Abstract
Introduction
Bupropion is a popular antidepressant due to its favorable side effect profile and indications for smoking cessation and weight loss. Due to the possibility of delayed onset seizure and other adverse outcomes after bupropion overdose, patients are often observed for periods of 12-24 hours following suspected ingestion. Tachycardia is a clinical predictor that holds promise in differentiating cases at risk for seizures from low-risk cases that do not require prolonged observation. This study assessed whether heart rate within the first eight hours of presentation can identify cases that do not require extended observation.
Methods
This is a retrospective cohort study of all supra-therapeutic bupropion cases from two hospital systems between 2010 and 2022.
Results
Data from 216 charts were included. Seizures, hypotension, and dysrhythmias occurred in 19 percent (n = 41), 1.4 percent (n = 3), 0.9 percent (n = 2) respectively. One patient died. Delayed adverse effects were rare (n = 4); they occurred from 14 hours to 28 hours post-ingestion. Maximum heart rate in eight hours was associated with a risk of adverse outcomes. (odds ratio, 1.07; 95 percent confidence interval: 1.05 to 1.09; P < 0.001). An eight hour maximum heart rate threshold of 104 beats/minute had a negative predictive value of 100 percent (95 percent confidence interval: 96.7 percent to 100 percent) for the occurrence of delayed adverse effects. All patients with delayed effects had tachycardia within five hours of emergency department arrival.
Discussion
Delayed adverse outcomes of seizures, hypotension, dysrhythmia, and death were uncommon in this cohort. Heart rate during the first eight hours of observation performs reliably as a screening test to identify patients at low risk for delayed adverse outcomes. This study is limited by its retrospective nature, the inability to ascertain time of ingestion for most cases and the lack of confirmatory laboratory testing.
Conclusion
This study supports the use of an eight hour observation period when there are no other clinical signs of toxicity to warrant admission and if no co-ingestion or administration of substances that mask tachycardia are present.
Introduction
Bupropion is an antidepressant that acts through norepinephrine and dopamine reuptake inhibition and is hepatically metabolized to active metabolites. It is prescribed frequently given its favorable side effect profile and additional use for smoking cessation and weight loss [Citation1]. It has a narrow therapeutic index and is associated with seizures, cardiotoxicity, and hemodynamic collapse in overdose [Citation2–5]. Bupropion is one of the most commonly ingested antidepressants and is among the class of antidepressants associated with the most severe outcomes since 2020 [Citation6,Citation7]. Seizures and other adverse events may be delayed for as long as 24 h after bupropion overdose, particularly with extended-release formulations [Citation8,Citation9]. Therefore, a minimum observation period of 12-24 h is often recommended after a suspected extended-release bupropion overdose [Citation8]. However, most patients who develop seizures following bupropion overdose do so within 8 h [Citation8,Citation10,Citation11]. A 24 h observation period after an overdose often commits a patient to hospital admission with associated costs and the potential to delay psychiatric care which may be unnecessary in low-risk patients. While central nervous system symptoms of agitation or tremors are often present before seizures and other adverse outcomes, seizures have occurred without preceding central nervous system toxicity [Citation8]. Previous studies have established tachycardia as a risk factor for seizures and delayed seizures [Citation8,Citation9,Citation11–14]. The purpose of this study was to assess the utility of using heart rate within the first 8 h of presentation to identify cases with low risk of delayed adverse outcomes and do not require extended observation.
Methods
Study design
This was a retrospective cohort study of patients identified as having ingested a supratherapeutic dose of bupropion who were evaluated at two hospital systems between January 1, 2010 and December 31, 2022. Patients were identified via a query of the electronic medical record of a single poison center (ToxSentry, Grady Memorial Hospital Corporation, Jacksonville FL), and cases were included if they were evaluated at one of the two hospital systems where chart abstractors had access to the electronic medical record. Cases with co-ingestion of substances that could have masked tachycardia (e.g., beta-adrenergic antagonists, calcium channel blockers, cardioactive steroids, cholinergics, and alpha2-adrenergic agonists) were excluded. The study sites included one tertiary care academic children’s hospital and one tertiary care adult hospital system with six affiliated community hospitals. The study received local institutional review board approval.
Data collection
Data was abstracted by two unblinded data abstractors (DI, KE) using a standardized data abstraction guide and survey form. Survey results were exported into Excel for analysis. Reviewers were trained in the systematic chart review process. Twelve percent of the charts were reviewed by both abstractors and kappa statistic was calculated to assess interrater reliability. Data abstracted included demographic information (age, gender, ethnicity, weight), ingestion details (dose, formulation, ingestion time when reported, ingestion reason, co-ingestion), clinical details (hospital arrival time, hospital length of stay, arrival vital signs, gastrointestinal decontamination performed, intensive care unit (ICU) admission, occurrence of hospital return within 24 h of discharge) as well as clinical variables and outcomes of interest. These included the highest recorded heart rate within 8 h of hospital arrival, presence of tachycardia, timing of tachycardia, altered mental status, electrocardiogram (ECG) QRS complex and QTc interval abnormalities, occurrence of clinical outcomes of seizures, hypotension, dysrhythmia, or death and timing of first occurrence of clinical outcomes. Both hospital systems use the same commercially available electronic medical record.
Data sharing
De-identified data is available upon request to outside qualified investigators pending approval from our local institutional review board.
Study definitions
Delayed adverse outcomes were defined as seizures, hypotension, dysrhythmia or death occurring more than 12 h after hospital presentation. Tachycardia was defined for ages ten years and older as heart rate greater than 100 beats/min. For ages less than ten years, tachycardia was defined according to Pediatric Advanced Life Support (PALS) guidelines (ages 4-9 years heart rate greater than 120 beats/min; ages 1-3 years, heart rate greater than 140 beats/min). Altered mental status was defined as the presence of documented agitation, hallucinations, tremulousness, or changes in orientation. Electrocardiograms obtained in the first 8 h of presentation were reviewed. QRS complex and QTc interval (Bazett’s) prolongation were defined as QRS complex greater than 120 ms and QTc interval greater than 460 ms in a female patient and 450 ms in a male patient that was new from prior ECGs or when no prior ECGs were available. Hypotension was defined as a documented significant drop in blood pressure below normal ranges for age that was sustained on two or more consecutive measurements and resulted in a critical intervention such as the initiation of fluid boluses or vasopressors. (systolic cut offs: ages ≥10 years, 90 mmHg, ages 1 − 3 years 76 mmHg, ages 4 − 6 years <80 mmHg, ages 7 − 9 years < 84 mmHg). Dysrhythmia was defined as a documented heart rhythm requiring intervention with cardioversion or defibrillation.
Outcomes
Our primary outcome was a composite endpoint of delayed seizure, hypotension, dysrhythmia, or death. Secondary outcomes were occurrences of the same adverse effects at any point, hospital length of stay, and ICU admission.
Data analysis
Descriptive statistics were reported for demographic data and clinical outcomes of interest. Nonnormally distributed continuous data were reported as medians (interquartile range [IQR]) and statistical difference was determined by Mann-Whitney U test. Differences between categorical data were determined by Fisher’s exact test. Logistic regression analysis was used to calculate odds ratios and confidence intervals to ascertain the relationship between heart rate, other clinical signs of toxicity, and adverse outcomes. Sensitivity and specificity were calculated for each recorded maximum heart rate in 8 h and a receiver-operating characteristic (ROC) curve was generated. Receiver-operating characteristic curve analysis and Youden’s J-statistic was used to determine a heart rate threshold that maximizes diagnostic accuracy while retaining 100% sensitivity for predicting the development of adverse outcomes. The negative predictive value (NPV) of this threshold for the occurrence of adverse outcomes and delayed adverse outcomes was calculated with confidence intervals. Statistical analysis was performed using SPSS software (Statistics Package for Social Sciences; IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp). For all analyses, a “P” value of 0.05 was used to demonstrate statistical significance, and confidence intervals were reported at the 95% level.
Results
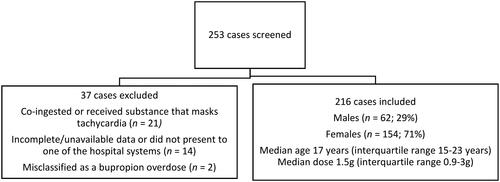
A total of 253 cases were screened for inclusion out of which 37 cases were excluded because of the presence of substances that mask tachycardia, incomplete or unavailable data on chart review, or the case did not have a reported supratherapeutic bupropion ingestion on chart review (). A total of 216 cases were included in the final analysis. Most cases involved female patients (71%, n = 154) and the median age was 17 (IQR 15-23) years. Ingestions involving extended-release, 24 h, sustained-release, 12 h and immediate-release formulations comprised 72% (n = 155), 15% (n = 32), 2% (n = 5) of all cases respectively. Ingestion reason was reported as intentional, unintentional, and unknown in 85% (n = 183), 12.5% (n = 27), and 3% (n = 6) respectively. Co-ingestion occurred in 50% (n = 109) of cases. Gastrointestinal decontamination with activated charcoal occurred in 29% of cases. No patients received gastrointestinal decontamination with orogastric lavage or whole bowel irrigation. The dose ingested was reported in 146 cases and the median dose ingested was 24.2 (IQR 14.3-50.8) mg/kg. The median hospital length of stay was 32 (IQR 20-54) h. Admission to the ICU occurred in 40% (n = 86) of all cases. No hospital returns after discharge were captured on chart review.
Seizures, hypotension, and dysrhythmias occurred in 19% (n = 41), 1.4% (n = 3), 0.9% (n = 2) respectively. One patient died from complications associated with extracorporeal membrane oxygenation cannulation after sustaining pulseless refractory ventricular tachycardia. Of all the cases with an adverse outcome, 76% (n = 30) involved an extended-release formulation, 14% (n = 6) involved a sustained-release formulation and one case involved an immediate-release formulation. There were five cases with delayed adverse effects.
Their characteristics are summarized in . There were no statistically significant differences in the gender demographic, median age, formulation ingested, ingestion reason, and presence of co-ingestion between patients with adverse outcomes and those without (). Patients with adverse outcomes had a greater median dose ingested than those without: 80.4 (IQR 26.6-115.7) mg/kg versus 22 (IQR 12.4-41.6) mg/kg, P = 0.01. Gastrointestinal decontamination with activated charcoal was performed more frequently in patients without adverse outcomes than those with adverse outcomes (33% versus 14%, P = 0.014).
Table 1. Characteristics of cases with delayed adverse outcomes.
Table 2. Clinical and demographical data with comparison between adverse effect and no adverse effect groups.
Of the clinical markers of toxicity evaluated, tachycardia, altered mental status, and QRS complex prolongation each had a statistically significant association with the development of adverse outcomes (). Using logistic regression, the presence of altered mental status had an odds ratio of 2.62 (95% CI: 1.28 − 5.37), and QRS complex prolongation had an odds ratio of 4.08 (95% CI: 1.21 − 13.79) for the development of adverse outcomes (). Every increase of one beat per min in the fastest heart rate in 8 h was associated with 7% increased odds of adverse outcomes. (OR 1.07, 95% CI: 1.05 − 1.10). The presence of QRS complex prolongation had a sensitivity of 14.2% (95% CI: 5.4% − 28.5%) and specificity of 96.5% (95% CI: 92.7% − 98.7%) while the presence of altered mental status had a sensitivity of 66.7% (95% CI: 50.5% − 80.4%) and a specificity of 58.1% (95% CI: 50.3% − 65.5%) for the development of adverse outcomes. The ROC analysis for peak 8 h heart rate for the development of adverse outcomes had an area under the curve (AUC) of 0.843 (95% CI: 0.782 − 0.903) (). A peak 8 h heart rate cutoff of 104 beats/min determined by Youden index had 100% sensitivity (95% CI: 91.6% − 100%) and 37% specificity (95% CI: 29.6% − 44.4%) for all adverse outcomes and 100% sensitivity (95% CI: 47.8% − 100%) and 53% specificity (95% CI: 45.6% − 59.5%) for delayed adverse outcomes. It had a NPV of 100% (95% CI: 96.7% − 100%) for the occurrence of delayed adverse outcomes.
Figure 2. Receiver operating characteristic curve for fastest heart rate in 8 h and adverse outcomes.
Area under the curve = 0.843; 95% confidence interval: 0.782 to 0.903.
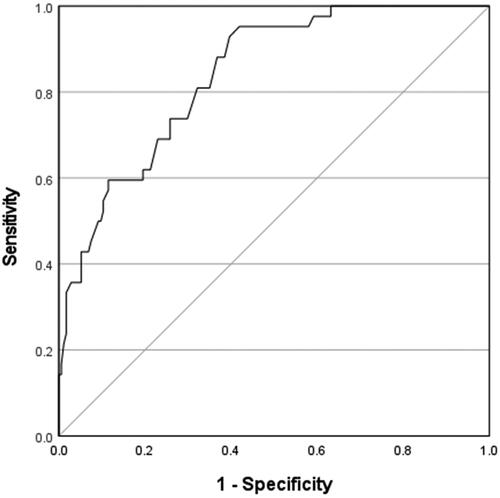
Table 3. Association of clinical signs of toxicity with development of adverse outcomes.
Table 4. Univariate logistic regression model results for association of clinical signs of toxicity with outcome of adverse effects.
Tachycardia was present on arrival in 66% of all cases (n = 143). Of all the patients with adverse outcomes, 100% had documented tachycardia within 8 h of arrival. Tachycardia was present on arrival in 93% (n = 39) of those cases. The longest time before tachycardia occurred regardless of outcome and reported formulation ingested was 5 h after hospital arrival in a patient who had delayed adverse effects at 14 h (). All other cases with delayed effects had tachycardia within 30 min of arrival.
A subgroup analysis was performed on cases with reported ingestion times (n = 173). Amongst cases with adverse outcomes, 26 had ingestion time reported. All 26 cases had documented seizures and the majority had tachycardia on arrival (85%, n = 22). The median time to seizure from ingestion time was 8 h (IQR 3.7 – 11 h) and among the patients who were not tachycardic on arrival, the median time to tachycardia was 5 h (IQR 3.0 − 6.3 h). Hypotension occurred in one patient 16 h post-ingestion who developed tachycardia at 5.5 h post-ingestion. Dysrhythmia occurred in one patient at 16 h post-ingestion who later died at 32 h and was tachycardic on arrival. The longest time to develop tachycardia from ingestion time among all 26 patients with adverse outcomes was 6 h.
Kappa statistics ranged from 0.4 to 0.7 for each variable evaluated.
Discussion
This study sought to determine if heart rate within 8 h of observation could be used to determine which patients may be medically cleared after a bupropion overdose and differentiate them from patients who need extended observation. Our findings show that heart rate during the first 8 h of observation performs reliably as a screening test to identify patients at low risk for delayed adverse outcomes. Offerman and colleagues [Citation11] suggested that an 8 h observation may be sufficient for the majority of bupropion overdose including extended-release formulations and found that arrival tachycardia and sustained tachycardia were strongly associated with the development of delayed seizures. Our study found that the absence of tachycardia within 8 h of observation was associated with the absence of delayed seizures and other adverse effects.
Our findings support conclusions from previous studies that the majority of patients develop adverse outcomes (most commonly seizures) within 8 h of presentation [Citation8–11,Citation15]. Cases of delayed adverse outcomes were rare in this cohort. In the Offerman [Citation11] study cohort, all patients who had delayed seizures had tachycardia on arrival or developed tachycardia shortly after arrival. Our study corroborates this finding: all patients with delayed adverse effects except one were tachycardic on arrival, with one patient developing tachycardia at 5 h after arrival.
Several reports have described the occurrence of delayed seizures or cardiotoxicity after bupropion overdose that have occurred at 24 h and beyond [Citation8,Citation9,Citation11,Citation12,Citation16,Citation17]. Delayed seizures occurred as late as 28 h in our study. This patient had co-ingestions with multiple anticholinergic drugs and benzodiazepines () which likely contributed to the delayed occurrence of seizures. The patient was tachycardic on hospital arrival and remained tachycardic preceding the occurrence of the first seizure at 28 h. Previous studies that have suggested the need for a 24 h minimum observation period for all overdoses involving extended-release formulations were limited by the lack of data on vital signs leading up to this adverse outcome [Citation8]. Our study conclusion that an 8 h observation period is sufficient is strengthened by our report on the maximal time to onset of tachycardia which is a vital clinical consideration for using heart rate as a triaging tool.
Hospital length of stay was often prolonged (>24 h) in this cohort even in patients without adverse outcomes. The period evaluated in this study included a period when our poison center guideline recommended 24 h observation for ingestions involving extended-release formulation. Our poison center later adopted a 12 h observation for these cases but some variation (12 versus 24 h observation) remained among consulting toxicologists. Some of the patients admitted may have been safely cleared with a shorter observation window. Using the heart rate cut-off of 104 beats/min in the first 8 h of observation to clear patients who did not proceed to have an adverse outcome would have led to 24% reduction in hospital admissions in this cohort.
Different heart rate cutoffs have been used in different studies to define tachycardia. While the most common cutoff is heart rate > 100 beats/min for ages >12 years old, some studies have used a cut-off as high as 140 beats/min. Rianprakaisang and colleagues [Citation13], found that tachycardia defined at heart rate >140 beats/min was associated with the outcome of seizures but did not reach statistical significance. Their study did not evaluate if a heart rate <140 beats/min predicted a clinical course with no seizures and the limited time window captured by the data registry used would have limited such conclusions. Starr and colleagues [Citation8], found that tachycardia defined as a heart rate >100 had 91.2% sensitivity and 92.9% NPV for predicting seizures. In our study, a heart rate cut-off of 104 beats/min had a 100 percent sensitivity and NPV for the predetermined composite endpoint of adverse outcomes which included seizures. One patient in our study had a peak heart rate of 105 beats/min and proceeded to have delayed seizures. Since cases that had the presence of substances that significantly blunt heart rate were excluded, the findings of this study support using a lower heart rate cut-off (100-104 beats/min) if using heart rate as a test in an otherwise asymptomatic patient.
Altered mental status was found to be associated with delayed adverse outcomes. This replicates similar findings from other studies [Citation8,Citation11,Citation14]. However, altered mental status is not a sensitive enough marker to exclude a risk of adverse outcomes and is less important as a triaging tool since a patient with an abnormal mental status is unlikely to be medically cleared. Bupropion overdose is associated with QRS complex prolongation via cardiac gap junction inhibition and QTc prolongation via Ikr inhibition [Citation18]. Rianprakaisang and colleagues [Citation13] found that QTc interval >500 ms had a statistically significant association with the development of seizures. However, no QTc interval cut off could be identified on ROC curve analysis for predicting seizures. Stewart and colleagues [Citation12] defined ECG interval changes as QTc interval >450 ms or QRS complex prolongation >110 ms and found that ECG interval changes were more common in patients who had an outcome of seizures [Citation12]. This study used slightly different definitions for QRS complex and QTc interval prolongation which makes direct comparison to these prior studies limited. A QRS complex duration >120 ms reached statistical significance for association with adverse outcomes while QTc interval prolongation as defined elsewhere in this paper did not reach statistical significance, however QRS complex prolongation as defined in our study was not a sensitive marker for predicting adverse outcomes. More studies are required to further evaluate the prognostic value of ECG intervals for predicting a benign clinical course.
Limitations
This study is limited by its retrospective nature and geographic limitation. Misclassification and recording bias are well-described limitations of retrospective studies [Citation19–21]. Although our patient sample was limited to two hospital systems in which electronic medical record access was available, the patients represented a large catchment of the geographical region. The included hospital systems are two of the largest tertiary care centers in the state and the pediatric hospital is one of only two hospitals that admits pediatric patients in that region of the state. This systematic chart review depended on data entered into the electronic medical recordat the time of care, often based on patient or family member reports, with no way to confirm accuracy. The bupropion exposures in this cohort were not confirmed by laboratory testing. Additionally, chart abstractors were unblinded to study hypotheses. Error in data entry and differences in interpretation of subjective clinical data is a limitation of manual chart review. However, assessment of interrater reliability using kappa statistic suggested an acceptable consistency between chart abstractors. This study cohort was skewed towards an adolescent population with a median age of 17 years which may limit generalizability. Previous studies have suggested that age may be associated with risk of seizures and adverse outcomes [Citation11–13] with some studies suggesting higher risk in the adolescent (13-18 year old) group [Citation13,Citation14]. Despite a skew towards an adolescent population, seizure and adverse effects were lower in our cohort (19%) than previously reported, possibly indicating a less sick population. One of the biggest limitations of this study was the inability to ascertain time of ingestion for most cases. Primary study analyses were performed with hospital arrival times since ingestion times were often unknown and the accuracy of ingestion times even when recorded could not be confirmed. Another limitation was that observation periods were not controlled and varied significantly. While we found no hospital readmissions on chart review, it is conceivable that some patients may have had an adverse effect after discharge and presented to different hospital systems which were not reflected in the electronic medical record. This would have been a rare occurrence, however, as most hospital visits within the region are visible in the electronic medical record through a feature that displays visit summaries across other institutions. Our decision to exclude patients with a known co-ingestion of substances that masks tachycardia is a potential limitation of this study. Ruling out certain co-ingestions is difficult in practice and many intentional ingestions involve polypharmacy. Our method of excluding these cases was based on history reported and the medications to which the patient had access. This reflects the realities of excluding these ingestions in clinical practice and thus expands the applicability of our study findings in a typical overdose scenario. The findings of this study should not be extrapolated to cases in which a co-ingestion of a substance that masks tachycardia is suspected.
Conclusion
In this retrospective cohort study of 216 patients after supratherapeutic bupropion ingestion, delayed adverse outcomes of seizures, hypotension, dysrhythmia, and death were uncommon. A heart rate less than 104 beats/min within 8 h of observation was associated with a benign clinical course and tachycardia occurred within 5 h of hospital admission in all patients with adverse outcomes. This study supports using absence of tachycardia to make the decision to medically clear a patient at 8 h if there are no other clinical signs of toxicity to warrant longer observation and if no xenobiotic that may mask tachycardia was co-ingested or administered. Prospective validation of study findings is warranted.
Supplemental Material
Download MS Word (29.4 KB)Disclosure statement
The authors report no conflicts of interest associated with this publication.
Additional information
Funding
References
- Patel K, Allen S, Haque MN, et al. Bupropion: a systematic review and meta-analysis of effectiveness as an antidepressant. Ther Adv Psychopharmacol. 2016;6(2):99–144. doi: 10.1177/2045125316629071.
- Heise CW, Skolnik AB, Raschke RA, et al. Two cases of refractory cardiogenic shock secondary to bupropion successfully treated with Veno-Arterial extracorporeal membrane oxygenation. J Med Toxicol. 2016;12(3):301–304. doi: 10.1007/s13181-016-0539-7.
- Sirianni AJ, Osterhoudt KC, Calello DP, et al. Use of lipid emulsion in the resuscitation of a patient with prolonged cardiovascular collapse after overdose of bupropion and lamotrigine. Ann Emerg Med. 2008;51(4):412–415, 415.e1. doi: 10.1016/j.annemergmed.2007.06.004.
- Shrier M, Díaz JE, Tsarouhas N. Cardiotoxicity associated with bupropion overdose. Ann Emerg Med. 2000;35(1):100. doi: 10.1016/s0196-0644(00)70119-3.
- Chhabra N, DesLauriers C, Wahl M, et al. Management of severe bupropion poisoning with intravenous lipid emulsion. Clin Toxicol (Phila). 2018;56(1):51–54. doi: 10.1080/15563650.2017.1337909.
- Spyres MB, Aldy K, Farrugia LA, Toxicology Investigators Consortium Study Group., et al. The toxicology investigators consortium 2020 annual report. J Med Toxicol. 2021;17(4):333–362. doi: 10.1007/s13181-021-00854-3.
- Gummin DD, Mowry JB, Beuhler MC, et al. 2022 Annual report of the national poison data system (NPDS) from america’s poison centers: 40th annual report. Clin Toxicol (Phila). 2023;61(10):717–939. doi: 10.1080/15563650.2023.2268981.
- Starr P, Klein-Schwartz W, Spiller H, et al. Incidence and onset of delayed seizures after overdoses of extended-release bupropion. Am J Emerg Med. 2009;27(8):911–915. doi: 10.1016/j.ajem.2008.07.004.
- Shepherd G, Velez LI, Keyes DC. Intentional bupropion overdoses. J Emerg Med. 2004;27(2):147–151. doi: 10.1016/j.jemermed.2004.02.017.
- Spiller HA, Ramoska EA, Krenzelok EP, et al. Bupropion overdose: a 3-year multi-center retrospective analysis. Am J Emerg Med. 1994;12(1):43–45. doi: 10.1016/0735-6757(94)90195-3.
- Offerman S, Gosen J, Thomas SH, et al. Bupropion associated seizures following acute overdose: who develops late seizures. Clin Toxicol (Phila). 2020;58(12):1306–1312. doi: 10.1080/15563650.2020.1742919.
- Stewart E, Grewal K, Hudson H, et al. Clinical characteristics and outcomes associated with bupropion overdose: a Canadian perspective. Clin Toxicol (Phila). 2020;58(8):837–842. doi: 10.1080/15563650.2019.1699658.
- Rianprakaisang TN, Prather CT, Lin AL, Toxicology Investigators Consortium (ToxIC)., et al. Factors associated with seizure development after bupropion overdose: a review of the toxicology investigators consortium. Clin Toxicol (Phila). 2021;59(12):1234–1238. doi: 10.1080/15563650.2021.1913180.
- Offerman S, Levine M, Gosen J, et al. Pediatric bupropion ingestions in adolescents vs. Younger children-a tale of two populations. J Med Toxicol. 2020;16(1):6–11. doi: 10.1007/s13181-019-00738-7.
- Balit CR, Lynch CN, Isbister GK. Bupropion poisoning: a case series. Med J Aust. 200320;178(2):61–63. doi: 10.5694/j.1326-5377.2003.tb05064.x.
- Liss DB, Phillips TM, Pizon AF, et al. Delayed seizures in bupropion overdose with concomitant ingestion of alpha-2 agonist: a case report. Toxicology Communications. 2017;1(1):15–17. doi: 10.1080/24734306.2017.1364839.
- Al-Abri SA, Orengo JP, Hayashi S, et al. Delayed bupropion cardiotoxicity associated with elevated serum concentrations of bupropion but not hydroxybupropion. Clin Toxicol (Phila). 2013;51(10):1230–1234. doi: 10.3109/15563650.2013.849349.
- Caillier B, Pilote S, Castonguay A, et al. QRS widening and QT prolongation under bupropion: a unique cardiac electrophysiological profile. Fundam Clin Pharmacol. 2012;26(5):599–608. ´ doi: 10.1111/j.1472-8206.2011.00953.x.
- Worster A, Bledsoe RD, Cleve P, et al. Reassessing the methods of medical record review studies in emergency medicine research. Ann Emerg Med. 2005;45(4):448–451. doi: 10.1016/j.annemergmed.2004.11.021.
- Kaji AH, Schriger D, Green S. Looking through the retrospectoscope: reducing bias in emergency medicine chart review studies. Ann Emerg Med. 2014;64(3):292–298. doi: 10.1016/j.annemergmed.2014.03.025.
- Gilbert EH, Lowenstein SR, Koziol-McLain J, et al. Chart reviews in emergency medicine research: where are the methods? Ann Emerg Med. 1996;27(3):305–308. doi: 10.1016/s0196-0644(96)70264-0.