Abstract
Introduction
The epidemiological and clinical characteristics of acute poisoning with liquid laundry detergent capsules have been comprehensively reported. However, studies of laboratory test results in these exposures are uncommon. This study analyzed the impact of the ingestion of liquid laundry detergent capsules on admission laboratory tests in paediatric patients.
Methods
This retrospective study was conducted in the clinical toxicology unit of a paediatric poison centre between 2015 and 2021. Paediatric patients (less than 18 years of age) who ingested liquid laundry detergent capsules were included. The relationship between the European Association of Poisons Centers and Clinical Toxicologists/European Commission/International Programme on Chemical Safety Poisoning Severity Score and admission laboratory test results was assessed using Fisher’s exact test or analysis of variance.
Results
A total of 156 patients were included in the study. A considerable proportion of patients presented with leucocytosis, acidosis, hyperlactataemia or base deficit. The median values of white blood cell count (P = 0.042), pH (P = 0.022), and base excess (P = 0.013) were significantly different among the Poisoning Severity Score groups. Hyperlactataemia was strongly associated with the Poisoning Severity Score (P = 0.003).
Discussion
Leucocytosis is a non-specific marker of severity following ingestion of liquid laundry detergent capsules. The incidence of metabolic acidosis and hyperlactataemia was higher in this study than in previous reports, but these metabolic features were not related to the severity of exposure. The exact mechanisms of toxicity are not yet known, but the high concentration of non-ionic and anionic surfactants, as well as propylene glycol and ethanol, in the capsule are likely contributing factors.
Conclusions
Pediatric patients who ingest liquid laundry detergent capsules may develop leucocytosis, metabolic acidosis, hyperlactataemia, and a base deficit.
Introduction
Liquid laundry detergent capsules, commonly referred to as pods, packets, or sacs, are concentrated, single-use units of detergent enclosed in water-soluble, polyvinyl alcohol-based membranes [Citation1–3]. Liquid laundry detergent capsules were first commercialized in Europe in 2001 [2] and subsequently introduced in the United States in 2010 [Citation4]. Following their worldwide availability in 2012 and owing to their user-friendly design [Citation4], liquid laundry detergent capsules have gained significant popularity as a household cleaning product [Citation5]. Therefore, exposure to liquid laundry detergent capsules has led to a substantial increase in the rate of detergent poisoning, particularly in children under 5 years of age [Citation1,Citation6].
The ingestion of liquid laundry detergent capsules is most commonly associated with vomiting, coughing, and central nervous system depression [Citation7,Citation8]. Previous studies have reported that liquid laundry detergent capsules are more harmful than conventional liquid laundry detergents [Citation9–12], which may be due to their lower water content [Citation8], higher surfactant concentrations [Citation2], and the presence of glycol solvents [Citation13,Citation14].
The medical community has raised concerns regarding poisoning with liquid laundry detergent capsules [Citation15–17], which has resulted in the development of preventive measures, such as child-resistant containers and opaque containers or packaging [Citation5,Citation18,Citation19]. In addition, Consumer Reports in the United States removed liquid laundry detergent capsules from their recommended product lists in 2015 based on the associated safety concerns [Citation20]. Although these measures have shown temporary effectiveness, liquid laundry detergent capsule exposure remains a persistent health issue requiring constant surveillance and intervention [Citation21–25].
The existing literature regarding poisoning with liquid laundry detergent capsules is substantial, but it mostly covers epidemiological and clinical aspects, with limited data on laboratory investigations [Citation7,Citation8,Citation26]. The results of laboratory investigations in patients with liquid laundry detergent capsule poisoning are primarily presented in case reports and case series [Citation27–29]. Observational studies have reported some laboratory features, including uncertain metabolic effects [Citation30,Citation31], respiratory, metabolic, or mixed acidosis [Citation7,Citation32], lactic acidosis [Citation8], and hyperlactataemia [Citation2,Citation7]. The laboratory parameters associated with poisoning from liquid laundry detergent capsules may aid in the understanding of the toxicity of liquid laundry detergent capsules [Citation8]. Therefore, this study aimed to describe the laboratory characteristics of paediatric patients diagnosed with liquid laundry detergent capsule poisoning. We also examined the relationship between the results of laboratory tests and poisoning severity.
Methods
Study design
Data were collected retrospectively from a clinical toxicology unit of a pediatric poison centre to analyze the laboratory parameters of pediatric patients who had been hospitalized for liquid laundry detergent capsule poisoning.
Electronic health records of the patients were screened using the T55 code (toxic effect of soaps and detergents) of the ICD-10-AM (International Classification of Diseases, 10th Revision, Australian Modification), which corresponds to the T55.0 × 1–4 and T55.1 × 1–4 codes of ICD-10-CM (International Classification of Diseases, 10th Revision, Clinical Modification). Subsequently, all patients who were admitted to the centre between 1 January 2015 and 31 December 2021 and were under the age of 18 were analyzed (n = 291). Patients poisoned with other detergent types (n = 126), co-exposed to other products (n = 1), exposed to liquid laundry detergent capsules via routes other than ingestion (n = 3), or with unrecorded laboratory values (n = 5) were excluded from the study. All patients who were not included in the study because of the absence of laboratory values had a European Association of Poisons Centers and Clinical Toxicologists/European Commission/International Programme on Chemical Safety Poisoning Severity Score (PSS) of 0.
The primary aim of this study was to evaluate the prevalence of altered laboratory test results, including leucocytosis, acidosis, hyperlactatemia, and base deficit. The secondary objective was to determine the relationship between the severity of poisoning and certain laboratory parameters. This study conformed to the ethical principles of the Declaration of Helsinki for medical research [Citation33]. The guardians of all patients provided informed consent for their participation in the study. Approval was obtained from the Ethical Committee of the hospital prior to the commencement of this study.
Data collection
Data were recorded in a spreadsheet using a set coding system. The extracted information included age, sex, the time interval between exposure and admission, clinical manifestations, laboratory parameters at admission, hospitalization time, and the PSS. Clinical manifestations included drowsiness, dehydration, gastrointestinal manifestations, respiratory manifestations, ocular lesions, and skin lesions. The ocular and skin lesions were caused by direct contact with the components of the liquid laundry detergent capsules after the patient broke them open in their mouth. Laboratory data included white blood cell count, pH, plasma lactate concentration, serum base excess, serum electrolyte concentrations, liver and kidney function tests.
The accuracy of the PSS reported during hospitalization and documented in the medical records was determined by the first author, a clinical toxicologist. Differences were assessed by a senior investigator. The patients were grouped based on the PSS to perform a comparative analysis.
Definitions
Dehydration was evaluated based on clinical examination at the time of admission and classified as mild, moderate, or severe [Citation34]. Mild dehydration was characterized by normal physical findings in children with decreased weight, applicable only to those with a clearly known weight. Moderate dehydration was indicated by the presence of dry mucous membranes, mild reduction in skin elasticity, sunken eyes, and/or a capillary refill time between 1.5 and 3 s. Severe dehydration was characterized by poor skin turgor, very dry mucous membranes, very sunken eyes, and or a capillary refill time above 3 sec [Citation34].
The PSS was jointly developed by the European Association of Poisons Centers and Clinical Toxicologists, the International Programme on Chemical Safety, and the European Commission to grade poisoning using patient signs and symptoms [Citation35]. A PSS 0 represents no signs or symptoms; PSS 1 indicates mild signs or symptoms (vomiting, diarrhoea, pain, cough, dyspnea, drowsiness, irritation, mild acid-base disturbances); PSS 2 represents moderate signs or symptoms that persist and need medical intervention (vomiting, diarrhoea, pain, dysphagia, cough, dyspnea, stridor, hypoxemia, unconsciousness, pronounced acid-base disturbances); PSS 3 represents severe or life-threatening signs or symptoms (respiratory distress, severe bronchospasm, glottal oedema, severe dysphagia, severe lesions, severe acidosis with pH <7.15); and PSS 4 indicates the patient died [Citation35].
Leucocytosis was defined as a white blood cell count count greater than 12 × 109/L. Acidosis was defined as a pH less than 7.37. Alkalosis was defined as a pH greater than 7.45. Hyperlactataemia was defined as a lactate concentration greater than 2 mmol/L. Base deficit was defined as a serum base excess less than −2 mmol/L. In accordance with the guidelines set forth by Kalas and colleagues [Citation36], abnormal liver function was defined as an activity of alanine aminotransferase and/or aspartate aminotransferase exceeding twice the upper limit of normal.
Statistical analysis
All statistical analyses were performed using XL-STAT (Addinsoft, France), VassarStats (Vassar College, United States of America), and DATAtab (DATAtab e.U, Austria). Continuous data were expressed as median with interquartile range (IQR), whereas categorical data were presented as numbers (n) and/or percentages (%). The percentages were calculated based on the valid number of cases for each variable, and the reported numbers were rounded without decimal places, following common practice for studies with a sample size below 200 [Citation37].
The relationships between PSS and abnormal laboratory test results (categorical variables) were assessed using the Freeman-Halton extension [Citation38] of Fisher’s exact two-tailed test and the standardized residuals analysis [Citation39].
A single-factor analysis of variance was conducted to compare the median values of laboratory investigations (continuous variables) among the PSS groups. The significance level was set at P < 0.05, with an alpha level of 5%. Post-hoc power analyses were not performed in this study. The relationship between two continuous variables was evaluated using Pearson’s correlation test.
Patients were screened for the presence of infection within one week of admission or upon admission. A paediatrician determined this diagnosis using the medical history, clinical examination, and a C-reactive protein greater than 20 mg/L. White blood cell counts excluded patients with recent or ongoing infections (n = 36).
A single patient with alanine and aspartate aminotransferase activities exceeding ten times the upper limit of normal was not considered in the analysis of these parameters. Despite referral to the paediatric hepatology department to investigate other potential causes of hepatic cytolysis aside from liquid laundry detergent poisoning, no follow-up was documented.
Missing variables were removed from the statistical analysis to ensure the strength of the findings.
Results
The study included 156 patients who ingested liquid laundry detergent capsules, with over half (56%) of the patients being male (n = 87). The median patient age was 1.92 years (IQR: 1.25–2.83 years). The age distribution was right-skewed, with one outlier showing that 97% (n = 151) of the cases were under the age of 5.
The admission year data was left-skewed, with 17 patients admitted in 2015, 28 in 2016, 29 in 2017, 33 in 2018, 23 in 2019, 14 in 2020, and 12 in 2021. The median length of time between exposure and admission was 2 h (IQR 1–3 h), which was not different between the patient age groups. The median hospitalization time was 22 h (IQR 17–26 h). All patients were discharged in satisfactory condition and did not require any home treatment or follow-up examinations.
Clinical manifestations
Seven patients (4%) experienced drowsiness, 32 (21%) had moderate dehydration, 24 (15%) had sialorrhea, three (2%) experienced odynophagia, 133 (85%) experienced vomiting, 10 (6%) had abdominal pain, 27 (17%) had diarrhoea, 49 (31%) had cough, three (2%) had dyspnoea, and nine (6%) had dysphonia.
Simultaneously with ingestion, 14 (9%) patients were exposed by the ocular and dermal routes after they broke the laundry detergent capsules in their mouths, leading to lesions at those sites.
All patients had a recorded PSS. Specifically, 14 (9%) patients had a PSS 0, 122 (78%) had a PSS 1, 18 (12%) had a PSS 2, and two (1%) had a PSS 3. No patient died (PSS 4).
Laboratory variables
presents the median age, sex distribution, and PSS distribution of the patients with abnormal laboratory results.
Table 1. The age, sex, and PSS of the patients and their laboratory test results. The age and sex of patients with normal and abnormal laboratory test results were comparable.
White blood cell count
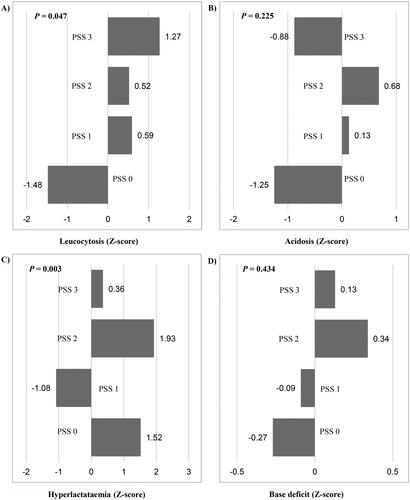
The white blood cell count was available for 155 patients, of whom 36 were excluded due to recent or ongoing infections. Among the remaining patients (n = 119), 50 (42%) had leucocytosis. The prevalence of leucocytosis was associated with the PSS (P = 0.047). Residual analysis revealed a negative deviation in the PSS 0 group and a positive deviation in the PSS 3 group (). Additionally, the median white blood cell count varied significantly among the PSS groups in the analyzed cohort (P = 0.042).
Figure 1. The frequencies of abnormal laboratory test results among the poison Severity Score groups: (A) leucocytosis (n = 50), (B) acidosis (n = 43), (C) hyperlactataemia (n = 24), and (D) base deficit (n = 106). The bars in the figure represent the standardized residuals analysis, with the Z-scores presented alongside. The P-values from the Fisher exact test are provided in the Figure. PSS: Poisoning Severity Score.
pH
The pH value was available for 117 patients, of whom 43 (37%) had acidosis, and five (4%) had alkalosis. The relationship between acidosis and PSS was not significant (P = 0.225; ). However, there was a statistically significant difference in the median pH value between the PSS groups (P = 0.022).
Lactate concentration
The lactate concentration was available for 69 patients, of whom 24 (35%) had hyperlactataemia. Hyperlactataemia was significantly associated with the PSS (P = 0.003), with a lower prevalence observed in the PSS 1 group and a higher prevalence in the PSS 2 group (). However, there was no statistically significant difference in the median serum lactate concentration between the PSS groups (P = 0.096).
Serum base excess
The base excess value was available for 116 patients, of whom 106 (96%) had a base deficit. The relationship between base deficit and PSS groups was not significant (P = 0.434) (). However, there was a statistically significant difference in the median base excess value between the PSS groups (P = 0.013).
Additional laboratory tests
Serum electrolyte analysis revealed no significant changes in sodium or chloride concentrations among all patients. The chloride concentration was increased by only 1 mmol/L (1 mEq/L) in five patients, whereas it was mildly decreased in three patients, falling within the 6 mmol/L range. Four patients had elevated potassium concentrations ranging from 5.17 to 6.39 mmol/L.
The median alanine aminotransferase activity was 19 U/L (IQR 15–24 U/L), and the median aspartate aminotransferase activity was 40 U/L (IQR 33–46 U/L). Aspartate aminotransferase activity was mildly increased in 46 patients, with 38 of them having a PSS of 1, six having a PSS of 2, and two having a PSS of 3. Two patients had minor increases in the alanine aminotransferase activity, one of whom had a PSS of 1 and the other had a PSS of 2.
Kidney function was within normal limits for all patients in this study, as indicated by the median creatinine concentration of 26.52 μmol/L [0.30 mg/dL] (IQR 17.54–28.98 μmol/L [0.23–0.38 mg/dL]).
Discussion
The majority of the patients in this study were under the age of 5 years (97%), which is consistent with previous reports (94.1–96.1%) [Citation7,Citation8,Citation40]. A greater proportion of patients in this study experienced vomiting (85%) than that reported in previous studies (46.5–56%) [Citation7,Citation8,Citation15,Citation40]. Similarly, the presence of cough was more frequent in this study (31%) than in previous studies (4.2–14.6%) [Citation7,Citation8,Citation15,Citation40]. Moreover, the proportion of patients with PSS 0 was lower, and that of patients with PSS 2 was greater than those in previous studies [Citation7,Citation8]. These differences may be attributed to the inclusion criteria in this study, which was limited to inpatients, in contrast to other studies that included patients managed at home. Additionally, the age range of the patients in the current study may have played a role, as previous studies were not limited to paediatric patients.
Our study revealed that many patients had leucocytosis, acidosis, or hyperlactataemia on admission. Furthermore, of the 74.4% of patients with a measured base excess, almost all (96%) exhibited a base deficit after ingesting a liquid laundry detergent capsule.
Substantial variations in median white blood cell count and the prevalence of leucocytosis were observed among the PSS groups. White blood cell count and the presence of leucocytosis can serve as a non-specific marker of severity in exposures to liquid laundry detergent capsules.
The incidence of metabolic acidosis was greater in this study (37%) than in previous reports (0.3–6%) [Citation2,Citation15] and was not related to the severity of exposure. Metabolic acidosis was observed in 1.9% of 690 cases by Huntington and colleagues [Citation30] and in 6% of 50 cases reported to the ToxIC Registry [Citation41]. In other studies, the lowest recorded pH values ranged from 7.1 to 7.28 [Citation27,Citation28,Citation32,Citation42,Citation43].
The incidence of hyperlactataemia (35%) in our study was much greater than that reported by Day and colleagues [Citation2] and was not related to the severity of exposure. Banner and colleagues [Citation8] described a similar frequency of hyperlactatemia (27.9%), though the lactate concentration was only reported in patients with acidosis. Reported peak lactate concentrations ranged from 4.1 to 14 mmol/L in eight patients with hyperlactataemia [Citation27,Citation42–44].
Previous studies did not consistently report base excess values in patients with liquid laundry detergent capsule poisoning [Citation7,Citation27,Citation29]. The majority of participants in this study exhibited substantial base deficits, yet the prevalence of base deficits was not associated with the PSS. Nevertheless, the incidence of base deficits and the median base excess values were decreased in subjects with more severe poisoning.
Liquid laundry detergent capsules contain concentrated detergent in a transparent water-soluble polyvinyl alcohol membrane. European products typically contain anionic surfactants (20–35%), non-ionic surfactants (10–20%), propylene glycol (8–20%) and ethanol (2–5%), and have a pH of 7–9 [Citation2]. United States formulations contain similar constituents, but the non-ionic surfactant concentration is generally higher [Citation2]. The mechanisms of toxicity are not completely understood, but it is probable that the primary cause is the high concentration of non-ionic surfactants present in some capsules, though anionic surfactants, propylene glycol and ethanol may also contribute [Citation2].
Propylene glycol is usually present in a capsule at a maximum concentration of 20%, more usually 15%, that is 6.5–8.6 g per 43 mL capsule, or 538–720 mg/kg for a 12 kg child, if all was ingested which is unlikely. Propylene glycol has been suggested as an explanation for the observed acid-base disturbances, as substantial ingestion of this glycol is known to cause metabolic acidosis, hyperglycaemia, and raised anion gap [Citation45]. As infants have a lower renal clearance of propylene glycol than adults, this could result in them presenting with these features more readily than adults following the same mg/kg dose.
Limitations
This study has some limitations, as its retrospective design may limit the generalizability of the findings. The population assessed in this study may not entirely reflect those with mild or no symptoms, as it is unclear how many individuals with such conditions were not admitted to the hospital but were instead treated at home or in the emergency department.
The study did not consider minor fluctuations in laboratory test results that could occur in patients without causative factors. Additionally, the study only analyzed laboratory test results at the time of admission and did not conduct follow-up tests after the resolution of symptoms.
The data presented in this study should be interpreted with caution and requires further validation. Future prospective multicenter studies that incorporate patients managed in a primary care facility may prove valuable in validating the findings of this research.
Conclusions
Pediatric patients who ingest liquid laundry detergent capsules may develop leucocytosis, metabolic acidosis, hyperlactataemia, and base deficit.
Acknowledgements
The authors would like to express their gratitude to the staff of the Pediatric Poisoning Center at the Grigore Alexandrescu Clinical Emergency Hospital for Children in Bucharest, Romania, for their diligent evaluation and treatment of patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Dart RC, Bronstein AC, Spyker DA, et al. Poisoning in the United States: 2012 emergency medicine report of the national poison data system. Ann Emerg Med. 2015;65(4):416–422. doi: 10.1016/j.annemergmed.2014.11.001.
- Day R, Bradberry SM, Thomas SHL, et al. Liquid laundry detergent capsules (PODS): a review of their composition and mechanisms of toxicity, and of the circumstances, routes, features, and management of exposure. Clin Toxicol. 2019;57(11):1053–1063. doi: 10.1080/15563650.2019.1618466.
- de Groot R, Brekelmans P, Desel H, et al. New legal requirements for submission of product information to poisons centres in EU member states. Clin Toxicol. 2018;56(1):1–6. doi: 10.1080/15563650.2017.1339888.
- O’Donnell KA. Pediatric toxicology: household product ingestions. Pediatr Ann. 2017;46(12):e449–e453. doi: 10.3928/19382359-20171120-04.
- de la Oliva Urieta S, Mencías Rodríguez E, Ucha Domingo MS, et al. Toxic exposure to laundry detergent capsules in Spain. Span J Legal Med. 2016;42(1):17–23. doi: 10.1016/j.remle.2015.10.001.
- Rosenfield D, Eltorki M, VandenBerg S, et al. Single-use detergent sacs: a retrospective multicenter Canadian review of emergency department cases. Pediatr Emerg Care. 2018;34(10):736–739. doi: 10.1097/PEC.0000000000000835.
- Day R, Bradberry SM, Jackson G, et al. A review of 4652 exposures to liquid laundry detergent capsules reported to the United Kingdom National Poisons Information Service 2008–2018. Clin Toxicol. 2019;57(12):1146–1153. doi: 10.1080/15563650.2019.1590586.
- Banner W, Yin S, Burns MM, et al. Clinical characteristics of exposures to liquid laundry detergent packets. Hum Exp Toxicol. 2020;39(1):95–110. doi: 10.1177/0960327119874451.
- Forrester MB. Comparison of pediatric exposures to concentrated "pack" and traditional laundry detergents. Pediatr Emerg Care. 2013;29(4):482–486. doi: 10.1097/pec.0b013e31828a3262.
- Claudet I, Honorat R, Casasoprana A, et al. Expositions des enfants aux lessives capsules, écodoses ou pods: plus toxiques que les lessives traditionnelles? [Pediatric exposures to laundry pods or capsules: more toxic than traditional laundry products?]. Arch Pediatr. 2014;21(6):601–607. doi: 10.1016/j.arcped.2014.03.020.
- Rigaux-Barry F, Patat AM, Cordier L, et al. Risks related to pods exposure compared to traditional laundry detergent products: study of cases recorded by French PCC from 2005 to 2012. Toxicol Anal et Clin. 2017;29(3):257–266. doi: 10.1016/j.toxac.2017.03.122.
- Swain TA, McGwin G, Griffin R. Laundry pod and non-pod detergent related emergency department visits occurring in children in the USA. Inj Prev. 2016;22(6):396–399. doi: 10.1136/injuryprev-2016-041997.
- Fowles JR, Banton MI, Pottenger LH. A toxicological review of the propylene glycols. Crit Rev Toxicol. 2013;43(4):363–390. doi: 10.3109/10408444.2013.792328.
- Wiener SW. Toxicologic acid-base disorders. Emerg Med Clin North Am. 2014;32(1):149–165. doi: 10.1016/j.emc.2013.09.011.
- Valdez AL, Casavant MJ, Spiller HA, et al. Pediatric exposure to laundry detergent pods. Pediatrics. 2014;134(6):1127–1135. doi: 10.1542/peds.2014-0057.
- Rocka A, Piędel F, Madras D, et al. Dark side of laundry pods: analysis of exposure to laundry detergent capsules in children. J Paediatr Child Health. 2021;57(12):1912–1916. doi: 10.1111/jpc.15608.
- Sjogren PP, Skarda DE, Park AH. Upper aerodigestive injuries from detergent ingestion in children. Laryngoscope. 2017;127(2):509–512. doi: 10.1002/lary.26184.
- Richmond A, Liang Z, Mulaj V, et al. The importance of an ecologically valid method in the evaluation of toddler interaction with coloured liquid laundry detergent capsules. PLoS One. 2018;13(7):e0199976. doi: 10.1371/journal.pone.0199976.
- Settimi L, Giordano F, Lauria L, et al. Surveillance of paediatric exposures to liquid laundry detergent pods in Italy. Inj Prev. 2018;24(1):5–11. doi: 10.1136/injuryprev-2016-042263.
- Wang A, Law R, Lyons R, et al. Assessing the public health impact of using poison center data for public health surveillance. Clin Toxicol. 2018;56(7):646–652. doi: 10.1080/15563650.2017.1413194.
- Day R, Eddleston M, Thomas SHL, et al. The impact of an international initiative on exposures to liquid laundry detergent capsules reported to the United Kingdom National Poisons Information Service between 2008 and 2015. Clin Toxicol. 2017;55(3):213–216. doi: 10.1080/15563650.2016.1267359.
- Gaw CE, Spiller HA, Casavant MJ, et al. Safety interventions and liquid laundry detergent packet exposures. Pediatrics. 2019;144(1):e20183117. doi: 10.1542/peds.2018-3117.
- Gulamhusein H, Sabri K. Detergent pods and children: a health hazard on the rise. Cjem. 2021;23(1):137–138. doi: 10.1007/s43678-020-00032-4.
- Reynolds KM, Burnham RI, Delva-Clark H, et al. Impact of product safety changes on accidental exposures to liquid laundry packets in children. Clin Toxicol. 2021;59(5):392–399. doi: 10.1080/15563650.2020.1817478.
- Wiener RC, Waters C, Bhandari R. Detergent pod-related oral-aerodigestive/ocular injuries in children, ages >0 to <18 years [abstract]. J Dent Hyg. 2023;97(1):18–32.
- Singh A, Anderson M, Altaf MA. Clinical and endoscopy findings in children with accidental exposure to concentrated detergent pods. J Pediatr Gastroenterol Nutr. 2019;68(6):824–828. doi: 10.1097/MPG.0000000000002270.
- Smith E, Liebelt E, Nogueira J. Laundry detergent pod ingestions: Is there a need for endoscopy? J Med Toxicol. 2014;10(3):286–291. doi: 10.1007/s13181-014-0414-3.
- Sidhu N, Jaeger MW. Concentrated liquid detergent pod ingestion in children. Pediatr Emerg Care. 2014;30(12):892–893. doi: 10.1097/PEC.0000000000000292.
- Roh DE. Laundry detergent pod: a rising cause of household poisoning. Pediatr Emerg Med J. 2020;7(2):131–134. doi: 10.22470/pemj.2020.00199.
- Huntington S, Heppner J, Vohra R, et al. Serious adverse effects from single-use detergent sacs: report from a U.S. statewide poison control system. Clin Toxicol. 2014;52(3):220–225. doi: 10.3109/15563650.2014.892122.
- Vohra R, Huntington S, Fenik Y, et al. Exposures to single-use detergent sacs reported to a statewide poison control system, 2013–2015. Pediatr Emerg Care. 2018;36(12):e690–e694. doi: 10.1097/PEC.0000000000001490.
- Schneir AB, Rentmeester L, Clark RF, et al. Toxicity following laundry detergent pod ingestion. Pediatr Emerg Care. 2013;29(6):741–742. doi: 10.1097/PEC.0b013e318294eb1d.
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053.
- Greenbaum LA. Chapter 70: deficit Therapy. In: Behrman RE, editor. Nelson textbook of paediatrics, Twenty-one Edition. Philadelphia (PA): Elsevier; 2020. p. 429–432.
- Persson HE, Sjöberg GK, Haines JA, et al. Poisoning severity score. Grading of acute poisoning. J Toxicol Clin Toxicol. 1998;36(3):205–213. doi: 10.3109/15563659809028940.
- Kalas MA, Chavez L, Leon M, et al. Abnormal liver enzymes: a review for clinicians. World J Hepatol. 2021;13(11):1688–1698. doi: 10.4254/wjh.v13.i11.1688.
- Christiansen S, Iverson C, Flanagin A, et al. AMA Manual of Style: a guide for authors and editors, 11th ed. Oxford: Oxford University Press; 2020.
- Freeman GH, Halton JH. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika. 1951;38(1-2):141–149. doi: 10.1093/biomet/38.1-2.141.
- Ahad NA, Okwonu FZ, Apanapudor JS, et al. Chi-square and adjusted standardized residual analysis. ASMScJ. 2023;18:1–11. doi: 10.32802/asmscj.2023.985.
- Williams H, Jones S, Wood K, et al. Reported toxicity in 1486 liquid detergent capsule exposures to the UK National Poisons Information Service 2009–2012, including their ophthalmic and CNS effects. Clin Toxicol. 2014;52(2):136–140. doi: 10.3109/15563650.2013.855315.
- Troncoso A, Calello D. Pediatric laundry detergent pod exposures: report from the Toxicology Investigator’s Consortium (ToxIC registry). Clin Toxicol. 2014;52:723.
- Lim R, Forward KE. Laundry detergent pod ingestion. Pediatr Emerg Care. 2013;29(9):1053–1054. doi: 10.1097/PEC.0b013e3182a36217.
- Fontane E. Ingestion of concentrated laundry detergent pods. J Emerg Med. 2015;49(1):e37–e38. doi: 10.1016/j.jemermed.2015.01.007.
- Romero S, Levitan R, Rosenberg R, et al. Abstract 196: previously unreported adverse events following ingestion of laundry detergent pods in children. Pediatr Crit Care Med. 2014;15(4_suppl):48. doi: 10.1097/01.pcc.0000448922.26069.9a.
- Lim TY, Poole RL, Pageler NM. Propylene glycol toxicity in children. J Pediatr Pharmacol Ther. 2014;19(4):277–282. doi: 10.5863/1551-6776-19.4.277.
