Abstract
Introduction
Snakebite incidence varies across Europe. However, there is limited research from Central and Southeastern Europe. These regions are notable for the presence of the common European adder (Vipera berus) and the more venomous nose-horned viper (Vipera ammodytes). No standard European antivenom protocol exists. The aim was to assess the epidemiology and treatment of viper bites in this region, focusing on a comparison of bites from Vipera berus and Vipera ammodytes.
Methods
We conducted a prospective multicenter study in Central and Southeastern Europe from 2018 to 2020. This study included poison centres and toxicology-associated hospital wards in Poland, the Czech Republic, Slovakia, Hungary, Slovenia, Croatia, Serbia, and Bulgaria. The following data were collected: age, gender, Vipera species, snakebite site, clinical picture, laboratory results, Audebert’s clinical severity grading score, and antivenom therapy.
Results
The annual incidence of viper bites in Central and Southeast Europe was estimated at 2.55 bites per million population. Within their respective geographical distribution areas, the incidence of Vipera ammodytes bites (1.61 bites per million population) was higher than Vipera berus bites (1.00 bites per million population). Patients bitten by Vipera ammodytes more frequently reported local pain and developed thrombocytopenia. Antivenom treatment was more commonly administered in Vipera ammodytes bites (72%) compared to Vipera berus bites (39%). The incidence of Vipera ammodytes bites treated with antivenom within its geographical distribution area was three times higher than Vipera berus bites treated with antivenom (1.16 bites per million population versus 0.39 bites per million population). No deaths were reported.
Conclusions
The estimated incidence of viper bites in Central and Southeastern Europe is at least 2.55 per million population. Vipera ammodytes bites are more common and severe, characterized by higher frequencies of pain and thrombocytopenia. Antivenom is needed more often for Vipera ammodytes bites. It is vital that enough European Medicines Agency-approved Vipera ammodytes antivenom is produced and offered affordably.
Introduction
Snakebite is a relatively rare event in Europe, but it can pose a significant threat. Across Europe, snakebites affect 4–11 people per million population per year, but we must be cautious when assessing viper bites in Europe, as the majority of epidemiological studies have been conducted in Northern, Western, and Southern Europe [Citation1]. The focus of these studies has primarily been on bites from the common European adder (Vipera berus) () and, to a lesser extent to, Vipera aspis and Vipera ursinii in Southern Europe. Unfortunately, there is a lack of comprehensive research on bites from Vipera ammodytes (nose-horned viper) (), which is found in Central and Southeastern Europe. These regions are particularly intriguing regions for snakebites, as they are home to both V.berus and the more venomous V. ammodytes.
Figure 1. Vipera berus (common European adder)(A) and Vipera ammodytes (nose horned viper)(B) (photo: M Brvar).
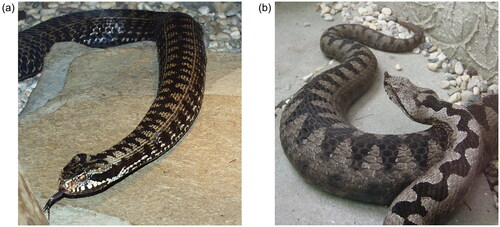
Vipera ammodytes stands out as the most dangerous among the European vipers, owing to its considerable size (length reaching up to 95 cm), long fangs (up to 13 mm), and potent venom characterized by its proteolytic, hemorrhagic, and neurotoxic properties [Citation2]. It primarily inhabits the Balkan Peninsula, where it is easily identifiable by its distinctive nose horn [Citation3].
Clinical manifestations of V. berus and V. ammodytes bites are similar and include local pain, swelling and bruising, and systemic symptoms, such as tachycardia and hypotension. The cardiotoxic effects of viper envenomation are primarily attributed to the action of the secretory phospholipase A2, which has a wide spectrum of toxic effects, including myotoxicity, cardiotoxicity, neurotoxicity, cytotoxicity and anticoagulation. The haemostatic system and vessels are primarily affected by other major toxins found in Vipera species, including snake venom serine proteases, metalloproteinases, snake c-type lectin-like proteins (snaclecs), and disintegrins [Citation4, Citation5].
The venom of V. berus is less complex in terms of its proteome compared to that of V. ammodytes [Citation6]. Specifically, it has lower concentrations of snaclecs and lacks neurotoxic phospholipases A2 (ammodytoxins) [Citation6]. The absence of ammodytoxins in V. berus venom explains the absence of neurotoxic symptoms that commonly occur after V. ammodytes envenomation, while snaclecs are likely to be the cause of thrombocytopenia, as they can induce platelet agglutination [Citation7, Citation8]. However, a direct comparison of the clinical and laboratory findings between V. berus and V. ammodytes envenomation has not yet been performed.
There is currently no standardized protocol for antivenom administration throughout Europe, resulting in variations from country to country. Furthermore, no antivenom has been approved by the European Medicines Agency [Citation9]. In the Southeast European region, the availability of antivenom is even more critical, as the antivenoms developed against V. berus venom are not sufficiently effective in treating severe cases of V. ammodytes bites. Therefore, specific antivenoms raised solely against V. ammodytes venom have been produced in certain Balkan countries.
The aim of this study was to evaluate the epidemiology and treatment of viper bites in Central and Southeastern Europe, with a specific focus on comparing bites caused by V. berus and V. ammodytes.
Methods
This was a prospective multicenter survey of snakebites from the Vipera spp. and the use of antivenom therapy set up in poison centres and toxicology-associated hospital wards across Central and Southeast Europe from 1 April 1 2018, to 30 November 30 2020. The study was organized by the Central European and Accession Countries Working Group of the European Association of Poison Centres and Clinical Toxicologists (EAPCCT).
Participating poison centres and toxicology departments
This study included poison centres and toxicology-associated hospital wards (e.g. toxicology or infectious disease departments) in several countries in Central and Southeast Europe, namely Poland, the Czech Republic, Slovakia, Hungary, Slovenia, Croatia, Serbia, and Bulgaria. However, some invited countries, such as Romania, North Macedonia and Greece, did not participate in the study. National data were provided by the national poison centres in the Czech Republic, Slovakia, Slovenia, Croatia, and Serbia. The poison centre in Łódź, Poland, and the toxicology departments in Budapest, Hungary, and Sofia, Bulgaria, provided data only for their respective service areas and not the entire country (). The population of the included countries and the areas covered by the poison centres and toxicology departments are shown in .
Figure 2. Central and Southeastern European countries included in the study [Citation10]). Legend: 
 local data provided by poison centres/toxicology departments
local data provided by poison centres/toxicology departments![Figure 2. Central and Southeastern European countries included in the study [Citation10]). Legend: Display full size national data provided by the national poison centres. Display full size local data provided by poison centres/toxicology departments](/cms/asset/7e104205-1b5c-4de4-af83-50ba2d6b0c29/ictx_a_2273761_f0002_c.jpg)
Table 1. Poison centres and toxicology departments included in the study, population covered by centres and departments, and reported viper bites (2018–2020).
Patients
The study included patients envenomated by native vipers who were either admitted to participating toxicology or toxicology-associated hospital departments or reported to participating poison centres (). Cases were only included if the viper envenomation was confirmed by visual identification of vipers, either by the patients themselves or by eyewitnesses or physicians using photographs. Alternatively, cases could be included if the bite clearly showed fang marks along with any typical local signs and symptoms (such as local swelling and bruising) occurring in areas inhabited by vipers, even if the patient did not have the opportunity to see and identify the snake. Dry snakebites in patients who were able to identify the snake as belonging to the genus Vipera were also included, except in Croatia.
However, patients bitten by non-native snake species (e.g. those kept in captivity) and clearly non-venomous snakes were excluded from the study. Similarly, patients who were bitten by an unidentified snake and did not develop any clinical symptoms were also excluded.
Data collection
Data were prospectively collected by using pre-designed Excel spreadsheets that were e-mailed to all participating centres and departments every three months for a period of three years. These spreadsheets included the following data on patients who were bitten by vipers: age; gender; Vipera species; site of the snakebite; local signs (pain, swelling, and bruising); systemic signs (vomiting, diarrhoea, fever, level of consciousness [somnolence or coma]); neurological symptoms (cranial nerve palsies, ataxia); tachycardia (heart rate greater than 100 beats per minute); hypotension (systolic blood pressure less than 90 mmHg) and dyspnoea; laboratory results including leukocytosis (white blood cell count greater than 10x109/L) and thrombocytopenia (platelet count less than 140x109/L); fatal outcome; severity score using Audebert’s clinical severity grading [Citation11] and administration of antivenom therapy (antivenom, number of doses, adverse reactions such as anaphylactic reactions or serum sickness). Data were obtained by telephone calls to poison centres or by review of discharge letters if they were admitted to associated toxicology departments or hospital wards.
Statistical analysis
Categorical variables are presented as frequencies (percentages) and odds ratios (ORs) with a 95% confidence interval (CI). The continuous variable (age) is presented as the mean and standard deviation, while the continuous non-parametric variable (severity score) is presented as the median and interquartile range (IQR). Fisher’s exact test was used for categorical data, the Mann-Whitney test was used for the non-parametric value, and unpaired t-test for the continuous variable to detect differences in the clinical presentation and laboratory findings between V. berus and V. ammodytes bites. A P value of 0.05 was considered statistically significant. Analyses were performed using IBM SPSS Statistics for Windows, Version 23.0, Armonk, NY, USA.
Results
The survey was conducted by eight poison centres and toxicology departments in eight countries, covering a total population of 38.9 million individuals (46% of the combined population of the included countries) ().
A total of 298 snakebites attributed to the Vipera species were documented (), resulting in an average of 2.55 bites per million population annually (). Antivenom treatment was administered in 125 cases, with an average of 1.07 treated viper bites per million population per year ().
Table 2. Incidence of viper bites and antivenom therapy in the service area of all participating poison centres and toxicology departments (38.9 millions) (2018–2020).
A total of 117 patients were bitten by V. berus in seven countries over the three years (), of whom 45 (38%) received antivenom treatment. The incidence of V. berus bites was 1.00 per million population, and of V. berus bites treated with antivenom 0.39 per million population ().
In Slovenia, Croatia, Serbia and Bulgaria, a total of 74 patients were bitten by V. ammodytes (), of whom 53 (72%) were treated with antivenom. The incidence of V. ammodytes bites was 0.63 per million population, and the incidence of V. ammodytes bites treated with antivenom was 0.45 per million population in the service areas of all participating poison centres and toxicology departments (38.9 millions) (). Bites by V. aspis and V. ursinii were not reported.
The incidence of V. ammodytes bites within its geographical distribution area (Slovenia, Croatia, Serbia and Bulgaria) was higher than the incidence of V. berus bites within its geographical distribution area (all countries included) (1.61 bites per million population per year versus 1.00 bites per million population per year, respectively) (). In addition, the incidence of V. ammodytes bites treated with antivenom within its geographical distribution area was almost three times higher than the incidence of V. berus bites treated with antivenom within its geographical distribution area (1.16 bites per million population per year versus 0.39 bites per million population per year, respectively) ().
Table 3. Incidence of Vipera berus and Vipera ammodytes bites and antivenom treatment within their respective geographic distribution areas (2018–2020).
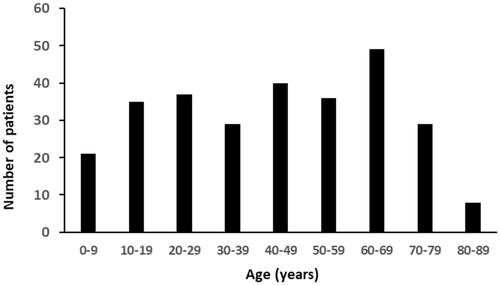
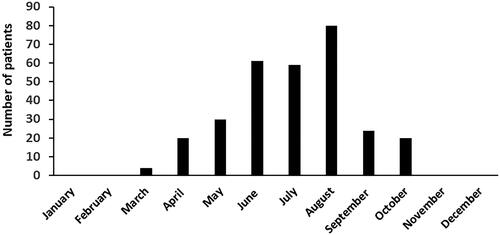
Patients bitten by V. ammodytes were older and more often bitten on their arms, whereas patients bitten by V. berus were more often bitten on the legs (). The age distribution of patients bitten by vipers and the monthly variation of viper bites are presented in and .
Table 4. General characteristics of patients bitten by Vipera berus and Vipera ammodytes.
The clinical presentation, laboratory findings, and treatment of V. berus and V. ammodytes bites are summarized in . Patients bitten by V. ammodytes more frequently reported experiencing local pain and developed thrombocytopenia (). The median severity score for V. ammodytes bites was 2/3, while it was 1/3 for V. berus bites, using the Audebert’s clinical severity score. Antivenom treatment with antivenom was more frequently administered to patients bitten by V. ammodytes (). No patient deaths were reported ().
Table 5. Symptoms and signs, laboratory results, treatment and outcome in Vipera ammodytes and Vipera berus bites.
Six different antivenoms were administered to patients bitten by vipers in eight countries between 2018 and 2020 (). ViperaTab® (MicroPharm Limited, Newcastle Emlyn, United Kingdom) and Viper venom antitoxin (Biomed Sera and Vaccines Manufacturer, Warsaw, Poland) were used in Poland, the Czech Republic, and Slovakia, where only V. berus bites occur. In Slovenia, Croatia, Serbia and Bulgaria with mixed populations of V. berus and V. ammodytes, antivenoms raised against V. ammodytes venom were predominantly used. European viper venom antiserum (Institute of Immunology Inc., Zagreb, Croatia), Bulbio snake venom antiserum (Bulbio, NCIPD ltd, Sofia, Bulgaria) and Viekvin® (Institute for Virology and Vaccines and Sera Torlak, Belgrade, Serbia) were used in Croatia, Bulgaria and Serbia, respectively, where they are/were produced. In Slovenia, Viperfav® was used for V. ammonodytes bites, while ViperaTab® was used when antivenoms specifically developed for V. ammonodytes were not available. However, up to four doses of ViperaTab® were required for V. ammodytes bites (). In all V. ammodytes bites, antivenoms raised against V. ammodytes venom were used in 87% of cases (). No adverse reactions related to antivenom administration were reported (). The route of administration was not collected in this study.
Table 6. Antivenoms used in Vipera berus, Vipera ammodytes and unspecified viper bites (2018–2020).
Discussion
In this study, the annual incidence of viper bites in Central and Southeastern Europe was 2.55 bites per million population; approximately half of these cases were given antivenom therapy (1.07 per million population). A meta-analysis predicted the annual incidence of native venomous snakebites (Vipera species) in Europe to be 10.6 per million population, with no significant differences between the incidence in the northern, central and southern regions of Europe [Citation1]. The observed discrepancy between the results of this study and the predicted incidence from the meta-analysis may be due to several factors. Firstly, it is possible that there was underreporting of viper bites in this study, as not all snakebite cases may have been treated in toxicology-associated hospital wards or reported to local poison centres. On the other hand, the model used in the meta-analysis may have overestimated the incidence by including data from several local studies [Citation1], since these studies may have included hospitals that treat a higher number of patients bitten by vipers due to their geographic location or specialization [Citation12]. Furthermore, the incidence of snakebites in countries such as Romania and Britain, where hospitals report snakebite cases to the national health services using a coding system, was found to be significantly lower—up to 5–10 times lower—than the incidence predicted by the meta-analysis [Citation12]. The average annual incidence of snakebites recorded in hospital registries across Europe was reported as 4.2 cases per million population, which is more in line with the incidence observed in this study [Citation12].
The Vipera spp. that is most commonly associated with snakebites in Europe is V. berus, accounting for 63.3% of cases [Citation13]. However, the proportion of V. berus bites in relation to all viper bites varies across different geographic areas and the presence of other Vipera species. This variation was also observed in this study, where the proportion of V. berus bites decreased from northern (100%) to southern (approximately 15%) countries. Interestingly, the incidence of V. ammodytes bites within its geographical distribution area was even higher (1.61 bites per million population) compared to the incidence of V. berus bites within its respective geographical distribution area (1.00 bites per million population). In this study, the patients and eyewitnesses were able to identify 64% of the vipers as either V. berus or V. ammodytes, whereas in France, only 13% of envenomations were identified by patients as V. berus or V. aspis bites [Citation14]. This higher identification rate may be due to the presence of only V. berus in northern countries and the distinctive and easily recognizable nose horn of V. ammodytes in southeastern countries.
Bites from V. aspis and V. ursinii were not reported in this study, likely because these viper species are only found in a limited part of the geographical area the study covered. Patients bitten by V. ammodytes were older and more likely to be bitten on their upper limbs, while the patients bitten by V. berus were more commonly bitten on their lower limbs. The reason for this difference is not known for certain, but it is probably related to the distribution patterns of the snakes. Viper berus is more commonly found in mountainous regions and tends to bite people on their legs, especially during hiking activities. Viper ammodytes, on the other hand, is more common in flat terrain, such as vineyards and fields, where it tends to bite people on their arms, especially during work-related activities. However, in countries where only V. berus is present, viper bites are evenly distributed between the upper and lower limbs [Citation15].
The median severity score for V. berus bites was lower (severity score 1/3) compared to V. ammodytes (severity score 2/3), but this difference was not statistically significant. Patients bitten by V. ammodytes were more likely to experience local pain, probably due to the greater amount of venom injected by V. ammodytes, which is the largest viper species in Europe.
The most notable difference in laboratory results between V. berus and V. ammodytes snakebites is the higher incidence of thrombocytopenia in V. ammodytes bites (28%). This is likely attributed to the higher content of snake c-type lectin-like proteins (snaclecs) in V. ammodytes venom [Citation6]. The incidence of thrombocytopenia in V. berus bites has been reported to range from 1.3% in Britain [Citation15] and from 1.5% to 19% in Poland [Citation16, Citation17]. Balkan adder (V. berus bosniensis) envenomation leads to thrombocytopenia in 14% of cases [Citation18], which is similar to the results of this study (12%). In France, thrombocytopenia occurred in 6% of patients bitten by either V. berus or V. aspis [Citation14], while in patients requiring antivenom therapy, thrombocytopenia occurred in 15% of cases [Citation19]. The reported incidence of thrombocytopenia in V. ammodytes bites also shows variability, possibly influenced by geographic variations in venom composition and the presence of different V. ammodytes subspecies. Thrombocytopenia rates in V. ammodytes bites have been reported to be 2% in Croatia [Citation20, Citation21] and 8% in Greece, where V. ammodytes is responsible for the majority of cases [Citation22]. However, among patients envenomated by V. ammodytes requiring antivenom treatment, thrombocytopenia developed in half of the cases in Croatia and Slovenia [Citation23]. The absence of clinically significant bleeding in V. ammodytes envenomed patients suggests that platelet function is preserved despite transient and severe thrombocytopenia [Citation8, Citation23–25]. Severe thrombocytopenia in V. ammodytes envenomed patients should be treated with antivenom, and platelets transfusion should be used in life-threatening bleeding after viper bites [Citation23].
In this study, antivenom was used more frequently for V. ammodytes bites (72%) than for V. berus bites (39%) (). The incidence of V. ammodytes bites treated with antivenom within its geographical distribution area treated with antivenom was three times higher than that of V. berus bites treated with antivenom (1.16 bites per million population compared to 0.39 bites per million population) (). Consequently, antivenom availability is particularly challenging in this region because the antivenoms raised against V. berus venom (ViperaTab® and Viper venom antitoxin) are not sufficiently effective in severe cases of V. ammodytes envenomation. Accordingly, these two antivenoms have been used primarily in Central European countries such as Poland, the Czech Republic, and Slovakia, where only V. berus is present. On the other hand, the Balkan countries have produced and used antivenoms raised against V. ammodytes venom: European viper venom antiserum (Croatia), Viekvin® (Serbia) and Bulbio snake venom antiserum (Bulgaria). These antivenoms consist of monovalent equine F(ab)2 fragments and are administered intramuscularly. Similar to other antivenoms, they are not licensed for use within the European Union [Citation9]. In addition, European viper venom antiserum has not been produced since 2014. Vipera ammodytes snakebites can also be effectively treated with intravenous Viperfav® (MicroPharm Limited, Newcastle Emlyn, United Kingdom), which is a formulation containing polyvalent equine F(ab’)2 fragments raised against the venoms of V. aspis, V. berus, and V. ammodytes [Citation24], but it is also currently unavailable due to changing the production site. Considering the limited availability of Viperfav® and European viper venom antiserum, a pharmaceutical formulation containing monospecific ovine Fab fragments targeting V. berus venom has also been used to treat V. ammodytes bites [Citation25, Citation26]. However, multiple doses of antivenom were required in these cases (). Previous research has shown that Fab fragments targeting V. berus venom can only partially and temporarily increase platelet count in thrombocytopenia caused by V. ammodytes envenomation [Citation23, Citation25].
This study has several limitations. Firstly, snakebite reporting to poison centres is not mandatory. As a result, not all cases of viper envenomation may have been referred to the participating poison centres during the study period. In addition, trained emergency physicians are capable of treating patients who have been bitten by vipers without the need to consult poison centres. Therefore, it is possible that the actual incidence of Vipera species envenomation may be higher than that reported in this study. Secondly, the study included telephone consultations, which introduces the possibility of missing or incomplete data, especially on clinical effects and treatment after the call. On the other hand, data from hospitalized patients in the included toxicology-associated hospital wards are more reliable. However, it is important to note that toxicology-associated hospital wards without a national poison service typically serve only a limited area around the hospital where they are located, such as in Budapest and Sofia. Thirdly, in accordance with the practice of the participating poison centres, only some patients were followed up by telephone until the clinical symptoms of envenomation resolved. Finally, the diagnosis of viper envenomation was based primarily on the patient’s self-report of being bitten, along with additional information such as the snake’s known distribution area, the patient’s local and systemic clinical symptoms of envenomation, laboratory abnormalities, and the response to specific antivenom treatment. As a result, we likely did not include certain dry bites from vipers when patients were unable to identify the snake and had no symptoms. The neurological symptoms reported in a patient bitten by V. berus could be inaccurately attributed to V. berus bite due to this limitation. The diagnosis was rarely confirmed by evidence of venom in the patient’s blood.
Conclusions
The estimated incidence of viper bites in Central and Southeastern Europe is at least 2.55 per million population. Bites from V. ammodytes are more common and more severe than bites from V. berus in the areas where each snake is found. Vipera ammodytes bites are characterized by a higher incidence of pain and thrombocytopenia. Antivenom administration is more frequently required for V. ammodytes bites. Therefore, it is crucial to ensure sufficient production of European Medicines Agency-approved V. ammodytes-specific antivenom and to make it available at an affordable price.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Chippaux JP. Epidemiology of snakebites in Europe: a systematic review of the literature. Toxicon. 2012;59(1):86–99. doi:10.1016/j.toxicon.2011.10.008.
- Mallow D, Ludwig D, Nilson G. True vipers: natural history and toxinology of Old-World vipers. ; Krieger Publishing Company: malabar, FL, USA, 2003; p. 359.
- Tomović L. Systematics of the nose horned viper (vipera ammodytes, linnaeus, 1758). Herpetological Journal. 2006;16:191–201.
- Di Nicola MR, Pontara A, Kass GEN, et al. Vipers of major clinical relevance in Europe: taxonomy, venom composition, toxicology and clinical management of human bites. Toxicology. 2021;453:152724. doi:10.1016/j.tox.2021.152724.
- Karabuva S, Lukšić B, Brizić I, et al. Ammodytin L is the main cardiotoxic component of the vipera ammodytes ammodytes venom. Toxicon. 2017;139:94–100. doi:10.1016/j.toxicon.2017.10.003.
- Leonardi A, Sajevic T, Pungerčar J, et al. Comprehensive study of the proteome and transcriptome of the venom of the most venomous european viper: discovery of a new subclass of ancestral snake venom metalloproteinase Precursor-Derived proteins. J Proteome Res. 2019;18(5):2287–2309. doi:10.1021/acs.jproteome.9b00120.
- Latinovic Z, Leonardi A, Šribar J, et al. Venomics of vipera berus berus to explain differences in pathology elicited by vipera ammodytes ammodytes envenomation: therapeutic implications. J Proteomics. 2016;146:34–47. doi:10.1016/j.jprot.2016.06.020.
- Dobaja Borak M, Grenc D, Reberšek K, et al. Reversible and transient thrombocytopenia of functional platelets induced by nose-horned viper venom. Thromb Res. 2023;229:152–154. doi:10.1016/j.thromres.2023.07.005.
- Lamb T, de Haro L, Lonati D, et al. Antivenom for european vipera species envenoming. Clin Toxicol (Phila). 2017;55(6):557–568. doi:10.1080/15563650.2017.1300261.
- Google Maps. 2023. Europe. Google Maps [online] Available at https://www.google.com/maps. [Accessed 17 July 2023]
- Audebert F, Sorkine M, Bon C. Envenoming by viper bites in France: clinical gradation and biological quantification by ELISA. Toxicon. 1992;30(5–6):599–609. doi:10.1016/0041-0101(92)90854-x.
- Chippaux JP, Saz-Parkinson Z, Amate Blanco JM. Epidemiology of snakebite in Europe: comparison of data from the literature and case reporting. Toxicon. 2013;76:206–213. doi:10.1016/j.toxicon.2013.10.004.
- Paolino G, Di Nicola MR, Avella I, et al. Venomous bites, stings and poisoning by european vertebrates as an overlooked and emerging medical problem: recognition, clinical aspects and therapeutic management. Life (Basel). 2023;13(6):1228. doi:10.3390/life13061228.
- Jollivet V, Hamel JF, de Haro L, et al. European viper envenomation recorded by french poison control centers: a clinical assessment and management study. Toxicon. 2015;108:97–103. doi:10.1016/j.toxicon.2015.09.039.
- Lamb T, Stewart D, Warrell DA, et al. Moderate-to-severe vipera berus envenoming requiring ViperaTAb antivenom therapy in the UK. Clin Toxicol (Phila). 2021;59(11):992–1001. doi:10.1080/15563650.2021.1891245.
- Dyląg-Trojanowska KE, Hodorowicz-Zaniewska D, Zybaczyńska J, et al. Is coagulopathy a common consequence of a vipera berus bite? A retrospective single Centre study. Ann Agric Environ Med. 2018;25(4):630–634. doi:10.26444/aaem/75941.
- Magdalan J, Trocha M, Merwid-Lad A, et al. Vipera berus bites in the region of southwest Poland–a clinical analysis of 26 cases. Wilderness Environ Med. 2010;21(2):114–119. doi:10.1016/j.wem.2010.01.005.
- Malina T, Krecsák L, Jelić D, et al. First clinical experiences about the neurotoxic envenomings inflicted by lowland populations of the Balkan adder, vipera berus bosniensis. Neurotoxicology. 2011;32(1):68–74. doi:10.1016/j.neuro.2010.11.007.
- Boels D, Hamel JF, Bretaudeau Deguigne M, et al. European viper envenomings: assessment of viperfav™ and other symptomatic treatments. Clin Toxicol (Phila). 2012;50(3):189–196. doi:10.3109/15563650.2012.660695.
- Luksić B, Bradarić N, Prgomet S. Venomous snakebites in Southern Croatia. Coll Antropol. 2006;30(1):191–197.
- Lukšić B, Karabuva S, Markić J, et al. Thrombocytopenic purpura following envenomation by the nose-horned viper (vipera ammodytes ammodytes): two case reports. Medicine (Baltimore). 2018;97(52):e13737. doi:10.1097/MD.0000000000013737.
- Frangides CY, Koulouras V, Kouni SN, et al. Snake venom poisoning in Greece. Experiences with 147 cases. Eur J Intern Med. 2006;17(1):24–27. doi:10.1016/j.ejim.2005.10.001.
- Kurtović T, Karabuva S, Grenc D, et al. Intravenous vipera berus Venom-Specific fab fragments and intramuscular vipera ammodytes Venom-Specific F(ab’)2 fragments in vipera ammodytes-Envenomed patients. Toxins (Basel). 2021;13(4):279. doi:10.3390/toxins13040279.
- Kurtović T, Brvar M, Grenc D, et al. A single dose of viperfav(TM) may be inadequate for vipera ammodytes snake bite: a case report and pharmacokinetic evaluation. Toxins (Basel). 2016;8(8):244. doi:10.3390/toxins8080244.
- Brvar M, Kurtović T, Grenc D, et al. Vipera ammodytes bites treated with antivenom ViperaTAb: a case series with pharmacokinetic evaluation. Clin Toxicol (Phila). 2017;55(4):241–248. doi:10.1080/15563650.2016.1277235.
- Casewell NR, Al-Abdulla I, Smith D, et al. Immunological cross-reactivity and neutralization of european viper venoms with the monospecific vipera berus antivenom ViperaTAb. Toxins (Basel). 2014;6(8):2471–2482. doi:10.3390/toxins6082471.