Abstract
Introduction
Intestinal toxicity can occur following ingestion of various drugs, chemicals, and toxins. Intestinal fatty acid binding protein is a cytosolic protein specific to intestinal epithelial cells released into the systemic circulation following intestinal injury. Understanding intestinal toxicity in poisoning has the potential to explain mechanisms of toxicity and gastrointestinal symptoms.
Methods
Plasma samples were retrospectively analysed for intestinal fatty acid binding protein in 25 healthy controls and in those poisoned with Gloriosa superba (n = 18), Thevetia peruviana (n = 26), organophosphates (in various solvents) (n = 17), paracetamol (n = 14), glyphosate (n = 20), 2-methyl-4-chlorophenoxyacetic acid (n = 18) and propanil (n = 19).
Results
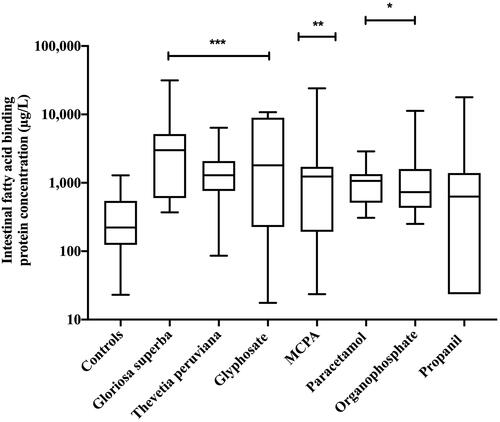
Median peak plasma intestinal fatty acid binding protein concentrations were significantly higher in patients poisoned with Gloriosa superba (2,994.1 µg/L; interquartile range 600.0–5,158.2, P < 0.001), Thevetia peruviana (1,292.5 µg/L; interquartile range 760.3 − 2,076.2; P < 0.001), glyphosate (1,803.6 µg/L; interquartile range 225.7–8,927.7; P < 0.001), 2-methyl-4-chlorophenoxyacetic acid (1,236.2 µg/L; interquartile range 192.6 − 1,709.7; P = 0.010), paracetamol (1,066.5 µg/L; interquartile range 512.9 − 1,336.9; P = 0.035), and organophosphate poisoning (729.8 µg/L; interquartile range 431.5 − 1,588.2; P = 0.046) than in healthy controls (221.6 µg/L; interquartile range 134.8 − 460.1). Median intestinal fatty acid binding protein was not statistically significantly increased compared to controls after propanil poisoning (630.0 µg/L; interquartile range 23.5 − 1,390.3; P = 0.423).
Conclusions
Our pilot study describes intestinal injury assessed by elevated plasma intestinal fatty acid binding protein concentrations following the ingestion of several poisons. This serves as a foundation for further exploration into enterocyte damage in toxicology.
Introduction
Intestinal toxicity following poisoning is not frequently quantified, and its contribution to overall toxicity remains poorly understood in human studies. Emerging biomarkers offer new avenues for assessing enterocyte damage and its implications in toxicological presentations.
Intestinal fatty acid binding protein, a cytosolic protein specific to intestinal epithelial cells, is found mainly in the duodenum and jejunum [Citation1]. Following intestinal injury, intestinal fatty acid binding protein is rapidly released into the systemic circulation. It has a short half-life in the plasma compartment of 11 min [Citation2] and thus enables real-time detection of intestinal injury. Its high specificity and abundance in the intestinal mucosa make intestinal fatty acid binding protein a potential biomarker for assessing intestinal toxicity. Enzyme-linked immunosorbent assay (ELISA) allows easy measurement, and the stability of intestinal fatty acid binding protein in plasma and urine permits testing in stored samples [Citation1,Citation3]. Reference ranges for intestinal fatty acid binding protein vary based on the laboratory assay method and tested population groups. Broadly, plasma intestinal fatty acid binding protein concentrations range from 400 µg/L to over 3,000 µg/L in inflammatory bowel disease, from 1,000 µg/L to over 5,000 µg/L in mesenteric ischemia, and from 50 µg/L to 200 µg/L in healthy controls [Citation1].
We conducted a pilot study with the objective of evaluating intestinal injury induced by various drugs, chemicals and toxins. We utilised plasma intestinal fatty acid binding protein as a potential biomarker to assess intestinal toxicity in toxicological presentations.
Methods
Study design and sample selection
We conducted a retrospective observational cohort study on patients with serial plasma samples after poisonings with Gloriosa superba (n = 18), Thevetia peruviana (n = 26), organophosphate in various solvents (n = 17), paracetamol (n = 14), glyphosate (n = 20), 2-methyl-4-chlorophenoxyacetic acid (MCPA) (n = 18) and propanil (n = 19). These were the remaining samples from previously published studies [Citation4–6]. We compared values to those from 25 healthy controls recruited at a similar time. Samples from patients with mixed overdoses or known gastrointestinal conditions (coeliac disease, inflammatory bowel disease and active peptic ulcer disease) were excluded.
We enrolled patients over 18 years of age with confirmed poisonings at three toxicology centres in Sri Lanka. Written consent for blood sample collection and clinical information was obtained from patients, and the patients consented to the future use of their blood specimens. The research protocol was approved by the Ethical Review Committee, Faculty of Medicine University of Peradeniya, and University of Sydney 2017/EC/79.
Data collection
Demographic data, including age, sex, time of presentation from ingestion, clinical symptoms and baseline blood tests, were collected on admission and information was stored in a password-protected database. Blood samples (5-10 mL) were collected at admission and at various time points during hospitalisation. After centrifugation, plasma was obtained, initially frozen to −20 °C within 1-2 days, and within two weeks stored at −80 °C. Sample selection within each tested drug, chemical or toxin was based on sample adequacy.
Measurement of plasma intestinal fatty acid binding protein
Plasma intestinal fatty acid binding protein was measured using an ELISA with kits from Hycult Biotechnology, Netherlands. Serial samples were assayed in duplicate, and standard operating procedures were followed as per the manufacturer’s recommendations.
Statistical analysis
Statistical analysis was conducted using Prism GraphPad 7.0b (GraphPad Software, La Jolla, CA). Continuous data are presented as median and interquartile range (IQR), while categorical data are reported as numbers and percentages. Comparisons between groups were performed using ANOVA or non-parametric Kruskal-Wallis test.
Results
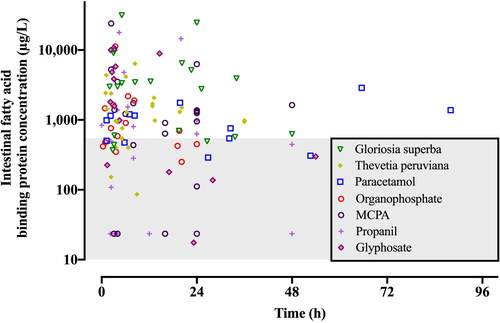
Median peak plasma intestinal fatty acid binding protein concentrations () were significantly higher in patients poisoned with Gloriosa superba (2,994.1 µg/L; IQR 600.0 – 5,158.2, P < 0.001), Thevetia peruviana (1,292.5 µg/L; IQR 760.3 − 2,076.2; P < 0.001), glyphosate (1,803.6 µg/L; IQR 225.7 – 8,927.7; P < 0.001), MCPA (1,236.2 µg/L; IQR 192.6 − 1,709.7; P = 0.010), paracetamol (1,066.5 µg/L; IQR 512.9 − 1,336.9; P = 0.035), and organophosphate poisoning (729.8 µg/L; IQR 431.5 − 1,588.2; P = 0.046) than in healthy controls (221.6 µg/L; IQR 134.8 − 460.1). Median plasma intestinal fatty acid binding protein concentrations were not statistically significantly increased compared to controls after propanil poisoning (630.0 µg/L; IQR 23.5 − 1390.3; P = 0.423). Peak plasma concentrations of intestinal fatty acid binding protein relative to time varied by drug, chemical or toxin ().
Discussion
Intestinal toxicity after acute poisoning can be measured using biomarkers such as intestinal fatty acid binding protein, marking the initial phase for studying the impact of drugs, chemicals and toxins on the gastrointestinal system.
Colchicine is known to cause intestinal injury in overdose. Gloriosa superba contains high concentrations of colchicine [Citation4]. The intestinal fatty acid binding protein concentrations in patients with Gloriosa superba are comparable to those in mesenteric ischemia. Thevetia peruviana contains highly toxic cardiac glycosides and causes prominent gastrointestinal symptoms after poisoning [Citation7]. Elevation in plasma intestinal fatty acid binding protein concentrations of this magnitude has been associated with increased intestinal permeability [Citation8]. This raises the possibility that intestinal injury associated with Thevetia peruviana poisoning may increase cardiac glycoside absorption and subsequent toxicity.
Enterocyte damage leading to increased serum drug concentrations has been shown in pharmacokinetic modelling studies involving paracetamol and patients with peptic ulcer disease [Citation9]. Intestinal toxicity in paracetamol overdose is plausible given that at high gut concentrations, paracetamol is a weak reversible inhibitor of both cyclooxygenase isoforms [Citation10]. Intestinal toxicity may alter drug absorption following overdose and may contribute to early metabolic acidosis.
Raised plasma intestinal fatty acid binding protein concentrations in glyphosate poisoning suggest potential intestinal injury. Animal studies have shown that glyphosate exposure leads to decreased messenger ribonucleic acid expression of zonula occludens-1 and claudin-1; both are critical enterocyte tight junction proteins that maintain intestinal integrity [Citation11].
Organophosphate poisoning has prominent gastrointestinal manifestations, and there are case reports of intestinal micro-perforations following ingestion [Citation12]. As a result, elevated plasma intestinal fatty acid binding protein concentrations would be anticipated. The variability in plasma intestinal fatty acid binding protein concentrations within this group could be attributed to differences in the ingested quantity or the presence of specific solvents rather than a general class effect.
Non-specific gastrointestinal symptoms are common (44%) after MCPA herbicide poisoning, and this may be due to the organic solvents or emulsifiers used rather than the MCPA compound itself [Citation13]. The wide variation in plasma intestinal fatty acid binding protein concentrations supports a possible effect specific to some products. Propanil, another herbicide, does not typically cause significant gastrointestinal effects, and hence, the lack of raised plasma intestinal fatty acid binding protein concentrations was not unexpected.
Conclusions
Our pilot study describes elevated plasma intestinal fatty acid binding protein concentrations in several poisonings. Intestinal fatty acid binding protein holds potential as a gut-specific biomarker that can be used in clinical and research settings. Larger studies focussing on specific drugs, chemicals or toxins that correlate the extent of intestinal fatty acid binding protein elevation with specific outcomes are needed to establish conclusive findings.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Additional information
Funding
References
- Piton G, Capellier G. Biomarkers of gut barrier failure in the ICU. Curr Opin Crit Care. 2016; 22(2):152–160. doi: 10.1097/MCC.0000000000000283.
- Derikx JP, Luyer MD, Heineman E, et al. Non-invasive markers of gut wall integrity in health and disease. World J Gastroenterol. 2010;16(42):5272–5279. doi: 10.3748/wjg.v16.i42.5272.
- Blaser A, Padar M, Tang J, et al. Citrulline and intestinal fatty acid-binding protein as biomarkers for gastrointestinal dysfunction in the critically ill. Anaesthesiol Intensive Ther. 2019;51(3):230–239. doi: 10.5114/ait.2019.86049.
- Wijerathna TM, Gawarammana IB, Mohamed F, et al. Epidemiology, toxicokinetics and biomarkers after self-poisoning with gloriosa superba. Clin Toxicol. 2019;57(11):1080–1086. Novdoi: 10.1080/15563650.2019.1581939.
- Mohamed F, Endre ZH, Pickering JW, et al. Mechanism-specific injury biomarkers predict nephrotoxicity early following glyphosate surfactant herbicide (GPSH) poisoning. Toxicol Lett. 2016;258:1–10. doi: 10.1016/j.toxlet.2016.06.001.
- Wijerathna TM, Buckley NA, Gawarammana IB, et al. Epidemiology and renal injury following 2-methyl-4-chlorophenoxyacetic acid (MCPA) poisoning. Sci Rep. 2022;12(1):21940. doi: 10.1038/s41598-022-25313-z.
- Rajapakse S. Management of yellow oleander poisoning. Clin Toxicol. 2009; 47(3):206–212. doi: 10.1080/15563650902824001.
- Rahman SH, Ammori BJ, Holmfield J, et al. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg. 2003;7(1):26–36. Jandoi: 10.1016/S1091-255X(02)00090-2.
- Wojcicki J, Gawronska-Szklarz B. Pharmacokinetics of paracetamol in patients with gastric and duodenal ulcers. Pol J Pharmacol Pharm. 1984; 36(1):59–63.
- Catella-Lawson F, Reilly MP, Kapoor SC, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345(25):1809–1817. doi: 10.1056/NEJMoa003199.
- Qiu S, Fu H, Zhou R, et al. Toxic effects of glyphosate on intestinal morphology, antioxidant capacity and barrier function in weaned piglets. Ecotoxicol Environ Saf. 2020;187:109846. doi: 10.1016/j.ecoenv.2019.109846.
- Mahajan RK, Rajan SJ, Peter JV, et al. Multiple small intestine perforations after organophosphorous poisoning: a case report. J Clin Diagn Res. 2016;10(3):GD06–7.
- Roberts DM, Seneviratne R, Mohammed F, et al. Intentional self-poisoning with the chlorophenoxy herbicide 4-chloro-2-methylphenoxyacetic acid (MCPA). Ann Emerg Med. 2005;46(3):275–284. doi: 10.1016/j.annemergmed.2005.03.016.