţ LE, Mărginean MO. Mushroom intoxication, a fatal condition in romanian children: Two case reports. Medicine. 2019;98(41):e17574.
- Wang Q, Sun M, Lv H, et al. Amanita fuliginea poisoning with thrombocytopenia: a case series. Toxicon. 2020;174(October 2019):43–47.
- Ye Y, Liu Z, Zhao M. CLIF-OF >9 predicts poor outcome in patients with amanita phalloides poisoning. Am J Emerg Med. 2021;39:96–101.
- Ganzert M, Felgenhauer N, Zilker T. Indication of liver transplantation following amatoxin intoxication. J Hepatol. 2005;42(2):202–209.
- Letschert K, Faulstich H, Keller D, et al. Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol Sci. 2006;91(1):140–149.
- Khunnala A, Narongchai S, Butkrachang S, et al. Anti-Oxidative stress activities of silibinin on Į-Amanitin in vitro. Thai J Toxicol. 2009;24(2):106–112.
- Magdalan J, Piotrowska A, Gomułkiewicz A, et al. Benzylpenicyllin and acetylcysteine protection from α-amanitin-induced apoptosis in human hepatocyte cultures. Exp Toxicol Pathol. 2011;63(4):311–315.
- Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Investig. 2002;22(1):51–65.
- Vargas-Mendoza N, Madrigal-Santillán E, Morales-González Á, et al. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6(3):144–149.
- Berczyński P, Kładna A, Kruk I, et al. Radical-scavenging activity of penicillin G, ampicillin, oxacillin, and dicloxacillin. J Biol Chem Lumin. 2017;32(3):434–442.
- Hellerbrand C, Schattenberg JM, Peterburs P, et al. The potential of silymarin for the treatment of hepatic disorders. Clin Phytosci. 2017;2(1):7.
- Mengs U, Torsten Pohl R, Mitchell T. Legalon® SIL: the antidote of choice in patients with acute hepatotoxicity from amatoxin poisoning. Curr Pharm Biotechnol. 2012;13(10):1964–1970.
- Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61(14):2035–2063.
- Chughlay MF, Kramer N, Werfalli M, et al. N-acetylcysteine for non-paracetamol drug-induced liver injury: a systematic review protocol. Syst Rev. 2015;4(1):1–6.
- Sklar GE, Subramaniam M. Acetylcysteine treatment for Non-Acetaminophen-Induced acute liver failure. Ann Pharmacother. 2004;38(3):498–501.
- Rank N, Michel C, Haertel C, et al. N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: Results of a prospective, randomized, double-blind study. Crit Care Med. 2000;28(12):3799–3807.
- Poucheret P, Fons F, Doré JC, et al. Amatoxin poisoning treatment decision-making: Pharmaco-therapeutic clinical strategy assessment using multidimensional multivariate statistic analysis. Toxicon. 2010;55(7):1338–1345.
- Connors NJ, Gosselin S, Hoffman RS. Comment on “N-acetylcysteine as a treatment for amatoxin poisoning. Clin Toxicol. 2021;59(6):534–535.
- Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96.
- Eren SH, Demirel Y, Ugurlu S, et al. Mushroom poisoning: Retrospective analysis of 294 cases. Clinics. 2010;65(5):491–496.
- Dutta A, Bora K. Elevated transaminases as predictor of mortality in mushroom poisoning patients. J Evol Res Med Biochem. 2016;2(2):1–3.
- Mydlik M, Derzsiova K. Liver and kidney damage in acute poisonings. Bantao J. 2006;4(1):30–33.
- Easterbrook PJ, Gopalan R, Berlin JA, et al. Publication bias in clinical research. Lancet. 1991;337(8746):867–872.
- Hoffman RS. Understanding the limitations of retrospective analyses of poison center data. Clin Toxicol (Phila). 2007;45(8):943–945.

