Abstract
Introduction
Acute metamfetamine toxicity is characterized by stimulant effects and neuropsychiatric disturbance, which is attenuated by gamma-aminobutyric acid type A receptor agonists including benzodiazepines. We utilized clinical registry data to examine the effect of co-exposure to a gamma-aminobutyric acid type B receptor agonist (gamma-hydroxybutyrate) in illicit drug cases with analytically confirmed exposure to metamfetamine.
Methods
The Emerging Drugs Network of Australia Victoria is an ethics board-approved prospective registry collecting clinical and analytical data (utilising blood samples) on emergency department illicit drug presentations. Comparison groups were defined by analytically confirmed exposure: lone metamfetamine, metamfetamine plus gamma-hydroxybutyrate, metamfetamine plus benzodiazepine, metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine. Cases with co-exposure to other stimulants or sedatives were excluded.
Results
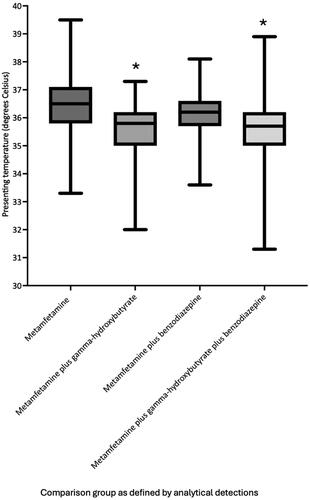
Median metamfetamine blood concentrations were significantly greater in metamfetamine plus gamma-hydroxybutyrate (n = 153, median = 0.20 mg/L, interquartile range: 0.10–0.32 mg/L, 95 per cent confidence interval: 0.20–0.23 mg/L) and metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine (n = 160, median = 0.20 mg/L, interquartile range: 0.10–0.30 mg/L, 95 per cent confidence interval: 0.20–0.30 mg/L) positive groups compared to gamma-hydroxybutyrate negative groups including metamfetamine (n = 81, median = 0.10 mg/L, interquartile range: 0.05–0.21 mg/L, 95 per cent confidence interval: 0.09–0.18 mg/L) and metamfetamine plus benzodiazepine (n = 73, median = 0.10 mg/L, interquartile range: 0.06–0.20 mg/L, 95 per cent confidence interval: 0.09–0.20 mg/L) groups (P < 0.0004). Presenting heart rate in metamfetamine plus gamma-hydroxybutyrate cases (n = 153, median = 72 beats per minute, interquartile range: 63–86 beats per minute, 95 per cent confidence interval: 70–78 beats per minute) was significantly lower than metamfetamine plus benzodiazepine cases (n = 73, median = 84 beats per minute, interquartile range: 73–98 beats per minute, 95 per cent confidence interval: 80–90 beats per minute, P < 0.0001), and lone metamfetamine cases (n = 81, median = 110 beats per minute, interquartile range: 87–131 beats per minute, 95 per cent confidence interval: 93–120 beats per minute, P < 0.0001). Presenting temperature in metamfetamine plus gamma-hydroxybutyrate cases (median = 35.8 °C, interquartile range: 35.0–36.2 °C, 95 per cent confidence interval 35.6–35.9 °C) was significantly lower than metamfetamine plus benzodiazepine cases (median 36.2 °C, interquartile range 35.7–36.6 °C, 95 per cent confidence interval, 36.0–36.4 °C, P = 0.017), and lone metamfetamine cases (median = 36.5 °C, interquartile range: 35.8–37.1 °C, 95 per cent confidence interval: 36.2–36.7 °C, P < 0.0001). Median presenting systolic blood pressure was significantly (P ≤ 0.001) lower in benzodiazepine positive groups (metamfetamine plus benzodiazepine median = 120 mmHg, interquartile range: 109–132 mmHg, 95 per cent confidence interval: 116–124 mmHg and metamfetamine plus benzodiazepine plus gamma-hydroxybutyrate median = 124 mmHg, interquartile range: 110–137 mmHg, 95 per cent confidence interval: 120–129 mmHg). Incidence of sedation (Glasgow Coma Scale less than 9) was significantly greater in metamfetamine plus gamma-hydroxybutyrate cases (63 per cent) compared to metamfetamine plus benzodiazepine cases (27 per cent, P < 0.0001) and lone metamfetamine cases (15 per cent, P < 0.0001). Incidence of agitation was significantly lower in metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine cases (17 per cent, P < 0.0001) and metamfetamine plus gamma-hydroxybutyrate cases (34 per cent, P = 0.0004) compared to lone metamfetamine cases (58 per cent).
Discussion
Differences in gamma-aminobutyric acid type A and B receptor physiology may offer a gamma-aminobutyric acid type B agonist-facilitated alternative pharmacodynamic mechanism able to attenuate metamfetamine stimulant and neuropsychiatric toxicity.
Conclusion
Metamfetamine intoxicated patients with analytically confirmed co-exposure to gamma-hydroxybutyrate had significantly reduced heart rate, body temperature and incidence of agitation compared to patients with lone metamfetamine exposure. Metamfetamine intoxicated patients with analytically confirmed co-exposure to a benzodiazepine had significantly reduced systolic blood pressure compared to patients with lone metamfetamine exposure. We hypothesize that gamma-aminobutyric acid type B receptor agonists may be beneficial in the management of acute metamfetamine toxicity.
Introduction
Acute metamfetamine (methylamphetamine, methamphetamine) toxicity is associated with stimulant and neuropsychiatric disturbance, including agitation [Citation1]. Severe stimulant effects can produce life-threatening physiological derangements including cardiac dysrhythmias, hyperthermia, disseminated intravascular coagulation, rhabdomyolysis, and hypertension with secondary vascular accidents [Citation1–4]. Acute neuropsychiatric disturbances including agitation, psychosis and impaired judgement can place intoxicated individuals and health care providers at risk of harm [Citation1,Citation5].
Sympatholytics, including benzodiazepine receptor agonists are established interventions in managing acute metamfetamine intoxication [Citation6–8]. Limited evidence demonstrates that benzodiazepines including diazepam, lorazepam, and midazolam reduce metamfetamine induced agitation and sympathomimetic stimulation [Citation9–12]. Benzodiazepines enhance gamma-aminobutyric acid type A (GABAA) central nervous system inhibition [Citation6,Citation7]. Non-benzodiazepine GABAB receptor agonists may also provide positive treatment effects in acute metamfetamine toxicity via GABAB-mediated central nervous system inhibition. Although baclofen may provide benefit in treating metamfetamine dependence [Citation13,Citation14], the potential benefit of non-benzodiazepine GABAB agonists in treating acute metamfetamine toxicity has not been extensively studied.
The Emerging Drugs Network of Australia Victoria is a prospective study collecting clinical and analytical data on cases of illicit drug toxicity presenting to a network of hospital emergency departments in the state of Victoria, Australia [Citation15]. Metamfetamine and gamma-hydroxybutyrate are the commonest illicit drugs detected within the Emerging Drugs Network of Australia Victoria study. Anecdotally, Emerging Drugs Network of Australia Victoria investigators have noted that clinical features are predominantly sedative in cases with co-detection of metamfetamine and gamma-hydroxybutyrate. The availability of a series of cases with analytical quantification of both gamma-hydroxybutyrate and metamfetamine concentrations provided the opportunity to study the pharmacodynamic interaction between these drugs in more detail. We hypothesized that gamma-hydroxybutyrate may attenuate acute metamfetamine stimulatory and neuropsychiatric effects.
Methods
Study dataset
The Emerging Drugs Network of Australia Victoria is a multi-institutional, prospective clinical study utilizing comprehensive analysis of blood samples obtained from a purposive sample of individuals presenting to Victorian emergency departments with illicit substance related toxicity [Citation15]. Under an ethics committee approved waiver of consent, de-identified clinical and analytical data are collated within a secure registry (EDNAV Clinical Registry, HREC/66506/Austin-2020). Full Emerging Drugs Network of Australia Victoria study methodology has been published in detail elsewhere [Citation16]. Since initial study commencement in September 2020, an additional seven hospital sites have been added, with the study now operational across seventeen hospitals (13 metropolitan and four regional). All Emerging Drugs Network of Australia Victoria data are stored in a secure online Research Electronic Data Capture (REDCap) database [Citation16,Citation17].
Analytical methodology
Blood samples obtained from cases at the time of hospital presentation undergo comprehensive toxicological analysis at the Victorian Institute of Forensic Medicine Toxicology Department. Samples are analysed using two separate liquid chromatography-tandem mass spectrometric (LC-MS/MS) screens of 575 pharmaceutical, illicit, and novel substances [Citation18]. Samples are subsequently analysed using untargeted screening via liquid chromatography quadrupole-time-of-flight mass spectrometry (LC-QTOF-MS) using the crowd-sourced HighResNPS.com database [Citation19,Citation20]. Ethanol is not routinely measured as part of the comprehensive screen but is measured at the attending physician’s discretion at study hospital sites.
Case selection and study groups
All Emerging Drugs Network of Australia Victoria registry cases for the period September 2020 – November 2023 aged 16 years and over were considered potentially eligible for study inclusion. To examine the effect of co-exposure to gamma-hydroxybutyrate in cases of metamfetamine exposure, four comparison groups were defined based on analytical results of toxicological analysis: Cases with lone metamfetamine exposure, cases with metamfetamine and gamma-hydroxybutyrate exposure, cases with metamfetamine and benzodiazepine exposure, and cases with metamfetamine, gamma-hydroxybutyrate and benzodiazepine exposure.
Quantitative metamfetamine and gamma-hydroxybutyrate blood concentrations were available for analysis. In cases of metamfetamine reported as ‘detected’, a concentration at the lower limit of reporting was utilised for analysis (0.05 mg/L). Quantitative benzodiazepine blood concentrations were available for detections of common pharmaceutical benzodiazepines but were not available for designer/novel benzodiazepine detections.
Cases in which a sedative medication was administered prior to the recording of initial physiological observations were excluded. To avoid potential confounding effects of other pharmaceutical or illicit drug exposures, cases were excluded if any drug (other than metamfetamine, gamma-hydroxybutyrate, or a benzodiazepine) known to have stimulant or depressant properties was co-detected. Co-detected classes of illicit drugs that resulted in case exclusion included stimulants (including all phenethylamine derivatives other than metamfetamine), synthetic cannabinoid receptor agonists, hallucinogens, dissociatives, opioids, and miscellaneous novel psychoactive substances including mitragynine. Co-detected classes of pharmaceuticals that resulted in case exclusion included GABA receptor agonists other than gamma-hydroxybutyrate/benzodiazepines, opioids, pharmaceutical stimulants, antidepressants, centrally acting alpha2 adrenergic receptor agonists, antihistamines, and antipsychotics.
Comparative measures of neuropsychiatric and sympathomimetic effects of metamfetamine
Metamfetamine blood concentrations were analysed to assess any difference in baseline exposure concentration between groups. To assess differences in metamfetamine sympathomimetic effects, heart rate (beats per minute, systolic blood pressure (mmHg), diastolic blood pressure (mmHg), and temperature (degrees Celsius) measured at the time of hospital presentation were compared. Incidence of agitation was compared between groups as a measure of neuropsychiatric toxicity. The incidence of sedation (defined in this study as a Glasgow Coma Scale <9) at the time of hospital presentation was also compared.
Statistical analysis
Statistical analysis was undertaken using Microsoft Excel (version 16.65, Microsoft Corporation, Redmond, WA, USA) and GraphPad Prism (version 10.0.3 GraphPad Software inc., San Diego, CA, USA). Groups were described using descriptive statistics including median and inter-quartile range (IQR) for non-normally distributed data. The Shapiro-Wilk test was used to test for normality. Mann–Whitney U-test was used to compare groups. Fisher’s exact test was used to compare proportions. GraphPad Prism (version 10.0.3 GraphPad Software inc., San Diego, CA, USA) was used to present comparison of group data as scatter plots and proportions as bar graphs.
Results
There were 2,473 documented Emerging Drugs Network of Australia Victoria presentations with comprehensive toxicological analysis completed during the study period. Metamfetamine was detected in 68.2% (n = 1,687) of presentations. Following exclusion of cases with co-detection of potential confounding drugs there remained 81 metamfetamine cases, 153 metamfetamine plus gamma-hydroxybutyrate cases, 73 metamfetamine plus benzodiazepine cases, and 160 metamfetamine plus benzodiazepine plus gamma-hydroxybutyrate cases.
Baseline demographic and exposure characteristics
There were no significant differences in median age between groups (). The proportion of females within the metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine group was significantly greater than the metamfetamine plus benzodiazepine group (48.1 % versus 36.2%, P = 0.039) and the metamfetamine plus gamma-hydroxybutyrate group (48.1 % versus 32.9%, P = 0.033). In the metamfetamine positive detection group 49% of cases had exposure to metamfetamine recorded in the clinical record. In the metamfetamine plus gamma-hydroxybutyrate group 30% had exposure to metamfetamine recorded and 82% had gamma-hydroxybutyrate recorded. In the metamfetamine plus benzodiazepine group 47% had exposure to metamfetamine recorded, while 44% had exposure to a benzodiazepine recorded. In the metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine group 22% had exposure to metamfetamine recorded in the clinical record, 60% for gamma-hydroxybutyrate and 5% for a benzodiazepine.
Table 1. Comparison of baseline characteristics between groups defined by analytical detections.
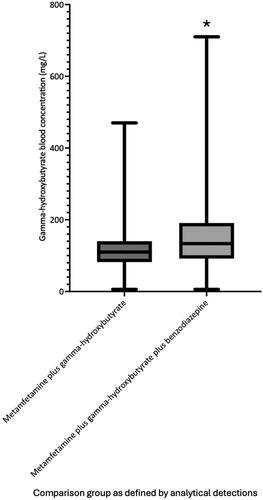
Metamfetamine blood concentrations are shown in . As illustrated in , the median metamfetamine blood concentration was significantly greater (P values ≤0.0004) in the two groups with gamma-hydroxybutyrate positive detections (metamfetamine plus gamma-hydroxybutyrate, median = 0.20 mg/L, interquartile range 0.10–0.32 mg/L, 95% confidence interval 0.20–0.23) and metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine (median = 0.20 mg/L, interquartile range 0.10–0.30 mg/L, 95% confidence interval 0.20–0.30), compared to metamfetamine (median = 0.10 mg/L, interquartile range 0.05–0.21 mg/L, 95% confidence interval 0.09–0.18) and metamfetamine plus benzodiazepine groups (median = 0.10 mg/L, interquartile range 0.06–0.20 mg/L, 95% confidence interval 0.09–0.20). The median gamma-hydroxybutyrate blood concentration was significantly greater in the metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine group compared to the metamfetamine plus gamma-hydroxybutyrate group (133 mg/L, interquartile range 82–140 mg/L, 95% confidence interval 100–120 mg/L versus 110 mg/L, interquartile range 92–191 mg/L, 95% confidence interval 120–150 mg/L, P = 0.0004, ).
Figure 1. Median, interquartile range and range of metamfetamine blood concentrations at the time of hospital admission for each analytically defined exposure groups. *Median metafetamine blood concentration in groups with gamma-hydroxybutyrate detections was significantly greater than groups without gamma-hydroxybutyrate detected (P < 0.0004).
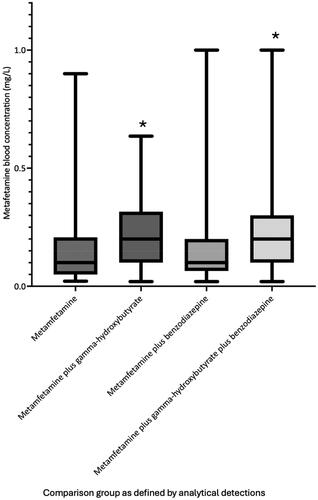
Figure 2. Median, interquartile range and range of gamma-hydroxybutyrate blood concentrations at the time of hospital admission for the analytically defined exposure groups. *Median gamma-hydroxybutyrate blood concentration was significantly greater in the metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine group compared to the metamfetamine plus gamma-hydroxybutyrate group (P = 0.0004).
There were 367 separate benzodiazepine detections (metamfetamine plus benzodiazepine n = 128, metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine group n = 239). A benzodiazepine approved for therapeutic use in Australia (‘pharmaceutical benzodiazepine’) was detected in 81% of metamfetamine plus benzodiazepine cases (n = 59) versus 92% of cases within the metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine group (n = 148). A designer benzodiazepine was detected in 53% of metamfetamine plus benzodiazepine group cases (n = 39) versus 26% of metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine cases (n = 42). A pharmaceutical benzodiazepine and designer benzodiazepine were co-detected in 32% (n = 23) of metamfetamine plus benzodiazepine cases and 18% (n = 29) of metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine cases. Analytical benzodiazepine quantitative results were available in 70% (n = 258) of the 367 total benzodiazepine detections. Given the incomplete quantitative benzodiazepine analysis, variation in pharmaceutical and designer benzodiazepine detections between groups, poorly defined pharmacological properties of designer benzodiazepines, and high incidence of multi-benzodiazepine exposure, a statistical comparison of benzodiazepine blood concentrations between the metamfetamine plus benzodiazepine and metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine groups was not undertaken. A summary of benzodiazepine detections is illustrated in Supplementary Table 1.
Blood ethanol concentration was measured in 41% (n = 33) of metamfetamine cases and detected in 21% of these cases. Corresponding figures for the other three groups: metamfetamine plus benzodiazepine ethanol measured in 58% (n = 42) and detected in 10%, metamfetamine plus gamma-hydroxybutyrate ethanol measured in 48% (n = 78) and detected in 5%, metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine ethanol measured in 59% (n = 94) and detected in 5%. Ethanol was significantly less likely to be detected in the metamfetamine plus gamma-hydroxybutyrate and metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine groups compared to the metamfetamine exposure group (P = 0.015 and P = 0.013, respectively).
In the metamfetamine group, 17% of patients (n = 14) reported ethanol co-ingestion. Blood ethanol was measured in six of these cases and detected in 50% (n = 3). Corresponding figures for the other three groups: metamfetamine plus benzodiazepine ethanol reportedly co-ingested in 22% of patients (n = 16), measured in 12 and detected in 16% (n = 2), metamfetamine plus gamma-hydroxybutyrate ethanol reportedly co-ingested in 9% of patients (n = 14), measured in seven and detected in 29% (n = 2), metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine ethanol reportedly co-ingested in 12% of patients (n = 19), measured in 14 and detected in 14% (n = 2).
Comparison of clinical effects between groups
The median Poisons Severity Score (PSS) was 3 for all groups. Median values for heart rate, systolic blood pressure, diastolic blood pressure, temperature, and incidence of agitation and sedation for each group in addition to analytical comparisons are presented in . The median presenting heart rate was significantly lower in groups with co-detection of gamma-hydroxybutyrate or a benzodiazepine either alone or in combination, compared to the metamfetamine group (metamfetamine = 110 beats per minute, metamfetamine plus benzodiazepine = 84 beats/min, metamfetamine plus gamma-hydroxybutyrate = 72 beats per minute, metamfetamine plus benzodiazepine plus gamma-hydroxybutyrate = 72 beats per minute, P < 0.001). The median presenting heart rate in the metamfetamine plus gamma-hydroxybutyrate group was significantly lower than the metamfetamine plus benzodiazepine group (72 beats per minute versus 84 beats per minute, P < 0.001). The presenting heart rate in the metamfetamine plus benzodiazepine plus gamma-hydroxybutyrate group was significantly lower than the metamfetamine plus benzodiazepine group (72 beats per minute versus 84 beats per minute, P < 0.0001). There was no significant difference in presenting heart rate between the metamfetamine plus gamma-hydroxybutyrate group and metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine group (72 beats per minute versus 72 beats per minute, P = 0.61). Graphical comparison of heart rate across the comparison groups is shown in .
Figure 3. Median, interquartile range and range of presenting heart rates (beats per minute) for each analytically defined exposure group. *Median presenting heart rate in the gamma-hydroxybutyrate groups was significantly lower than groups without co-detection of gamma-hydroxybutyrate (P < 0.0001).
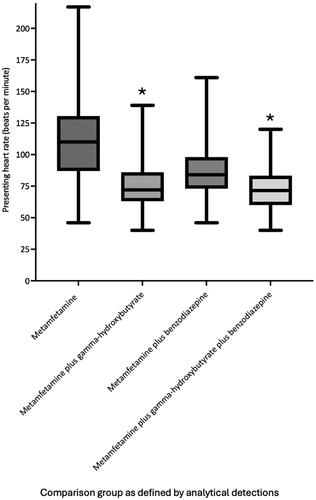
Table 2. Comparison of clinical effects recorded at the time of hospital presentation between groups defined by analytical detections.
Median systolic blood pressure in groups with benzodiazepine co-detected were significantly lower compared to the metamfetamine group (metamfetamine plus benzodiazepine 120 mmHg versus metamfetamine 130 mmHg, P < 0.0001, and metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine 124 mmHg versus metamfetamine 130 mmHg, P = 0.001). Median systolic blood pressure was significantly lower in the metamfetamine plus benzodiazepine group compared to the metamfetamine plus gamma-hydroxybutyrate group (120 mmHg versus 130 mmHg, P = 0.003). There was no significant difference in median systolic blood pressure between the metamfetamine and metamfetamine plus gamma-hydroxybutyrate group (130 mmHg versus 130 mmHg, P = 0.09). There were no significant differences in median presenting diastolic blood pressure between any groups.
Cases with metamfetamine and co-detections of gamma-hydroxybutyrate or a benzodiazepine alone or in combination had significantly lower median presenting body temperature compared with the metamfetamine detection group (). In addition, the median presenting body temperature in the metamfetamine plus gamma-hydroxybutyrate and metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine groups was significantly lower than the metamfetamine plus benzodiazepine group (metamfetamine plus gamma-hydroxybutyrate = 35.8 °C versus metamfetamine plus benzodiazepine = 36.2 °C P = 0.0001, metamfetamine plus gamma-hydroxybutyrate = 35.8 °C versus metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine = 35.7 °C, P < 0.0001). There was no significant difference in median presenting body temperature between the metamfetamine plus gamma-hydroxybutyrate and metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine groups (35.8 °C vs 35.7 °C, P = 0.59). Graphical comparison of temperature across the comparison groups is shown in .
Figure 4. Median, interquartile range and range of presenting temperature (degrees Celsius) for each analytically defined exposure group. *Median presenting temperature in the gamma-hydroxybutyrate groups was significantly lower than groups without co-detection of gamma-hydroxybutyrate (P ≤ 0.0001).
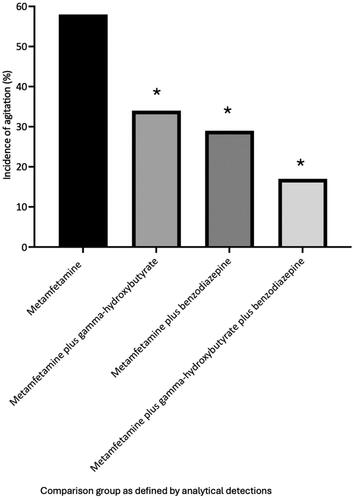
Agitation was reported in 58% of metamfetamine group cases. The recorded incidence of agitation was significantly lower in cases with co-detections of gamma-hydroxybutyrate or a benzodiazepine alone or in combination (benzodiazepine = 29%, P = 0.0003, gamma-hydroxybutyrate = 34%, P = 0.0004, benzodiazepine plus gamma-hydroxybutyrate= 17%, P < 0.0001). There was no significant difference in incidence of agitation between metamfetamine plus benzodiazepine and metamfetamine plus gamma-hydroxybutyrate groups (29% versus 34%, P = 0.45). The incidence of agitation was significantly lower in the metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine group compared to the metamfetamine plus gamma-hydroxybutyrate group (17 % versus 34%, P = 0.0007). In addition, the incidence of agitation was lower in the metamfetamine plus benzodiazepine plus gamma-hydroxybutyrate group compared to the metamfetamine plus benzodiazepine group (17 % vs 29%), however this result did not reach statistical significance (P = 0.54). Graphical comparison of incidence of agitation between exposure groups is shown in .
Figure 5. Reported incidence of agitation on hospital presentation for each analytically defined exposure group. *Incidence of agitation in groups with co-detection of gamma-hydroxybutyrate or benzodiazepines was significantly lower compared to the lone metamfetamine group (P ≤ 0.0004).
Sedation (Glasgow Coma Scale <9) was recorded at the time of ED presentation in 15% of metamfetamine cases. The incidence of sedation was significantly greater in metamfetamine plus gamma-hydroxybutyrate cases (63%, P < 0.0001). Metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine cases had a non-significantly greater overall incidence of sedation compared to metamfetamine cases (27 % versus 15%, P = 0.11). Incidence of sedation was significantly greater in metamfetamine plus benzodiazepine cases when gamma-hydroxybutyrate was co-detected (72 % versus 27%, P < 0.001).
Outcomes
There was one death in the metamfetamine group and one in the metamfetamine plus gamma-hydroxybutyrate group (PSS 4). Both patients were found collapsed in cardiac arrest in the community and suffered irreversible hypoxic brain injury.
Discussion
In this study of analytically confirmed illicit drug exposures requiring hospital presentation, patients with metamfetamine exposure and co-exposure to a benzodiazepine or gamma-hydroxybutyrate had significantly reduced heart rate, body temperature, and incidence of agitation compared to patients with lone metamfetamine exposure. Study groups that included gamma-hydroxybutyrate as an analytically confirmed co-exposure had significantly greater median metamfetamine blood concentration, but lower presenting heart rate and body temperature compared to all other groups. Patients with exposure to gamma-hydroxybutyrate were significantly more likely to be sedated (Glasgow Coma Scale <9) compared to patients with lone exposure to metamfetamine.
Evidence illustrating the efficacy of benzodiazepines in attenuating acute cardiovascular manifestations of toxicity caused by amphetamine derivatives and analogues is largely limited to case reports [Citation6,Citation21]. Our study found significantly lower heart rates in patients presenting with metamfetamine toxicity and co-exposure to a benzodiazepine, compared to patients presenting with lone metamfetamine exposure. In turn, patients with co-exposure to gamma-hydroxybutyrate also had significantly lower heart rates than patients with isolated metamfetamine exposure and metamfetamine-benzodiazepine co-exposure. In addition, presenting heart rate was significantly lower in metamfetamine exposures with gamma-hydroxybutyrate plus benzodiazepine co-exposure, compared to benzodiazepine co-exposure alone. These findings suggest gamma-hydroxybutyrate may be more efficacious than benzodiazepines in attenuating metamfetamine induced tachycardia. Tachycardia is normally well tolerated in patients with metamfetamine toxicity, although extreme tachycardia may lead to myocardial ischemia and more significant dysrhythmias [Citation22]. Although both gamma-hydroxybutyrate and benzodiazepine co-exposure appeared effective in attenuating tachycardia in this study, it is not possible to conclude that gamma-hydroxybutyrate or benzodiazepines were effective in preventing the development of dysrhythmias or myocardial ischemia.
Metamfetamine associated cerebrovascular and cardiovascular complications are well documented [Citation22–24]. Vasoconstriction and vasospasm may lead to tissue ischemia, and when combined with a hyperdynamic circulation can produce hypertension with secondary vascular haemorrhage or dissection. Benzodiazepines are commonly recommended for the initial management of hypertension associated with metamfetamine exposure, although evidence of efficacy is limited to case reports [Citation1,Citation21,Citation22]. In this study, metamfetamine-intoxicated patients with benzodiazepine co-exposure had statistically significantly lower presenting systolic blood pressure. Although systolic blood pressures within this cohort were not overtly high (medians of all groups were ≤130 mmHg, maximum measured systolic blood pressure was 149 mmHg), this is an important finding which adds indirect evidence supporting the benefit of benzodiazepines in managing stimulant-induced hypertension. There was no difference in diastolic blood pressure between metamfetamine exposure group and groups with benzodiazepine co-exposure. There was no difference in systolic or diastolic blood pressure between the metamfetamine exposure group and either group with gamma-hydroxybutyrate co-exposure.
Hyperthermia is a complication of stimulant drug toxicity, including metamfetamine, and carries a high mortality risk [Citation22,Citation25–27]. Rapid cooling techniques including ice-water immersion and pharmacological control of agitation are key components of managing stimulant-induced hyperthermia [Citation1,Citation28,Citation29]. Benzodiazepines are recommended as one of several pharmacological interventions to reduce stimulation and hence temperature, however, published evidence demonstrating efficacy is limited [Citation1,Citation22,Citation30]. In our study, metamfetamine-intoxicated patients with co-exposure to benzodiazepines had lower presenting body temperature compared to lone metamfetamine exposed patients. In turn, gamma-hydroxybutyrate co-exposure was associated with lower presenting body temperature compared to metamfetamine and metamfetamine plus benzodiazepine exposure groups. Although notable, it is not possible to conclude that this finding demonstrates efficacy of benzodiazepines or gamma-hydroxybutyrate in treating hyperthermia, as pre and post-exposure temperatures were not recorded and there were no patients within the cohort with severe hyperthermia. However, it does suggest that benzodiazepine and gamma-hydroxybutyrate co-exposure attenuate metamfetamine-induced increase in body temperature, and that gamma-hydroxybutyrate may be more efficacious compared to benzodiazepines. Indeed, hypothermia is a recognised clinical feature of gamma-hydroxybutyrate toxicity [Citation31]. This raises the possibility that gamma-hydroxybutyrate administration may be more effective than benzodiazepines in reducing body temperature in cases of severe hyperthermia. However, gamma-hydroxybutyrate pharmacokinetic properties suggest that administration as a therapeutic intervention may not lead to rapid clinical benefit, a highly desirable aspect for effective management of severe hyperthermia. Gamma-hydroxybutyrate is available for therapeutic use as an oral preparation and is rapidly although incompletely absorbed [Citation31]. Gamma-hydroxybutyrate is reliant on active transport via blood-brain-barrier mono-carboxylate transporters to enter the central nervous system [Citation32]. Mono-carboxylate transporter 1 is one of several mono-carboxylate transporters expressed at the human blood–brain barrier; however, mono-carboxylate transporter 1 is saturable [Citation32]. Time to peak cerebrospinal fluid concentrations in humans following intravenous gamma-hydroxybutyrate administration is unknown. Even if an intravenous preparation was available for therapeutic use, onset of action of central nervous system gamma-hydroxybutyrate effects may be slower than those provided by lipophilic benzodiazepines which generally require greater than five minutes to produce adequate sedation in the agitated patient following intravenous administration [Citation33,Citation34].
Metamfetamine-induced acute neuropsychiatric toxicity, particularly agitation, poses a significant risk to patients and healthcare providers [Citation1, Citation5]. Both benzodiazepines and antipsychotics are relatively safe and effective in treating acute agitation, with no clear evidence that one class is superior [Citation8,Citation30]. In our study, metamfetamine-exposed patients with benzodiazepine or gamma-hydroxybutyrate co-exposure had significantly reduced rates of agitation compared to lone metamfetamine-exposed patients. Although not statistically significant, rates of agitation were lower in patients with co-exposure to benzodiazepine compared to gamma-hydroxybutyrate. Patients with combined exposure to metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine had significantly lower rates of agitation compared to those with just metamfetamine plus gamma-hydroxybutyrate exposure. This data suggests that both benzodiazepines and gamma-hydroxybutyrate effectively attenuate metamfetamine-induced agitation, and that in this instance benzodiazepines may be superior to gamma-hydroxybutyrate. However, although gamma-hydroxybutyrate toxicity can produce profound sedation, intermittent agitation is a recognised aspect of clinical toxicity [Citation31] and recorded rates of agitation in gamma-hydroxybutyrate exposed groups in our study may reflect intermittent periods of agitation often observed as gamma-hydroxybutyrate central nervous system toxicity resolves. Of note, our data demonstrate significantly higher rates of sedation in patient groups with gamma-hydroxybutyrate exposure compared to lone metamfetamine and metamfetamine plus benzodiazepine exposure. Gamma-hydroxybutyrate may be superior compared to benzodiazepine in producing sedation in metamfetamine exposed patients.
Benzodiazepines and gamma-hydroxybutyrate are GABA receptor agonists [Citation31], and both appeared to attenuate metamfetamine stimulation and agitation in this study. However, we observed differences in efficacy of benzodiazepines and gamma-hydroxybutyrate when examining individual metamfetamine clinical effects. Given that benzodiazepines act via GABAA receptor agonism, and gamma-hydroxybutyrate via GABAB and gamma-hydroxybutyrate receptor agonism, this is perhaps not surprising. Endogenous gamma-hydroxybutyrate acts via G-protein coupled gamma-hydroxybutyrate receptors but following exogenous administration with subsequent increases in central nervous system gamma-hydroxybutyrate concentration, gamma-hydroxybutyrate acts both as a GABAB receptor agonist and indirectly through metabolism to form GABA [Citation32]. Gamma-aminobutyric acid type B receptors are G-protein coupled receptors that produce neuronal inhibition through a reduction in pre-synaptic calcium ion influx and activation of post-synaptic potassium ion channels. In contrast GABAA receptors are ligand gated ion channels, increasing post-synaptic chloride ion influx to produce neuronal inhibition. Gamma-hydroxybutyrate also reduces dopamine neurotransmission in the mesolimbic and substantia nigra regions of the brain and modulates serotonin neurotransmission [Citation35]. Serotonin and dopamine are important neurotransmitters that modulate body temperature. Gamma-hydroxybutyrate alteration of these pathways may contribute to gamma-hydroxybutyrate induced hypothermia and partially explain our observations that patients with co-exposure to metamfetamine and gamma-hydroxybutyrate have lower body temperature compared to lone metamfetamine and metamfetamine plus benzodiazepine co-exposed patients. Our results suggest differing mechanisms underlying gamma-hydroxybutyrate-metamfetamine and benzodiazepine-metamfetamine pharmacodynamic interactions. For example, despite a significantly greater median metamfetamine blood concentration, the metamfetamine plus benzodiazepine plus gamma-hydroxybutyrate group had significantly lower median heart rate, body temperature and higher incidence of sedation compared to the metamfetamine plus benzodiazepine group, but no difference in blood pressure or incidence of agitation.
Metamfetamine concentrations were significantly higher in the metamfetamine plus gamma-hydroxybutyrate and metamfetamine plus gamma-hydroxybutyrate plus benzodiazepine groups. This is likely to reflect differences in use and dosing patterns between individuals using these substances with differing intent. For example, gamma-hydroxybutyrate may be used simultaneously with metamfetamine to increase drug effects or following metamfetamine use to minimise its negative side effects [Citation36].
The findings of our study raise the possibility that administration of gamma-hydroxybutyrate, or other GABAB receptor agonists may provide beneficial effects in selected cases of metamfetamine toxicity. The apparent ability of gamma-hydroxybutyrate to attenuate rises in body temperature may provide a useful treatment modality to manage metamfetamine or other stimulant-induced severe hyperthermia, a condition associated with significant mortality [Citation25]. Gamma-hydroxybutyrate may also be effective in rapidly treating life-threatening stimulant drug-induced agitation and excited delirium. Although gamma-hydroxybutyrate administration is associated with deep sedation (a potentially desirable effect), respiratory depression, and possible secondary hypoxaemia, these complications are manageable in critical care settings and likely outweigh the possible complications associated with severe hyperthermia and extreme states of agitation. Of note, gamma-hydroxybutyrate may not induce hypotension in these critically unwell patient as is commonly observed with sedation initiated using benzodiazepines or barbiturates. At present therapeutic administration of gamma-hydroxybutyrate in cases of severe metamfetamine toxicity is limited by an absence of an approved intravenous formulation, however gamma-hydroxybutyrate is available as an oral preparation (sodium oxybate) in several jurisdictions and is used to treat a number of conditions including narcolepsy. Although other GABAB receptor agonists including baclofen possess variations in pharmacodynamic mechanisms compared to gamma-hydroxybutyrate, they may potentially provide benefit in treating acute metamfetamine toxicity.
Our study is subject to several limitations. In comparing groups, we have assumed that metamfetamine blood concentrations correlate with degree of clinical toxicity. A greater proportion of females in the metamfetamine plus benzodiazepine plus gamma-hydroxybutyrate group may have meant a lower mean body weight, with the theoretical possibility of more pronounced clinical effects at any given substance blood concentration compared to other groups [Citation37]. It was not possible to accurately quantify the severity of benzodiazepine exposures as measured by blood concentration due to a lack of quantitative analytical data in the case of novel/designer benzodiazepines, the difficulties in estimating pharmacological equivalence in cases of multi-benzodiazepine exposures, and the lack of published data describing pharmacological characteristics of novel/designer benzodiazepines. These factors mean that it was not possible to accurately assess comparative pharmacological contributions between gamma-hydroxybutyrate and benzodiazepine exposures. The study protocol describes blood sampling occurring at the time of initial venepuncture, undertaken at the time of hospital presentation (and therefore proximal to the measurement of presenting clinical features). However, it is possible that in a minority of cases there was a delay between recording of clinical observations and sample collection. Most patients in these cases did not exhibit severe clinical toxicity, and so it is not clear that the observed illicit drug interactions can be automatically extrapolated to cases of severe toxicity. Ethanol was only measured in 40-50% of cases within each group. This reflects local emergency department practice, where ethanol is not routinely measured in cases of suspected illicit drug toxicity. However, the low rate of ethanol concentration measurement is a limitation given ethanol’s sedative effects. In the groups where ethanol was measured, it was detected at relatively low rates. Ethanol was detected in only 5% of gamma-hydroxybutyrate exposed cases, making ethanol less likely to be acting as a confounder and increasing sedative or central nervous system inhibitory effects in the gamma-hydroxybutyrate exposure groups compared to other exposure groups. There is limited evidence from user focus groups to suggest that the rate of ethanol co-use with gamma-hydroxybutyrate is low [Citation36]. There was no clear definition available for clinicians to classify agitation introducing the possibility of variability in classification. Cases in this study were included via a purposive sampling method and may not be representative of the population from which they are drawn. The findings of our study may not be applicable to other settings.
Conclusion
In this study of analytically confirmed illicit drug exposures requiring hospital presentation, patients with metamfetamine exposure and co-exposure to a benzodiazepine or gamma-hydroxybutyrate had significantly reduced heart rate, body temperature and incidence of agitation compared to patients with lone metamfetamine exposure. Patient groups with co-exposure to gamma-hydroxybutyrate have significantly lower presenting body temperature and a higher incidence of sedation than patients with lone exposure to metamfetamine, or exposure to metamfetamine and a benzodiazepine. We hypothesize that gamma-hydroxybutyrate administration in cases of metamfetamine-induced severe hyperthermia or agitation may provide therapeutic benefit.
Supplemental Material
Download MS Word (15 KB)Acknowledgment
We acknowledge and thank the Emerging Drugs Network of Australia (EDNA) project investigators (chief investigator Professor Daniel Fatovich) for supporting this work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Greene SL, Kerr F, Braitberg G. Review article: amphetamines and related drugs of abuse. Emerg Med Australas. 2008;20(5):391–402. doi: 10.1111/j.1742-6723.2008.01114.x.
- Weiss SR, Raskind R, Morganstern NL, et al. Intracerebral and subarachnoid hemorrhage following use of methamphetamine ("speed"). Int Surg. 1970;53(2):123–127.
- Callaway CW, Clark RF. Hyperthermia in psychostimulant overdose. Ann Emerg Med. 1994;24(1):68–76. doi: 10.1016/s0196-0644(94)70165-2.
- Ginsberg MD, Hertzman M, Schmidt-Nowara WW. Amphetamine intoxication with coagulopathy, hyperthermia, and reversible renal failure. A syndrome resembling heatstroke. Ann Intern Med. 1970;73(1):81–85. doi: 10.7326/0003-4819-73-1-81.
- Harnett JT, Dargan PI, Dines AM, et al. Increasing emergency department attendances in Central london with methamphetamine toxicity and associated harms. Emerg Med J. 2022;39(6):463–466. doi: 10.1136/emermed-2020-209550.
- Richards JR, Albertson TE, Derlet RW, et al. Treatment of toxicity from amphetamines, related derivatives, and analogues: a systematic clinical review. Drug Alcohol Depend. 2015;150:1–13. doi: 10.1016/j.drugalcdep.2015.01.040.
- Wodarz N, Krampe-Scheidler A, Christ M, et al. Evidence-based guidelines for the pharmacological management of acute methamphetamine-related disorders and toxicity. Pharmacopsychiatry. 2017;50(3):87–95. doi: 10.1055/s-0042-123752.
- Gosselin S, Hoffman RS. The management of agitated toxidromes. Emerg Med Clin North Am. 2022;40(2):223–235. doi: 10.1016/j.emc.2022.01.009.
- Richards JR, Derlet RW, Duncan DR. Methamphetamine toxicity: treatment with a benzodiazepine versus a butyrophenone. Eur J Emerg Med. 1997;4(3):130–135. doi: 10.1097/00063110-199709000-00003.
- Derlet RW, Rice P, Horowitz BZ, et al. Amphetamine toxicity: experience with 127 cases. J Emerg Med. 1989;7(2):157–161. doi: 10.1016/0736-4679(89)90263-1.
- Ruha AM, Yarema MC. Pharmacologic treatment of acute pediatric methamphetamine toxicity. Pediatr Emerg Care. 2006;22(12):782–785. doi: 10.1097/01.pec.0000245179.51535.ab.
- Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med. 1998;16(4):567–573. doi: 10.1016/s0736-4679(98)00045-6.
- Heinzerling KG, Shoptaw S, Peck JA, et al. Randomized, placebo-controlled trial of baclofen and gabapentin for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85(3):177–184. doi: 10.1016/j.drugalcdep.2006.03.019.
- Phillips TJ, Reed C. Targeting GABAB receptors for anti-abuse drug discovery. Expert Opin Drug Discov. 2014;9(11):1307–1317. doi: 10.1517/17460441.2014.956076.
- Syrjanen R, Schumann J, Fitzgerald J, et al. The emerging drugs network of Australia - Victoria clinical registry: a state-wide illicit substance surveillance and alert network. Emerg Med Australas. 2022;35(1):82–88. doi: 10.1111/1742-6723.14059.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010.
- Harris PA, Taylor R, Minor BL, REDCap Consortium., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208.
- Di Rago M, Pantatan S, Hargreaves M, et al. High throughput detection of 327 drugs in blood by LC-MS-MS with automated data processing. J Anal Toxicol. 2021;45(2):154–183. doi: 10.1093/jat/bkaa057.
- von Cüpper M, Dalsgaard PW, Linnet K. Identification of new psychoactive substances in seized material using UHPLC-QTOF-MS and an online mass spectral database. J Anal Toxicol. 2021;44(9):1047–1051. doi: 10.1093/jat/bkaa028.
- Mardal M, Andreasen MF, Mollerup CB, et al. Corrigendum to "HighResNPS.com: an online Crowd-Sourced HR-MS database for suspect and non-targeted screening of new psychoactive substances. J Anal Toxicol. 2019;43(6):e7–e8. Erratum for: J Anal Toxicol. 2019 Aug;43:520-527. doi: 10.1093/jat/bkz049.
- Wood DM, Davies S, Puchnarewicz M, et al. Recreational use of mephedrone (4-methylmethcathinone, 4-MMC) with associated sympathomimetic toxicity. J Med Toxicol. 2010;6(3):327–330. doi: 10.1007/s13181-010-0018-5.
- Schep LJ, Slaughter RJ, Beasley DM. The clinical toxicology of metamfetamine. Clin Toxicol (Phila). 2010;48(7):675–694. doi: 10.3109/15563650.2010.516752.
- Osman S, Zhu Z, Farag M, et al. Intracerebral hemorrhage: who gets tested for methamphetamine use and why might it matter? BMC Neurol. 2020;20(1):392. doi: 10.1186/s12883-020-01967-y.
- Darke S, Duflou J, Kaye S, et al. Psychostimulant use and fatal stroke in young adults. J Forensic Sci. 2019;64(5):1421–1426. doi: 10.1111/1556-4029.14056.
- Grunau BE, Wiens MO, Brubacher JR. Dantrolene in the treatment of MDMA-related hyperpyrexia: a systematic review. CJEM. 2010;12(5):435–442. doi: 10.1017/s1481803500012598.
- Chan P, Chen JH, Lee MH, et al. Fatal and nonfatal methamphetamine intoxication in the intensive care unit. J Toxicol Clin Toxicol. 1994;32(2):147–155. doi: 10.3109/15563659409000444.
- Lan KC, Lin YF, Yu FC, et al. Clinical manifestations and prognostic features of acute methamphetamine intoxication. J Formos Med Assoc. 1998;97(8):528–533.
- Rusyniak DE, Sprague JE. Toxin-induced hyperthermic syndromes. Med Clin North Am. 2005;89(6):1277–1296. Erratum in: med Clin North Am. 2006;90:261-2. doi: 10.1016/j.mcna.2005.06.002.
- Laskowski LK, Landry A, Vassallo SU, et al. Ice water submersion for rapid cooling in severe drug-induced hyperthermia. Clin Toxicol (Phila). 2015;53(3):181–184. doi: 10.3109/15563650.2015.1009994.
- Connors NJ, Alsakha A, Larocque A, et al. Antipsychotics for the treatment of sympathomimetic toxicity: a systematic review. Am J Emerg Med. 2019;37(10):1880–1890. doi: 10.1016/j.ajem.2019.01.001.
- Schep LJ, Knudsen K, Slaughter RJ, et al. The clinical toxicology of γ-hydroxybutyrate, γ-butyrolactone and 1,4-butanediol. Clin Toxicol (Phila). 2012;50(6):458–470. doi: 10.3109/15563650.2012.702218.
- Felmlee MA, Morse BL, Morris ME. γ-Hydroxybutyric acid: Pharmacokinetics, pharmacodynamics, and toxicology. Aaps J. 2021;23(1):22. doi: 10.1208/s12248-020-00543-z.
- Knott JC, Taylor DM, Castle DJ. Randomized clinical trial comparing intravenous midazolam and droperidol for sedation of the acutely agitated patient in the emergency department. Ann Emerg Med. 2006;47(1):61–67. doi: 10.1016/j.annemergmed.2005.07.003.
- Taylor DM, Yap CYL, Knott JC, et al. Midazolam-Droperidol, droperidol, or olanzapine for acute agitation: a randomized clinical trial. Ann Emerg Med. 2017;69(3):318–326.e1. doi: 10.1016/j.annemergmed.2016.07.033.
- Kamal RM, van Noorden MS, Franzek E, et al. The neurobiological mechanisms of gamma-hydroxybutyrate dependence and withdrawal and their clinical relevance: a review. Neuropsychobiology. 2016;73(2):65–80. doi: 10.1159/000443173.
- Barker JC, Harris SL, Dyer JE. Experiences of gamma hydroxybutyrate (gamma-hydroxybutyrate) ingestion: a focus group study. J Psychoactive Drugs. 2007;39(2):115–129. doi: 10.1080/02791072.2007.10399870.
- Anderson GD. Gender differences in pharmacological response. Int Rev Neurobiol. 2008;83:1–10. doi: 10.1016/S0074-7742(08)00001-9.
