Abstract
Context
Acute toxicity caused by illicit substance use is a common reason for emergency department (ED) presentation. Knowledge of the substances involved is helpful for predicting and managing potential toxicity, but limited information is available about the accuracy of patient-reported substance exposure. This study assessed the accuracy of the history of exposure in those reporting use of a single substance by comparison with those identified by detailed toxicological analysis, focusing on synthetic cannabinoid receptor agonists (SCRA).
Methods
Adults (≥16 years) presenting between March 2015 and July 2021 to participating UK hospitals with toxicity after reporting use of a single illicit substance were included. Exposure details were documented from medical records and blood and/or urine samples analysed using high-resolution accurate mass liquid chromatography-mass spectrometry (HRAM LCMS). Sensitivity, specificity, and positive and negative predictive values of the exposure history were calculated by comparison with biological sample analysis (“gold standard”).
Results
Single substance exposure was reported for 474 (median age 33 years, IQR: 18 range 16–75, 80% males) patients. Analysis commonly identified multiple substances (Median 3, IQR 2–5). A history of exposure was documented for 121 of 151 patients where a SCRA or metabolite was detected on analysis (sensitivity 80.1%, 95% CI 72.9, 86.2%). Corresponding proportions were lower for 3,4-methylenedioxymethamphetamine (MDMA, 44/70, 62.9%., 95% CI 50.5%, 74.1%), heroin 41/108 (38.0% 95% CI 28.8–47.8%) and cocaine (22/56, 31.3%, 95% CI 20.9, 43.6%).
Conclusions
Multiple undeclared substances were detected analytically in most patients reporting single substance use. Clinicians should be alert to the potential presence and toxicity of unreported substances when managing patients presenting after substance misuse.
Keywords:
Introduction
Toxicity resulting from substance misuse (sometimes called recreational drug use/misuse) is a common reason for emergency department (ED) presentations and hospital admissions [Citation1,Citation2]. Knowledge of the substances involved helps to interpret clinical features, anticipate the predicted clinical course, and inform appropriate monitoring and management decisions, including in some cases the administration of antidotes. Urine drug screening using immunoassays is sometimes performed, but has substantial limitations, including low sensitivity and specificity and, in particular, may not detect new psychoactive substances (NPS) [Citation3], although methods have been developed to detect some specific compounds [Citation4]. While liquid chromatography-mass spectrometry can be very sensitive for detecting substances of misuse, it is time consuming and expensive to perform. As a result, real-time analytical information is rarely available at the time of presentation, so the history provided by the patient or other witnesses is often the only source of information on substances involved. Research and surveillance studies of reported substance use, important for developing national drug control policies [Citation5], may also rely on unvalidated user accounts for exposure information.
There is considerable evidence collected in different countries and settings that patient-reported substance use often underestimates drug use as determined by analytical screening [Citation5–9]. This may be affected by various factors including age, sex, study setting, education, socioeconomic status and race [Citation10–12]. Limited information, however, is available on the accuracy of patient exposure histories for substance users presenting to hospitals with toxicity, when the accuracy of the history may also be affected by acute drug effects on cognition. In the broader context of drug overdose, clinically important discrepancies between patient exposure histories and analytical findings were commonly observed [Citation13].
Considering patients with substance use, only 52% of 318 patients presenting to an ED in the United States (US) with pain who had analytically documented exposure to cocaine or methamphetamine had reported use of these substances [Citation14]. In 55 ED patients presenting with pain or nausea in Colorado, US, illicit drugs were detected by comprehensive drug screening in 14%, having not been reported in the history. Conversely, 4% reported an illicit drug that was not detected analytically [Citation15]. In a case series of patients with substance use in a single hospital in London, 9 of 18 patients (50%) in whom synthetic cannabinoid receptor agonists (SCRA) were detected had reported their use [Citation16]. Of 128 presenting to 2 EDs in Washington DC with reported SCRA intoxication, analytical testing detected a SCRA in 55% [Citation17]. Analysis of samples from 158 patients with substance use attending outpatient and inpatient facilities in Oslo, Norway, detected NPS in 8% (none of these a SCRA), although these were not suspected clinically in any [Citation18]. These studies suggest poor agreement between the clinical history and laboratory analysis, but some studies were not focused on patients attending after substance use and all were limited in terms of numbers of hospital sites and patients studied.
This study was therefore performed to assess the accuracy of the patient history of exposure in those reporting use of a single substance, by comparison with substances identified by detailed toxicological analysis in a large multicentre cohort of patients attending an ED after suspected non-medical substance use. This study focused on SCRA, because the data collection was initially restricted to patients with suspected NPS exposure. SCRA were the most commonly identified NPS (as was also the case in one US study [Citation19]) and these compounds cause significant clinical toxicity [Citation20], but we also compared data with other drugs or groups where sufficient information was available.
Methods
This research was conducted using data collected by the ongoing UK Identification Of Novel psychoActive substances (IONA) study, which has been collecting clinical information and analysing biological samples from adults presenting to participating EDs with suspected toxicity due to substance use (including counterfeit medicines) since March 2015. The study methods have been described previously [Citation21,Citation22]. By July 2021, 30 participating hospitals in England, Wales and Scotland were involved. The IONA study is open to those aged at least 16 years presenting to a participating ED with toxicity related to substance misuse. Initially (March 2015 to January 2017), inclusion required the presence of severe toxicity according to defined criteria (except in Scotland) and suspected use of a NPS. Inclusion criteria were broadened in January 2017 to include suspected exposure to non-pharmaceutical opioids (e.g., heroin), as a part of UK surveillance for the emergence of the use of fentanyl and its analogues. In January 2020 inclusion criteria were further broadened to include use of any psychoactive substance and any severity of toxicity. Inclusion and exclusion criteria are assessed by local research teams under the supervision of a principal investigator at that site (an ED physician or clinical toxicologist), in consultation with the treating clinical team.
This study has ethical approval and participants’ written consent is required for inclusion. Those lacking capacity for at least six hours after presentation can be included on the advice of a personal (e.g., family member) or professional (e.g., member of staff who is not involved in the study) consultee. On recovery of capacity, these patients are asked to provide consent; their data and samples are not retained if they decline.
Anonymised demographic data including age, sex and partial postcode (missing the final 2 digits to increase anonymity), clinical details (reported exposures as documented by the clinical team, admission observations, clinical features recorded during hospital stay, treatments administered, laboratory results, clinical outcomes) and linked biological samples (blood or urine with dates and times of collection) are recorded using a standardised data collection sheet and consistent methodology. Further clinical details can subsequently be requested from the clinical records, when necessary, without compromising anonymity.
Data and sample collection and processing
A code unique to each participant is used to identify biological samples and clinical data, allowing linkage of patient specific clinical and analytical information without compromising anonymity. Clinical information is sent by secure email to the study centre (Newcastle University). Biological samples (blood, plasma, serum, urine) collected at research sites are refrigerated prior to transfer to one of the analytical laboratories by first class post at ambient temperature. If this is delayed, samples are frozen (−20 °C or colder) pending transfer. The analysing laboratory passes on analytical results to the study centre, where the information is amalgamated with the previously provided clinical information. No identifiable patient information is sent to the laboratories or study centre.
Biological sample analysis
From March 2015 to September 2019 clinical samples from England and Wales were analysed in the Medical Toxicology Centre at Newcastle University, while those from Scotland were analysed at the Scottish Police Authority Forensic Laboratory in Edinburgh. Subsequently, all samples have been analysed by a single forensic laboratory, LGC Ltd in Fordham, Cambridgeshire. All laboratories used a similar methodology, analysing samples using high-resolution accurate-mass liquid chromatography-mass spectrometry (HRAM LCMS). Details of the methods used have been published previously [Citation22,Citation23]. The analytical data are currently generated using two validated methods, both accredited by the UK Accreditation Service (UKAS) and based on a full scan HRAM LCMS with All Ion Fragmentation (AIF) and data dependent MS2 based on an inclusion list containing all the analytes in the accurate mass databases supporting each method. The data are processed automatically using Thermo Tracefinder software using a mass tolerance of ± 1 mDa, a retention time window of ± 15 s compared to the reference retention time (where known), full scan HRAM data dependent MS2 spectra for matching against in house libraries and online libraries (e/g mzcloud), AIF fragment ions where data dependant MS2 spectra are not generated and isotopic matching of the molecular ion of unknown component against that generated from the elemental composition of the hit. The hits are then reviewed manually before reporting.
The in-house databases contain most NPS reported to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Early Warning System and other accessible forensic drug networks. For example, at the time of analysis the database contained 1176 synthetic cannabinoids and metabolites, both actual (where possible) and theoretical. For this research, retrospective review of HRAM data was not performed. This was considered unnecessary because the databases used were updated as soon as new drugs were reported internationally.
“Open” screening methods were used, but the limits of detection (LOD) of compounds representative of the major groups of NPS were calculated according to the approach recommended in the Lab 51 standards of the UK Accreditation Service (UKAS) [Citation24] and are provided in the supplementary material (Table S4). These are statistically derived LOD and as such, findings below the LOD where the analyte in question is present are still reported.
Prior to 2020, the analytical methods did not reliably detect gamma hydroxybutyrate (GHB) and related compounds or tetrahydrocannabinol (THC), so these compounds have been excluded from our statistical analysis.
In our primary analysis, the presence of metabolites was considered evidence of exposure to the parent drug. These included all SCRA metabolites, major cocaine metabolites (benzoylecgonine, norcocaine and ecgonine methyl ester) and the 3,4-methylenedioxymethamphetamine (MDMA) metabolite 3,4-Methylenedioxyamphetamine (MDA). The presence of monoacetyl morphine, morphine, or morphine conjugates (henceforward referred to as selected heroin/morphine metabolites) was considered evidence of heroin (diacetylmorphine) exposure. A separate analysis was performed considering only the parent drug for cocaine, because of the possibility that metabolites, some of which have long half-lives, might persist from an earlier episode of drug use.
Participants
IONA participants were eligible for inclusion in this specific research unless there was no reported exposure to any specific substance or exposure to more than one substance, the reported exposure was to an unknown or unidentifiable substance (including unspecified white powders), or the reported exposure was to a substance likely to contain more than one active ingredient, e.g., “snowball” or “speedball.”
We considered in detail the accuracy of the exposure history in those reporting use of SCRA as this was the focus of the study. For comparison we also planned to analyse data for substances where there were at least 50 patients with self-reported exposure. This number choice was arbitrary as we did not have any data on which to base a formal power calculation. Reported SCRA exposures included exposure to “spice” or products previously demonstrated to contain SCRA such as “Mamba,” “Pandora’s box,” “Vertex,” “Red Exodus,” etc. MDMA exposures included those where the use of “ecstasy,” “Molly,” or products known to contain MDMA (e.g., Ikea logo pills) was reported. Cocaine use included exposure to “powder cocaine” or “crack.”
Patient information was further categorized according to reported exposure routes (oral, insufflated/snorted, smoked, subcutaneous, intramuscular, inhaled, intravenous, buccal, and sublingual, multiple routes or routes unknown). Those reporting two routes of exposure were counted for both reported routes. Age and sex distribution were assessed for those reporting SCRA, heroin, cocaine, and MDMA use.
Statistical analysis
Data were expressed as frequencies and percentages or medians with interquartile ranges (IQRs). In analysing the accuracy of the patient exposure history, the history obtained from the patient or relative/witness, as recorded in the medical records, was considered analogous to the finding of an investigation and its accuracy is compared against the findings of sample analysis, which was considered the “gold standard.” Participants were classified into 4 groups for each substance analysed as follows: (a) substance reported and detected in at least one patient sample on analysis (“true positive”), (b) substance reported but not detected in any sample (“false positive”), (c) Substance not reported but detected in at least one patient sample (“false negative”) and (d) substance not reported and not detected in any sample “(true negative”). From these values sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), were calculated using a freely available online calculator [Citation25]. These terms are explained in more detail in the supplementary material (Table S1). Of these, the sensitivity and PPV were considered the most clinically important. For the included drug categories, sub-analyses were performed to determine the effect of considering the reported route of exposure along with the reported substance use. Note that those reporting use of a substance where that substance was also detected were counted as true positives for that substance, even if other substances were also detected.
Results
Clinical and analytical data were available for 1168 patients recruited between March 2015 and July 2021. Of these, 474 (median age 33 years, range 16–75; 80% male) reported use of a single illicit drug and were included in this research. The substances most commonly reported by patients as being involved and those detected on sample analysis were SCRA, selected heroin/morphine metabolites cocaine and MDMA (). The age and sex distribution of those using these substances and the routes of exposure reported are detailed in . The specific SCRA identified over the course of the study and the numbers of patients exposed to each were as follows: 5 F-MDMB-PINACA (5 F-ADB, n = 53), AMB-FUBINACA (FUB-AMB, n = 29), MDMB-CHMICA (n = 25), MDMB-4en-PINACA (n = 20), AB-FUBINACA (n = 13), 5 F-AMB (n = 12), 5 F MDMB-PICA (n = 9), 5 F-PB-22 (n = 9), 5 F-NPB-22 (n = 7), 4 F-MDMB-BUTINACA (4 F-MDMB-BINACA) (n = 7), 5 F-AKB-48 (n = 5), AB-CHIMINACA (n = 5), FUB-PB-22 (n = 3), 5 F-AMB-PICA (n = 2), AMB-CHMICA (n = 2) and 4 F-MDMB-BICA, ADB-HEXINACA, FUB NPB −22, AM-1248, 5 F-AEB, and AKB-48 4 F (each n = 1).
Table 1. Number and percentage of included participants reporting use of specific compoundsa at the time of admission and numbers in which these substances were detected by HRAM LCMS in at least one sample.
Table 2. Numbers of patients reporting single substance use by age group, sex and reported routes of exposure for the four most common reported drug types.
Detailed toxicology analysis
Multiple substances were detected on sample analysis in most patients (median 3, IQR 2, 5, ), even though inclusion was restricted to those where exposure to a single substance was reported. A larger number of substances were detected in those reporting exposure to heroin than those reporting SCRA, cocaine, or MDMA (all p < 0.001, ). Those reporting heroin use were also more likely to be exposed to codeine compared to those reporting SCRA, cocaine, or MDMA and were also commonly exposed to SCRA and cocaine ().
Figure 1. Numbers of separate substances identified by analysis in all those reporting single drug exposure (Panel A) and those reporting SCRA (Panel B), heroin (Panel C), cocaine (Panel D) and MDMA (Panel E).
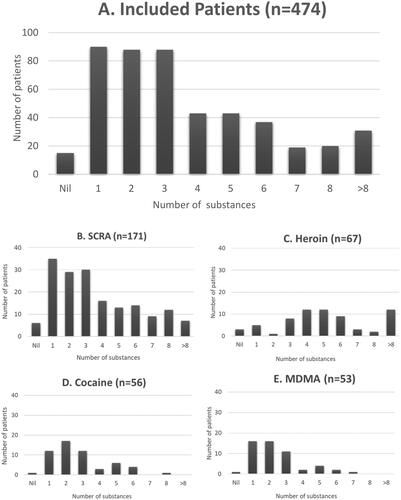
Table 3. Most commonly detected substances in biological samples of patients reporting use of a single drug when this was a SCRA, cocaine, heroin or MDMA.
Accuracy of patient exposure history
Sufficient patient numbers were available to assess the accuracy of the reported history of exposure for SCRA (n = 171), cocaine (n = 56), heroin (n = 65) and MDMA (n = 53) and results are shown in . Patient numbers for other substances were too low (n < 50) for reliable analysis. The high numbers reportedly exposed to SCRA reflect the focus of the study on suspected NPS toxicity until 2020.
Table 4. Accuracy of reported exposure to SCRA, cocaine, heroin or MDMA in 473 patients reporting single substance exposure and attending emergency departments with toxicity.
There were 121 patients who reported use of a SCRA and where a SCRA was also detected on sample analysis (“true positives”), 273 who did not report SCRA use and where SCRA were not found on sample analysis (“true negatives”), 30 where a SCRA was detected in samples without being recorded in the exposure history (false negatives, sensitivity 121/151 or 80%) and 50 who reported SCRA use without these compounds being detected on analysis (false positives). The PPV of the history was therefore 121/171 or 71% ().
Fewer patients in this series reported the use of cocaine, heroin or MDMA. These substances (or metabolites) were detected in many patients who did not report their use and the calculated sensitivity of the history was lower for these substances, especially cocaine (26.7%) and heroin (38.0%). For cocaine and MDMA, a high proportion of those reporting use had that substance (or metabolites) present on sample analysis (PPV 82.1% for cocaine and 83% for MDMA). For cocaine, however, of the 172 participants considered exposed on the basis of the analytical detection of cocaine or its metabolites, only 70 had the parent drug present in biological samples. Repeating the statistical analysis considering patients as exposed only if cocaine itself was present improved the sensitivity of the history, but this remained low and PPV was reduced substantially (). Considering a specific and commonly reported route of exposure for each substance improved the accuracy of the patient history as a reflection of analytical exposure. For example, a history of SCRA use by smoking was more sensitive and provided a slightly higher PPV than a history of SCRA use when the exposure route was not taken into consideration. Those patients who did not report smoking as the route of exposure were less likely to have SCRA detected in their biological samples. For the other three drugs, considering a primary common route of drug use increased sensitivity and NPV, with little effect on values for specificity (which were already high) or PPV ().
Discussion
In this research we have compared the recorded history of exposure with the results of detailed sample analysis in patients attending EDs in the UK after suspected use of a single substance. The results demonstrate that it is very common for multiple undeclared substances to be present; this is important because these undeclared substances may contribute to the clinical presentation and progress of the patients involved.
The majority of those with analytical evidence of exposure to SCRA also gave a history of SCRA use and calculated sensitivity in this study (80%) was higher than previously reported in a smaller cohort from London (50%) [Citation16]. However, almost 30% of those who gave a history of SCRA use did not have a SCRA detected in their samples (PPV 71%). Lower sensitivities applied to the exposure history for MDMA, heroin and especially cocaine, with results for cocaine consistent with those previously reported for cocaine or methamphetamine in patients presenting with chest pain [Citation14]. It is possible that patients are more reluctant to report the use of these compounds because of their legal status or associated stigma. MDMA, heroin and cocaine were all controlled as class A drugs, the UK’s highest legal classification, throughout the study. In contrast, some SCRAs were uncontrolled until 2016 and those that have been controlled are classified in the lower-class B category.
For cocaine, heroin or MDMA, the history of exposure demonstrated higher specificity than SCRA. This means that the proportion of those who were not exposed analytically but who had reported use of the substance in question was higher for SCRA than for cocaine, heroin or MDMA.
If commonly used routes of administration were also considered, sensitivity was improved for all four drugs or groups tested.
There are several reasons why patients may not report all the drugs that they have used or may report drugs that are not found on analysis. Intoxication may impair memory or cause confusion, making a reliable history more difficult to obtain. Witnesses who have further information may not be available in the ED. There may be a reluctance to report because of concerns about the confidentiality of medical records. Patients may also be unaware of the precise contents of preparations they have used as the constituents of illicit drugs may vary [Citation26–29]. For example, they may have been sold a product they believe to contain a specific drug when it contains others, or sometimes is an inert material [Citation30,Citation31].
It is also possible that some drugs or metabolites detected have persisted from an earlier episode of drug use that the patient does not consider relevant, and which may not be making an important contribution to the current clinical picture. This was a possible explanation for the very low sensitivity documented for cocaine, for which the benzoylecgonine metabolite may persist for several days, especially after repeated use [Citation32]. Sensitivity, however, remained low even if only the presence of the parent drug was considered as evidence of exposure and PPV was reduced substantially. For heroin, metabolites may have arisen from the consumption of other opioids and not heroin, such as codeine [Citation33] or poppy seeds. Morphine and its metabolites might also be detected as a result of therapeutic use, although prescribing would be unusual in those with opioid use disorders and no patients with positive samples had morphine or codeine administered in hospital prior to sample collection. Our methods, assuming that morphine and its metabolites arise exclusively from heroin exposure, may therefore result in some over-diagnosis, explaining, at least in part, the low observed sensitivity. Quantification of drugs was not possible in this study, so some analytical findings may reflect drugs at concentrations too low to be clinically relevant, for example due to unintended contamination of drug product, e.g., during the process of cutting, as well as arising from a previous episode of drug misuse. Conversely, other substances may disappear quickly so they may not be detected if there is a delay between exposure and sample collection, especially for those patients where urine samples were not provided. It is also possible that some drugs that were used were not detected because they were not included in our drug screen, or because concentrations fell below the limit of sensitivity of the assays used, especially if the interval between use and sampling was long. In particular, analytical methods used prior to 2020 were not able to detect the evidence of cannabis (i.e., presence of carboxy THC) or gamma hydroxybutyrate (GHB) and related compounds. Otherwise, the study has used a very extensive screening panel which includes almost all recently detected NPS and it is unlikely that clinically significant concentrations would not be detected in view of the high sensitivity of the mass spectrometry methods that were used (Table S4). We cannot exclude the possibility, however, that some highly potent substances may not have been detected at clinically important concentrations.
The care and time taken by the clinician to take the history may also affect accuracy; while more prolonged or repeated attempts to obtain a comprehensive exposure history may improve accuracy, this is often impractical due to other work pressures in busy EDs. In particular, information on previous drug use is often not collected. The data used here reflect the quality of information obtained in real-world settings across many EDs and the large number contributing data means findings are likely to be generalizable to other hospitals, although it is possible that the collection of data in the context of a research study may have improved data quality.
The study has further limitations that should be considered. For simplicity, we considered only those patients reporting a single substance exposure. Those who report use of multiple substances might provide less accurate histories because of the complexity of their exposure. The inclusion criteria have been limited to specific groups and recruiting hospitals and have changed during the data collection period, with a focus on substance users where NPS exposure is suspected. The selection of participants is not random and depends on the capacity of research staff and the willingness of patients to consent, so results may not be representative of all substance users attending EDs. Finally, samples were taken opportunistically, with timing in relation to substance use and the types of a sample taken (urine, blood) differing between patients.
In conclusion, we have demonstrated important inconsistencies between the reported history of drug use and drug exposure as detected by sample analysis in those attending EDs after substance use, specifically SCRA, cocaine, heroin and MDMA. Of particular importance, multiple undeclared substances were commonly present even though the research was restricted to those reporting single drug use. Clinicians need to be aware of this when planning appropriate monitoring and treatment, with management based on evolving toxidromes, irrespective of the history of exposure. The research also emphasises the potential value of sample analysis as part of the public health surveillance of illicit substance use, including NPS.
Supplemental Material
Download MS Word (30.9 KB)Acknowledgements
We acknowledge with gratitude the work done by the research staff in all IONA sites and thank all the participants for allowing their data and samples to be used for this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Binks S, Hoskins R, Salmon D, et al. Prevalence and healthcare burden of illegal drug use among emergency department patients. Emerg Med J. 2005;22(12):872–873.
- Vivolo-Kantor AM, Hoots BE, Scholl L, et al. Nonfatal drug overdoses treated in emergency departments - United States, 2016–2017. MMWR Morb Mortal Wkly Rep. 2020;69(13):371–376.
- Nelson ZJ, Stellpflug SJ, Engebretsen KM. What can a urine drug screening immunoassay really tell Us? J Pharm Pract. 2016;29(5):516–526.
- Deville M, Bailly R, Gauthier N, et al. Biochip array technology for new psychoactive substances detection in biological samples: evaluation of the specificity of the Randox Evidence Investigator®. Ann Clin Biochem. 2022;59(5):357–362.
- Magura S. Validating self-reports of illegal drug use to evaluate National Drug Control Policy: a reanalysis and critique. Eval Program Plann. 2010;33(3):234–237.
- Hamid R, Deren S, Beardsley M, et al. Agreement between urinalysis and self-reported drug use. Subst Use Misuse. 1999;34(11):1585–1592.
- Colón HM, Robles RR, Sahai H. The validity of drug use self-reports among hard core drug users in a household survey in Puerto Rico: comparison of survey responses of cocaine and heroin use with hair tests. Drug Alcohol Depend. 2002;67(3):269–279.
- Fendrich M, Johnson TP, Wislar JS, et al. The utility of drug testing in epidemiological research: results from a general population survey. Addiction. 2004;99(2):197–208.
- Harrison LD, Martin, SS, Enev T, Harrington, D. Comparing drug testing and self-report of drug use among youths and young adults in the general population (DHHS Publication No. SMA 07-4249, Methodology Series M-7). Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies. 2007. Available from: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.182.9980&rep=rep1&type=pdf
- Fendrich M, Mackesy-Amiti ME, Johnson TP. Validity of self-reported substance use in men who have sex with men: comparisons with a general population sample. Ann Epidemiol. 2008;18(10):752–759.
- Hunt DE, Kling R, Almozlino Y, et al. Telling the truth about drug use: How much does it matter? J Drug Issues. 2015;45(3):314–329.
- Li DH, Janulis P, Mustanski B. Predictors of correspondence between self-reported substance use and urinalysis screening among a racially diverse cohort of young men who have sex with men and transgender women. Addict Behav. 2019;88:6–14.
- Pohjola-Sintonen S, Kivistö KT, Vuori E, et al. Identification of drugs ingested in acute poisoning: correlation of patient history with drug analyses. Ther Drug Monit. 2000;22(6):749–752.
- Lee MO, Vivier PM, Diercks DB. Is the self-report of recent cocaine or methamphetamine use reliable in illicit stimulant drug users who present to the Emergency Department with chest pain? J Emerg Med. 2009;37(2):237–241.
- Monte AA, Heard KJ, Hoppe JA, et al. The accuracy of self-reported drug ingestion histories in emergency department patients. J Clin Pharmacol. 2015;55(1):33–38.
- Abouchedid R, Hudson S, Thurtle N, et al. Analytical confirmation of synthetic cannabinoids in a cohort of 179 presentations with acute recreational drug toxicity to an Emergency Department in London, UK in the first half of 2015. Clin Toxicol (Phila). 2017;55(5):338–345.
- Tebo C, Mazer-Amirshahi M, DeGeorge L, et al. Suspected synthetic cannabinoid receptor agonist intoxication: does analysis of samples reflect the presence of suspected agents? Am J Emerg Med. 2019;37(10):1846–1849.
- Vallersnes OM, Persett PS, Øiestad EL, et al. Underestimated impact of novel psychoactive substances: laboratory confirmation of recreational drug toxicity in Oslo, Norway. Clin Toxicol (Phila). 2017;55(7):636–644.
- Monte AA, Hopkinson A, Saben J, et al. The Psychoactive Surveillance Consortium and Analysis Network (PSCAN): the first year. Addict Abingdon Engl. 2020;115(2):270–278.
- Trecki J, Gerona RR, Schwartz MD. Synthetic cannabinoid-related illnesses and deaths. N Engl J Med. 2015;373(2):103–107.
- Hill SL, Najafi J, Dunn M, et al. Clinical toxicity following analytically confirmed use of the synthetic cannabinoid receptor agonist MDMB-CHMICA. A report from the Identification of Novel psychoActive substances (IONA) study. Clin Toxicol (Phila). 2016;54(8):638–643.
- Hill SL, Dunn M, Cano C, et al. Human toxicity caused by indole and indazole carboxylate synthetic cannabinoid receptor agonists: from horizon scanning to notification. Clin Chem. 2018;64(2):346–354.
- Haden M, Cashman J, Ketchin A, et al. Detection of flubromazolam in patients with suspected non-medical drug use attending emergency departments in the United Kingdom. Clin Toxicol. 2022;60:33–37.
- United Kingdom Accreditation Service. UKAS accreditation of laboratories performing analysis of toxicology samples [Internet]. [cited 2022 Aug 11]. Available from: 2021;https://www.ukas.com/wp-content/uploads/2021/06/LAB-51-UKAS-Accreditation-of-Laboratories-Performing-Analysis-of-Toxicology-Samples.pdf
- MedCalc Software Ltd. Diagnostic test evaluation calculator [Internet]. [cited 2022 Mar 15]. Available from: https://www.medcalc.org/calc/diagnostic_test.php
- Araújo AM, Valente MJ, Carvalho M, et al. Raising awareness of new psychoactive substances: chemical analysis and in vitro toxicity screening of “legal high” packages containing synthetic cathinones. Arch Toxicol. 2015;89(5):757–771.
- Brunt TM, Nagy C, Bücheli A, et al. Drug testing in Europe: monitoring results of the Trans European Drug Information (TEDI) project. Drug Test Anal. 2017;9(2):188–198.
- Davies S, Wood DM, Smith G, et al. Purchasing ‘legal highs’ on the Internet—is there consistency in what you get? QJM. 2010;103(7):489–493.
- Vogels N, Brunt TM, Rigter S, et al. Content of ecstasy in The Netherlands: 1993–2008. Addiction. 2009;104(12):2057–2066.
- Baron M, Elie M, Elie L. An analysis of legal highs: do they contain what it says on the tin? Drug Test Anal. 2011;3(9):576–581.
- Ti L, Tobias S, Maghsoudi N, et al. Detection of synthetic cannabinoid adulteration in the unregulated drug supply in three Canadian settings. Drug Alcohol Rev. 2021;40(4):580–585.
- Jufer RA, Wstadik A, Walsh SL, et al. Elimination of cocaine and metabolites in plasma, saliva, and urine following repeated oral administration to human volunteers. J Anal Toxicol. 2000;24(7):467–477.
- Braithwaite RA, Jarvie DR, Minty PS, et al. Screening for drugs of abuse. I: opiates, amphetamines and cocaine. Ann Clin Biochem. 1995;32(2):123–153.
