Abstract
Introduction
Cannabis is the most common recreational drug worldwide and synthetic cannabinoid receptor agonists are currently the largest group of new psychoactive substances. The aim of this study was to compare the clinical features and outcomes of lone acute cannabis toxicity with lone acute synthetic cannabinoid receptor agonist toxicity in a large series of presentations to European emergency departments between 2013-2020.
Methods
Self-reported drug exposure, clinical, and outcome data were extracted from the European Drug Emergencies Network Plus which is a surveillance network that records data on drug-related emergency department presentations to 36 centres in 24 European countries. Cannabis exposure was considered the control in all analyses. To compare the lone cannabis and lone synthetic cannabinoid receptor agonist groups, univariate analysis using chi squared testing was used for categorical variables and non-parametric Mann-Whitney U- testing for continuous variables. Statistical significance was defined as a P value of <0.05.
Results
Between 2013-2020 there were 54,314 drug related presentations of which 2,657 were lone cannabis exposures and 503 lone synthetic cannabinoid receptor agonist exposures. Synthetic cannabinoid receptor agonist presentations had statistically significantly higher rates of drowsiness, coma, agitation, seizures and bradycardia at the time of presentation. Cannabis presentations were significantly more likely to have palpitations, chest pain, hypertension, tachycardia, anxiety, vomiting and headache.
Discussion
Emergency department presentations involving lone synthetic cannabinoid receptor agonist exposures were more likely to have neuropsychiatric features and be admitted to a psychiatric ward, and lone cannabis exposures were more likely to have cardiovascular features. Previous studies have shown variability in the acute toxicity of synthetic cannabinoid receptor agonists compared with cannabis but there is little comparative data available on lone exposures. There is limited direct comparison in the current literature between lone synthetic cannabinoid receptor agonist and lone cannabis exposure, with only two previous poison centre series and two clinical series. Whilst this study is limited by self-report being used to identify the drug(s) involved in the presentations, previous studies have demonstrated that self-report is reliable in emergency department presentations with acute drug toxicity.
Conclusion
This study directly compares presentations with acute drug toxicity related to the lone use of cannabis or synthetic cannabinoid receptor agonists. It supports previous findings of increased neuropsychiatric toxicity from synthetic cannabinoid receptor agonists compared to cannabis and provides further data on cardiovascular toxicity in lone cannabis use.
Introduction
Cannabis is reported by the United Nations Office on Drugs and Crime (UNODC) as being the most commonly used recreational drug worldwide, with an estimated global annual prevalence of use of 3.98% in adults aged 15–64 years in 2019 [Citation1]. In Europe in 2022 cannabis use in young adults aged 15-34 years was estimated to be 15.1%, and 2.1% of young adults aged 15-34 years used cannabis on 20 or more days in the last month [Citation2]. Cannabis use is generally regarded as being associated with a low risk of acute toxicity [Citation3] and patients presenting to the emergency department with acute cannabis toxicity typically present with nausea and vomiting, anxiety, palpitations and agitation [Citation4,Citation5]. However, cases of severe toxicity are reported in most of the large series of acute cannabis toxicity with seizures, syncope, acute coronary syndrome, dysrhythmias and fatalities reported from lone cannabis use [Citation3–8]. Recent systematic reviews [Citation9,Citation10] confirm that cannabis use is associated with an increased risk of cardiac dysrhythmias and acute coronary syndrome.
In the last 15–20 years there has been increasing availability of a wide range of different new psychoactive substances, with 1,047 new psychoactive substances reported worldwide to the end of December 2020 [Citation1]. The synthetic cannabinoid receptor agonists (SCRAs) are the largest group of new psychoactive substances both in terms of the number of substances and in terms of the number and volume of new psychoactive substances seizures and, in Europe, 98 new synthetic cannabinoid receptor agonists were reported in 2021 and 24 in 2022 [Citation1,Citation2]. In addition to being the largest group of new psychoactive substances, synthetic cannabinoid receptor agonists are involved in the greatest proportion of deaths and emergency hospital presentations [Citation1,Citation2,Citation11]. Despite this, data do not suggest that synthetic cannabinoid receptor agonists are as commonly used as cannabis; for example, in England and Wales in 2018, 0.5% of the adult population reported use of synthetic cannabinoid receptor agonists in the past year compared to 7.6% reporting past year use of cannabis [Citation11]. The synthetic cannabinoid receptor agonists have evolved since they first emerged as recreational drugs in the mid-2000s [Citation12], specifically, the current third- and fourth-generation synthetic cannabinoid receptor agonists have much greater binding affinity and potency at the type 1 cannabinoid receptor (CB-1) compared to type 2 cannabinoid receptors (CB-2) while the main psychoactive component of cannabis, Δ9-tetrahydrocannabinol is a partial CB-1 and CB-2 agonist [Citation13,Citation14]. A number of case series have reported on the pattern of acute synthetic cannabinoid receptor agonist toxicity with a broad range of clinical features including tachycardia, hypertension, hypotension, chest pain, acute kidney injury, seizures, agitation, psychosis and coma [Citation15–19]. Synthetic cannabinoid receptor agonists are reported in large proportions of new psychoactive substance related harms in Europe. In 2019, three quarters of the new psychoactive substance related acute toxicity presentations in the European Drug Emergencies Network (Euro-DEN) were linked to synthetic cannabinoid receptor agonists [Citation20].
There have been two studies which have compared the acute toxicity of cannabis and synthetic cannabinoid receptor agonists in patients presenting to emergency departments. A small prospective cohort study compared clinical features in 17 patients with self-reported synthetic cannabinoid receptor agonist use to 70 patients with self-reported cannabis use [Citation21]. This found agitation (odds ratio 3.8) and dysrhythmia (odds ratio 9.2) were more common in the synthetic cannabinoid receptor agonist group compared to the cannabis group. The second study was a retrospective cohort study of 415 adolescent (aged between 13 and 19 years old) exposures investigating the neuropsychiatric sequelae of synthetic cannabinoid receptor agonist toxicity in comparison with cannabis exposures [Citation22]. This study found that lone synthetic cannabinoid receptor agonist exposure was associated with higher odds of a decreased level of consciousness (odds ratio 3.42) and seizures (odds ratio 3.89) but less agitation (odds ratio 0.18).
The European Drug Emergencies Network (Euro-DEN) is a European surveillance network which collects data on presentations to sentinel emergency departments with acute toxicity related to the use of recreational drugs, new psychoactive substances and the misuse of prescription medicines [Citation23]. Since 2015 Euro-DEN has expanded as Euro-DEN Plus and by the end of 2020 had data from 36 centres in 24 European countries [Citation24]. The aim of this study was to use these data to compare the clinical features and outcomes in a large series of presentations with lone acute cannabis toxicity and lone acute synthetic cannabinoid receptor agonist toxicity.
Methods
Euro-DEN data collection methodology
Data are collected from the hospital medical record using a purpose design minimum dataset on all presentations to each Euro-DEN Plus sentinel site with acute recreational drug toxicity [Citation25]. We have previously reported detailed information on the Euro-DEN Plus patient inclusion/exclusion criteria, data collection methodology and local regulatory approval processes [Citation23–25].
Data extraction
Data were extracted from the Euro-DEN Plus dataset for all presentations with acute toxicity related to self-reported lone exposure to cannabis or lone exposure to a synthetic cannabinoid receptor agonist from 1 October 2013 to 31 December 2020 (these are presentations where co-use of other recreational drug(s), new psychoactive substances or alcohol together with cannabis or a synthetic cannabinoid receptor agonist were excluded). The data extracted were: age and sex, whether the patient arrived in the emergency department by ambulance, drug(s) taken, clinical observations at presentation, whether pre-defined clinical features [Citation23–25] were present at any time during the presentation, and outcome (disposition from the emergency department, length of hospital stay, and death in hospital). Within the Euro-DEN Plus dataset, the drug(s) involved in the presentation are based on patient self-report together with the clinical assessment of the treating physician; if analytical toxicology screening is undertaken as part of routine clinical care these data are recorded.
Data analysis
Categorical variables were expressed as absolute numbers and percentages, and continuous variables as median and interquartile range (IQR). For level of consciousness, some centres record data as one of three categorical variables (alert, drowsy or coma); whilst others record data in the form of a Glasgow Coma Scale (GCS). In order to allow evaluation of the data as a whole, data expressed as a GCS was converted using the traditionally accepted ranges as alert (GCS 15), drowsy (GCS 9-14) and comatose (GCS ≤8). Cannabis exposure was considered the control in all analyses and to compare the two groups, univariate analysis using chi squared testing was used for categorical variables and non-parametric Mann-Whitney U- testing was used for continuous variables. Statistical testing was considered significant if the P value was less than 0.05. Statistical analysis was performed using RStudio 2022.07.1 Build 554.
Results
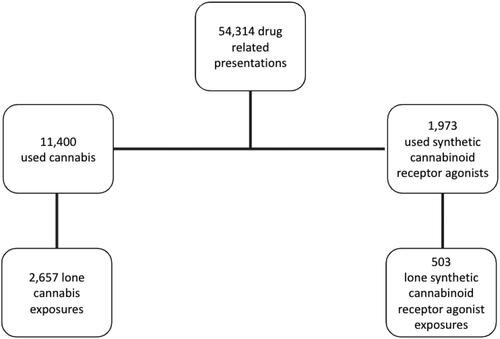
There were 54,314 acute drug toxicity presentations to the Euro-DEN Plus sentinel centres between 01 October 2013 and 31 December 2020. Of these, 11,400 (21%) reported use of cannabis and 1,973 (4%) reported use of synthetic cannabinoid receptor agonists (); after presentations with co-use of other substances were removed, there were 2,657 (5%) presentations with self-reported lone cannabis use and 503 (1%) presentations with self-reported lone synthetic cannabinoid receptor agonist use.
A total of 36 Euro-DEN Plus centres provided data on cannabis and synthetic cannabinoid receptor agonist presentations with the largest number of lone cannabis presentations to the centre in Amsterdam, the Netherlands (807) and the largest number of lone synthetic cannabinoid receptor agonist presentations to the centre in Msida, Malta (284). Supplementary Table S1 gives a breakdown of the number of cannabis, synthetic cannabinoid receptor agonist and total recreational drug toxicity presentations to each centre. Whilst lone cannabis presentations were reported at all Euro-DEN Plus centres, only 11 centres reported lone synthetic cannabinoid receptor agonist presentations and five of these (Gdansk, Poland; Izmir, Türkiye; London (St Thomas’ Hospital), United Kingdom; Msida, Malta; and Munich, Germany) had more lone synthetic cannabinoid receptor agonist presentations than lone cannabis presentations.
The lone synthetic cannabinoid receptor agonist presentations more frequently involved male patients (88.9% versus 70.7%; P < 0.01) but there was no difference in the median [IQR] age of presentations (26 [21-35] versus 25 [20-33], P = 0.054). The lone synthetic cannabinoid receptor agonist presentations were more likely to arrive in hospital by ambulance (85.1% versus 54.0%, P < 0.001).
The clinical features of the presentations are summarized in . With regards to neuropsychiatric features, the lone synthetic cannabinoid receptor agonist presentations were more likely to be drowsy (49.0% versus 17.2%, P < 0.001) or comatose (3.7% versus 1.9%, P < 0.001). They were also more likely to be agitated (42.4% versus 22.4%, P < 0.001), to have psychosis (16.4% versus 12.7%, P = 0.033), or have seizures (8.1% versus 2.6%, P < 0.001),; but the proportions with hallucinations were similar (11.5% versus 12.4%, P = 0.606), and anxiety was more common in the lone cannabis presentations (37.4% versus 17.8%, P < 0.001). Vomiting (20.6% versus 15.1%, P = 0.006) and headache (7.1% versus 2.7%, P < 0.001) were more common in the lone cannabis presentations.
Table 1. Clinical features of presentations.
There were two cardiac arrests in the cannabis group, one of whom died, and no cardiac arrests in the synthetic cannabinoid receptor agonist group. There were no differences in the proportion of presentations with dysrhythmias between the cannabis and synthetic cannabinoid receptor agonist groups (1.7% versus 0.8%, P = 0.253). The median heart rate on presentation was greater in the cannabis presentations (96 beats/min versus 88 beats/min, P < 0.001) and cannabis presentations were more likely to present tachycardic (heart rate ≥150 beats/min) compared to synthetic cannabinoid receptor agonist patients (1.9% versus 0.02%, P < 0.001). Cannabis presentations were less likely to be bradycardic (heart rate ≤50 beats/min) on presentation than the synthetic cannabinoid receptor agonist presentations (0.9% versus 2.2%, P = 0.028). The cannabis group were more likely to report palpitations (20.4% versus 5.2%, P < 0.001) or chest pain (10.2% versus 5.2%, P < 0.008). The proportion of presentations that developed hypertension (systolic ≥180 mmHg) was greater in the lone cannabis group (4.4% versus 1.2%, P < 0.001), whilst the proportion that developed hypotension was not significantly different.
Outcome data are presented in . Lone cannabis presentations were more likely to be medically discharged from the emergency department (74.2% versus 38.0%, P < 0.001), but less likely to self-discharge (5.6% versus 25.8%, P < 0.001). There was no difference in the proportion of presentations admitted to critical care, but the lone synthetic cannabinoid receptor agonist presentations were more likely to be admitted to psychiatry (15.5% versus 7.5%, P < 0.001). Cannabis presentations had a median length of stay that was 43 min shorter than synthetic cannabinoid receptor agonist presentations (3 h 22 min versus 4 h 5 min, P < 0.001). There were three deaths in the lone cannabis group and no deaths amongst synthetic cannabinoid receptor agonist presentations.
Table 2. Outcomes.
Discussion
This study has compared clinical features and outcomes of lone synthetic cannabinoid receptor agonist and lone cannabis acute toxicity presentations to the emergency department in the Euro-DEN Plus dataset. Lone synthetic cannabinoid receptor agonist presentations were more likely to have neuropsychiatric features including drowsiness/coma, seizures, agitation, and psychosis. Lone synthetic cannabinoid receptor agonist presentations were more likely to be admitted to a psychiatric ward from the emergency department. Conversely, lone cannabis presentations were more likely to have cardiovascular features including tachycardia, chest pain, palpitations, and hypertension. Although there was a statistical difference in the length of stay, with lone cannabis presentations having a median length of stay 43 min shorter than lone synthetic cannabinoid receptor agonist presentations, this difference in length of stay is unlikely to be of clinical significance.
Several retrospective reviews of data collected in calls made to poison information services have examined the clinical effects of exposure to synthetic cannabinoid receptor agonists and cannabis. A retrospective review of calls to the Texas poison centre network in 2010 compared clinical features in 418 lone synthetic cannabinoid receptor agonist and 99 lone cannabis cases [Citation26]. The cases identified in this poison centre study with lone synthetic cannabinoid receptor agonist toxicity had a similar increased neuropsychiatric toxicity (agitation/irritability, relative risk 2.37; hallucinations/delusions relative risk 5.57) that was reported in our study, however, this poisons centre study identified a higher relative risk of cardiovascular toxicity (tachycardia, relative risk 2.79) in the synthetic cannabinoid receptor agonist cases. Analysis of 1,353 lone synthetic cannabinoid receptor agonist exposure cases reported to the US National Poison Data System between 1 January and 1 October 2010, the most commonly reported clinical effects were cardiovascular [tachycardia (40%), hypertension (8.1%), chest pain (4.7%)], neuropsychiatric [agitation (23.4%), drowsiness (13.5%), confusion (12%), hallucinations (9.4%), dizziness (7.3%), and seizures (3.8%)] or gastrointestinal [nausea (10%), vomiting (15.3%)] [Citation27]. In another study, there were 615 synthetic cannabinoid receptor agonist, cannabis and medical cannabis cases referred to the Israeli Poison Information Centre between 2007 and 2018 [Citation28]. Although neurological toxicity was the most commonly reported issue, there was no difference in the frequency of neurological toxicity between the different cannabinoid presentations. It did find that synthetic cannabinoid receptor agonist cases were more likely to have cardiovascular (relative risk ratio 1.55, P < 0.01) and gastrointestinal (relative risk ratio 4.27, P < 0.001) toxicity than recreational cannabis cases [Citation28].
Direct comparative data between cases of acute cannabis and synthetic cannabinoid receptor agonist toxicity seen clinically rather than reported to poisons centres is limited. A literature review found two studies directly comparing the toxicity of cannabis and synthetic cannabinoid receptor agonists [Citation21,Citation22]. In 2016, a prospective cohort study of 87 patients in New York directly compared the toxicity of 70 patients presenting to an emergency department following the use of cannabis with 17 patients presenting following the use of synthetic cannabinoid receptor agonists; it is not clear from the published paper whether these were lone exposures or whether patients had also used other recreational drugs and/or new psychoactive substances [Citation21]. This study reported a greater proportion of cases with agitation (odds ratio 3.8) in the synthetic cannabinoid receptor agonist presentations, supporting our finding of an increased risk of neuropsychiatric toxicity related to synthetic cannabinoid receptor agonist use. However, unlike our finding of increased risk of cardiovascular toxicity in patients with lone cannabis use, this small United States cohort study found that dysrhythmias (odds ratio 9.2) were more common in synthetic cannabinoid receptor agonist presentations compared to cannabis presentations.
In 2019, a retrospective cohort study using data from the Toxicology Investigators Consortium (ToxIC) Case Registry looked at a total of 415 adolescent (aged between 13 and 19 years old) exposures to investigate neuropsychiatric sequelae of synthetic cannabinoid receptor agonist toxicity in comparison with cannabis exposures [Citation22]. The ToxIC registry included presentations where the patient had a direct bedside clinical/medical toxicology consultation. This identified four exposure subgroups: lone synthetic cannabinoid receptor agonist (n = 107); synthetic cannabinoid receptor agonist polydrug (n = 38); lone cannabis (n = 86); and cannabis polydrug (n = 117). Similar to our study, the ToxIC registry study reported that lone synthetic cannabinoid receptor agonist use was associated with a higher rate of seizures (odds ratio 3.89) and coma (odds ratio 3.89) compared to lone cannabis use. However, they reported that lone synthetic cannabinoid receptor agonist use was associated with a lower rate of agitation (odds ratio 0.18) in comparison to our study where we found a higher rate of agitation in the lone synthetic cannabinoid receptor agonist group. One potential explanation for the differences between the clinical features reported in the United States case series compared to our case series of presentations across Europe is the variability of the different individual synthetic cannabinoid receptor agonists within the product(s) that are available within different geographical regions [Citation29–31].
The limitations of the Euro-DEN Plus project have been previously discussed in other manuscripts published by our group and resources [Citation23,Citation32]. The study methodology of the Euro-DEN Plus registry, which uses self-reported drug(s) used for the majority of patient presentations included, is the same methodology as the ToxIC registry study [Citation22]. There is the potential that both our and the ToxIC registry studies may under-identify presentations involving synthetic cannabinoid receptor agonists as individuals may not be aware that they have used them. It is unlikely that patients and/or others will under-report cannabis use/exposure given the nature of cannabis, the way it is used and the smell associated with its use. Some cases might involve multiple drugs, despite only one being reported. For this analysis there is no information on the nature of the synthetic cannabinoid receptor agonists, the potency of the cannabis, the form or the route of administration. The potency of the cannabis used by the patients in this series might have changed over time, reflecting changing in the European drug market, but data were not available to enable investigation of these issues. However, it should be noted that the range of acute toxicity and frequency of occurrence seen in our patients with synthetic cannabinoid receptor agonist toxicity is similar to that described in previous case series of analytically confirmed synthetic cannabinoid receptor agonist cases of acute toxicity [Citation33].
Conclusion
This large study directly compares a series of presentations with acute drug toxicity related to the lone use of cannabis and the lone use of synthetic cannabinoid receptor agonists. It provides further support to previous literature findings that synthetic cannabinoid receptor agonists are more likely to be associated with significant neuropsychiatric toxicity, particularly in the form of seizures, agitation, drowsiness and coma when compared with cannabis; and further highlights the potential for cardiovascular toxicity associated with lone cannabis use. This provides clinicians working in emergency department settings data on the potential toxicity associated with synthetic cannabinoid receptor agonists that they should be aware of when managing these types of presentations. In addition, they enable those engaging with synthetic cannabinoid receptor agonist users to provide accurate harm reduction advice on the risks associated with their use.
Supplemental Material
Download MS Word (30.6 KB)Acknowledgements
We are extremely grateful to our colleagues at the Euro-DEN Plus centres who have spent years collecting data on recreational drug presentations, without which this research would not be possible. Thank you to Dr Ahmed Al-Hindawi for his assistance with statistical analyses.
Disclosure statement
The Euro-DEN and Euro-DEN Plus project received financial support from the Drug Prevention and Information Program (DPIP) and the Prevention of and Fight Against Crime (ISEC) Programme of the European Union from 2013-2015. It has received financial support from the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) since 2015. Additional funding for individual Euro-DEN Plus centres in Switzerland has been provided by The Swiss Centre of Applied Human Toxicology, Burgergemeinde Bern, Switzerland and the Scientific Research Advisory Board of the Ente Ospedaliero Cantonale Ticino, Switzerland.
Data availability
The data that support the findings of this study are available from Paul Dargan, upon reasonable request.
Additional information
Funding
References
- United Nations Office of Drugs and Crime. World drug report 2023. Vienna (Austria): United Nations Publication; 2023. Available from: https://www.unodc.org/unodc/en/data-and-analysis/world-drug-report-2023.html
- European Monitoring Centre for Drugs and Drug Addiction. European drug report 2023: trends and developments. Luxembourg: Publications Office of the European Union; 2023. Available from: https://www.emcdda.europa.eu/publications/european-drug-report/2023_en
- World Health Organisation. The health and social effects of nonmedicinal cannabis use. Geneva (Switzerland): World Health Organisation; 2016. ISBN 9789241510240e. Available from: https://www.who.int/publications/i/item/9789241510240
- Schmid Y, Scholz I, Mueller L, et al. Emergency department presentations related to acute toxicity following recreational use of cannabis products in Switzerland. Drug Alcohol Depend. 2020;206:107726. doi:10.1016/j.drugalcdep.2019.107726.
- Dines AM, Wood DM, Galicia M, et al. Presentations to the emergency department following cannabis use - a multi-centre case series from ten European countries. J Med Toxicol. 2015;11(4):415–421. doi:10.1007/s13181-014-0460-x.
- Schmid Y, Galicia M, Vogt SB, et al. Differences in clinical features associated with cannabis intoxication in presentations to european emergency departments according to patient age and sex. Clin Toxicol (Phila). 2022;60(8):912–919. doi:10.1080/15563650.2022.2060116.
- Casier I, Vanduynhoven P, Haine S, et al. Is recent cannabis use associated with acute coronary syndromes? An illustrative case series. Acta Cardiol. 2014;69(2):131–136. doi:10.1080/ac.69.2.3017293.
- Gresnigt F, van den Brink LC, Hunault C, et al. Incidence of cardiovascular symptoms and adverse events following self-reported acute cannabis intoxication at the emergency department: a retrospective study. Emerg Med J. 2023;40(5):357–358. doi:10.1136/emermed-2022-212784.
- Richards JR, Bing ML, Moulin AK, et al. Cannabis use and acute coronary syndrome. Clin Toxicol (Phila). 2019;57(10):831–841. doi:10.1080/15563650.2019.1601735.
- Richards JR, Blohm E, Toles KA, et al. The association of cannabis use and cardiac dysrhythmias: a systematic review. Clin Toxicol (Phila). 2020;58(9):861–869. doi:10.1080/15563650.2020.1743847.
- Office for National Statistics. Drugs misuse: findings from the 2018/19 crime survey for England and Wales. London (UK): Home Office Statistical Bulletin; 2019. Available from: https://www.gov.uk/government/statistics/drug-misuse-findings-from-the-2018-to-2019-csew
- Advisory Council for the Misuse of Drugs. Synthetic cannabinoid receptor agonists (SCRA) an updated harms assessment and a review of classification and scheduling under the misuse of drugs act 1971 and its regulations. London: Advisory Council on the Misuse of Drugs; 2020.
- Sachdev S, Vemuri K, Banister SD, et al. In vitro determination of the efficacy of illicit synthetic cannabinoids at CB1 receptors. Br J Pharmacol. 2019;176(24):4653–4665. doi:10.1111/bph.14829.
- Potts AJ, Cano C, Thomas SHL, et al. Synthetic cannabinoid receptor agonists: classification and nomenclature. Clin Toxicol (Phila). 2020;58(2):82–98. doi:10.1080/15563650.2019.1661425.
- Dargan P, Wood D. Novel psychoactive substances: classification, pharmacology and toxicology. London: Academic Press; 2022.
- Chung EY, Cha HJ, Min HK, et al. Pharmacology and adverse effects of new psychoactive substances: synthetic cannabinoid receptor agonists. Arch Pharm Res. 2021;44(4):402–413. doi:10.1007/s12272-021-01326-6.
- Lovett C, Wood DM, Dargan PI. The pharmacology and toxicology of the synthetic cannabinoid receptor agonists. Réanimation. 2015;24(5):527–541. doi:10.1007/s13546-015-1104-4.
- Adams AJ, Banister SD, Irizarry L, et al. Zombie" outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N Engl J Med. 2017;376(3):235–242. doi:10.1056/NEJMoa1610300.
- Tait RJ, Caldicott D, Mountain D, et al. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol (Phila). 2016;54(1):1–13. doi:10.3109/15563650.2015.1110590.
- Crulli B, Dines AM, Blanco G, et al. Novel psychoactive substances-related presentations to the emergency departments of the European drug emergencies network plus (Euro-DEN plus) over the six-year period 2014-2019. Clin Toxicol (Phila). 2022;60(12):1318–1327. doi:10.1080/15563650.2022.2137524.
- Zaurova M, Hoffman RS, Vlahov D, et al. Clinical effects of synthetic cannabinoid receptor agonists compared with marijuana in emergency department patients with acute drug overdose. J Med Toxicol. 2016;12(4):335–340. doi:10.1007/s13181-016-0558-4.
- Anderson SAR, Oprescu AM, Calello DP, et al. Neuropsychiatric sequelae in adolescents with acute synthetic cannabinoid toxicity. Pediatrics. 2019;144(2):e20182690. doi:10.1542/peds.2018-2690.
- Wood DM, Heyerdahl F, Yates CB, et al. The European drug emergencies network (Euro-DEN). Clin Toxicol (Phila). 2014;52(4):239–241. doi:10.3109/15563650.2014.898771.
- European Monitoring Centre for Drugs and Drug Addiction. Drug-related hospital emergency presentations in Europe: update from the Euro-DEN plus expert network. Luxembourg: European Monitoring Centre for Drugs and Drug Addiction; 2020. Available from: https://www.emcdda.europa.eu/publications/technical-reports/drug-related-hospital-emergency-presentations-in-europe_en
- EMCDDA. Frequently asked questions (FAQ) on acute drug toxicity presentations to hospital emergency services [internet]. Lisbon (Portugal): European Monitoring Centre for Drugs and Addiction. Available from: https://www.emcdda.europa.eu/publications/faq/euro-den_en
- Forrester MB, Kleinschmidt K, Schwarz E, et al. Synthetic cannabinoid and marijuana exposures reported to poison centers. Hum Exp Toxicol. 2012;31(10):1006–1011. doi:10.1177/0960327111421945.
- Hoyte CO, Jacob J, Monte AA, et al. A characterization of synthetic cannabinoid exposures reported to the national poison data system in 2010. Ann Emerg Med. 2012;60(4):435–438. doi:10.1016/j.annemergmed.2012.03.007.
- Sznitman SR, Pinsky-Talbi L, Salameh M, et al. Cannabis and synthetic cannabinoid exposure reported to the Israel poison information center: examining differences in exposures to medical and recreational compounds. Int J Drug Policy. 2020;77:102711. doi:10.1016/j.drugpo.2020.102711.
- Norman C, Halter S, Haschimi B, et al. A transnational perspective on the evolution of the synthetic cannabinoid receptor agonists market: comparing prison and general populations. Drug Test Anal. 2021;13(4):841–852. doi:10.1002/dta.3002.
- Thomas SHL, Vidler D, Officer J, et al. Changing patterns of synthetic cannabinoid receptor agonists encountered in UK emergency departments. Clin Toxicol. 2021;59(6):538–538.
- United Nations Office on Drugs and Crime. Regional diversity and the impact of scheduling on NPS trends. Global SMART Update. 2021;25:3–6
- Liakoni E, Yates C, Dines AM, et al. Acute recreational drug toxicity: comparison of self-reports and results of immunoassay and additional analytical methods in a multicenter european case series. Medicine (Baltimore). 2018;97(5):e9784. doi:10.1097/MD.0000000000009784.
- Hermanns-Clausen M, Kneisel S, Szabo B, et al. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction. 2013;108(3):534–544. doi:10.1111/j.1360-0443.2012.04078.x.