Abstract
Introduction
Carbon monoxide poisoning is associated with severe damage to various organs. In this study, we aimed to determine if previous carbon monoxide poisoning was associated with an increased risk of lung diseases.
Methods
The study population was derived from the National Health Insurance Service database of Korea between 1 January 2002 and 31 December 2021. Adults with carbon monoxide poisoning, with at least one visit to medical facilities between 2002 and 2021, were included. For comparison, an equal number of matched controls with the same index date were selected from the database.
Results
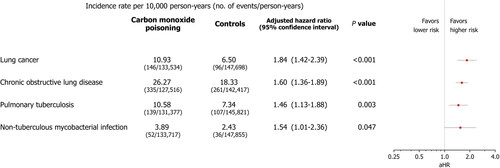
A total of 28,618 patients with carbon monoxide poisoning and 28,618 matched controls were included in this study. Approximately 42.8 per cent of the patient and control groups were female, with a mean age of 51.3 years. In patients with carbon monoxide poisoning, there was a significant increase in the risk of lung cancer (adjusted hazard ratio, 1.84; 95 per cent confidence interval, 1.42–2.39; P < 0.001), chronic obstructive pulmonary disease (adjusted hazard ratio, 1.60; 95 per cent confidence interval, 1.36–1.89; P < 0.001), pulmonary tuberculosis (adjusted hazard ratio, 1.46; 95 per cent confidence interval, 1.13–1.88; P = 0.003), and non-tuberculous mycobacterial infection (adjusted hazard ratio, 1.54; 95 per cent confidence interval, 1.01–2.36; P = 0.047).
Discussion
In this retrospective cohort study, previous carbon monoxide poisoning was associated with an increased risk of lung cancer, chronic obstructive pulmonary disease, pulmonary tuberculosis, and non-tuberculous mycobacterial infection. Further studies are needed to confirm such an association in other populations and the risk of lung diseases due to the toxic effect of carbon monoxide from different sources.
Conclusions
Previous carbon monoxide poisoning was associated with an increased risk of lung diseases, but the relative importance of the causes and sources of exposure was not known. The long-term management of survivors of acute carbon monoxide poisoning should include monitoring for lung cancer, chronic obstructive pulmonary disease, pulmonary tuberculosis, and non-tuberculous mycobacterial infection.
Introduction
The role of carbon monoxide as a potential therapeutic aid in pulmonary medicine is currently being evaluated despite being regarded as a highly toxic gas [Citation1]. Carbon monoxide is considered to have anti-inflammatory [Citation2], antiapoptotic, antioxidant [Citation3], and vasoactive [Citation4] characteristics. However, there are striking differences between being poisoned by exposure to high carbon monoxide concentrations and using low carbon monoxide concentrations for therapeutic purposes. Each year, over 50,000 people are admitted to hospital emergency departments in the United States for carbon monoxide poisoning, and an estimated 1,500 patients die from this condition [Citation5–7]. Moreover, neurocognitive sequelae occur in 25–50% of acute carbon monoxide poisoning survivors [Citation8,Citation9]. In addition, carbon monoxide poisoning can cause heart [Citation10–12] and kidney damage [Citation13,Citation14] and may increase the risk of mortality among patients with cardiac conditions [Citation15]. We recently reported in a rat model that carbon monoxide poisoning can lead to the development of emphysematous changes in the lungs [Citation16]. Currently, it is unclear if previous carbon monoxide poisoning is associated with an increased risk of lung diseases.
In this retrospective study, we aimed to determine if previous carbon monoxide poisoning was associated with an increased risk of lung diseases, using data from a nationwide population-based cohort.
Materials and methods
Data source and ethics statement
This nationwide population-based cohort study collected data from the administrative database of the National Health Insurance Service of Korea [Citation17]. The National Health Insurance Service of Korea covers more than 99% of the national population. The database contains data on demographics, socioeconomic profiles, diagnoses, and medical prescriptions of more than 50 million people [Citation18]. The general health examinations database was used to collect data on the annual or biannual health examinations that employees, householders, and all citizens over the age of 40 years are required to undergo. The study was approved by the Korean National Institute for Bioethics Policy (NHIS-2022-1-366) and Wonju Severance Christian Hospital’s institutional review board (approval number CR321347) and was conducted in accordance with the Declaration of Helsinki. The requirement for informed consent was waived owing to the use of deidentified data and the retrospective nature of this study.
Study population
The study population was identified from the National Health Insurance Service database between 1 January 2002 and 31 December 2021. Patients with acute carbon monoxide poisoning were included if they were 18 years of age or older and had any documented visits to medical facilities between 1 January 2002 and 31 December 2021 because of the toxic effects of carbon monoxide (International Classification of Diseases, Tenth Revision, code T58, as a principal or adjuvant diagnosis). If there was more than one visit for carbon monoxide poisoning, the first one was used for analysis. The date of the documented visit for carbon monoxide poisoning was defined as the index date for patients; that is, the first carbon monoxide exposure date is the index date. The matched controls, who did not have code T58 in their records during the entire observation period, were selected for the control group. The index date for matched controls was the same as that of the cases. Follow-up started at the index date and continued until death, emigration, or the end of the observation period (31 December 2021), whichever came first. Only those who had undergone chest radiography at least once during the general health examination were included in both groups.
Validation of patients with carbon monoxide poisoning
We first evaluated the reliability of our criteria for identifying patients with carbon monoxide poisoning from the administrative database based on a review of electronic medical records of the patients who visited our institution from January 2006 to August 2022. At our institution, the diagnosis of acute carbon monoxide poisoning was established by considering the patient’s medical history and the presence of elevated carboxyhemoglobin levels exceeding 5% for non-smokers and 10% for heavy smokers. Two board-certified specialists in emergency medicine reviewed each case to confirm the diagnosis and the positive predictive value (see Results).
Outcome measurements
The National Health Insurance Service in Korea has a Rare and Intractable Disease registration program for patients with lung cancer and idiopathic pulmonary fibrosis, which offers a co-payment reduction of 90%. For registration, all candidates are required to have their diagnosis certified by board-certified experts using uniform criteria set by the government. The National Health Insurance Service reviews the application and diagnosis of the Rare and Intractable Disease registration program to ensure that diagnostic criteria are met. The claims are then sent to and included in the claims database. This systematic process ensures that diagnoses of lung cancer and idiopathic pulmonary fibrosis are reliable.
The outcome was the incidence of lung diseases in patients with carbon monoxide poisoning and controls. The following lung diseases were investigated separately: lung cancer (International Classification of Diseases, Tenth Revision, code C34), chronic obstructive pulmonary disease (J44), bronchiectasis (J47), asthma (J45), idiopathic pulmonary fibrosis (J841 and V236), idiopathic interstitial pneumonia (interstitial lung diseases (J84) except idiopathic pulmonary fibrosis [J841 and V236] and connective tissue disease [M30-M36]), pulmonary tuberculosis (A15, A16, and A19), and non-tuberculous mycobacterial infection (A31).
Statistical analyses
The baseline demographic characteristics of the study population were described as frequencies and percentages or means with standard deviations. Differences in clinical characteristics were analyzed using an independent t-test or chi-squared test. The incidence rate for each outcome was calculated as the number of incident diseases per 10,000 person-years. After testing the proportional hazard assumption, multivariable Cox proportional hazards regression analyses were performed to estimate the adjusted hazard ratios and 95% confidence intervals for patients with carbon monoxide poisoning compared to controls during the observation period. Multivariable models were generated with the following covariates: matching variables (birth year, sex, insurance type, income level, location of residence, and alcohol and smoking status), body mass index, pulmonary tuberculosis history ascertained from the latest general health examination before the index date, and Charlson Comorbidity Index score, which was adjusted for comorbidities, at the index date. For each analysis, patients who had already been diagnosed with one of the eight lung diseases on or before the index date were excluded so that only at-risk patients and controls were included in the calculation of adjusted hazard ratios.
All statistical analyses were performed using the SAS statistical software (version 9.4; SAS Institute Inc.) and R statistical software (version 3.6.3, R Foundation) at a significance level of 5%.
Results
Validation of criteria for identifying patients
From January 2006 to August 2022, 1,991 patients visited our institution at least once because of the toxic effects of carbon monoxide poisoning (code T58). Based on our review, 1,920 patients were confirmed to have carbon monoxide poisoning. The positive predictive value was 96.4% (95% confidence interval 95.5–97.2%), indicating the reliability of our criteria for identifying patients with carbon monoxide poisoning from the National Health Insurance Service database.
Characteristics of the study population
A total of 28,618 patients with carbon monoxide poisoning and 28,618 matched controls were included in this study (). Their mean (±SD) age was 51.3 ± 15.3 years. There were 12,241 (42.8%) females and 12,369 (43.2%) never-smokers in each group. The mean (±SD) Charlson Comorbidity Index score was 2.3 ± 2.4 and 2.1 ± 2.3 in the carbon monoxide poisoning and control groups, respectively (P < 0.001). Among the prevalent comorbidities on the index date, ischaemic heart disease, stroke or transient ischaemic attack, chronic obstructive pulmonary disease, and pulmonary tuberculosis were more common in the carbon monoxide poisoning group. A history of malignancies was present in 1,326 (4.6%) and 1,226 (4.3%) patients in the carbon monoxide poisoning and control groups, respectively (P < 0.001). Hyperbaric oxygen therapy was administered to 6,529 (22.8%) patients in the carbon monoxide poisoning group. The mean (±SD) follow-up periods were 4.7 ± 3.5 years for patients with carbon monoxide poisoning and 5.2 ± 3.5 years for the controls. There were more deaths from all causes among patients with carbon monoxide poisoning than controls (3,388 versus 633; P < 0.001). Patients with carbon monoxide poisoning had a much higher cumulative incidence of all-cause mortality throughout the observation period ().
Figure 1. Cumulative incidence of all-cause mortality the cumulative incidence of all-cause mortality in patients with previous carbon monoxide poisoning and matched controls. The shaded area shows the 95% confidence interval of the cumulative incidence.
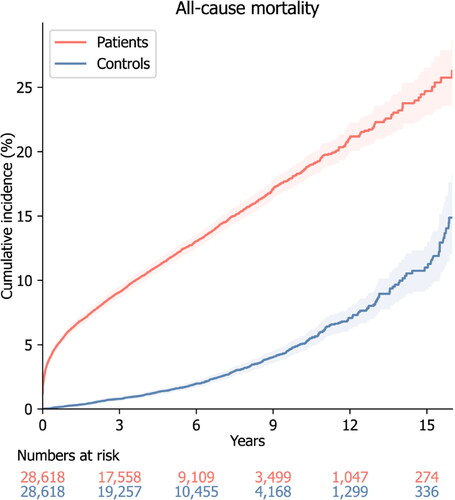
Table 1. Baseline characteristics of the study population.
Development of lung diseases
The cumulative incidence plots for incident lung diseases in patients and controls are shown in . The increased overall risk of lung cancer (adjusted hazard ratio, 1.84; 95% confidence intervals, 1.42–2.39; P < 0.001), chronic obstructive pulmonary disease (adjusted hazard ratio, 1.60; 95% confidence intervals, 1.36–1.89; P < 0.001), pulmonary tuberculosis (adjusted hazard ratio, 1.46; 95% confidence intervals, 1.13–1.88; P = 0.003), and non-tuberculous mycobacterial infection (adjusted hazard ratio, 1.54; 95% confidence intervals, 1.01–2.36; P = 0.047) in the patient group compared to the control group was observed (). With respect to bronchiectasis, asthma, idiopathic pulmonary fibrosis and idiopathic interstitial pneumonia, there was a prominent time-dependent effect with crossing of the cumulative incidence curves over time (). Cox proportional hazard analysis was not performed, since the overall adjusted hazard ratio could not be interpreted as an average over time.
Figure 2. Cumulative incidence plot of incident lung diseases. The cumulative incidence of incident lung diseases in patients with previous carbon monoxide poisoning and matched controls. The shaded area shows the 95% confidence interval of the cumulative incidence.
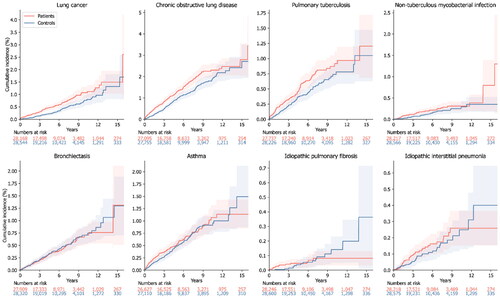
Figure 3. Risk of developing lung diseases associated with carbon monoxide poisoning. The plot presents incidence rates and adjusted hazard ratio (aHR) for each outcome in patients with previous carbon monoxide poisoning and matched controls. Multivariable cox proportional hazard analysis adjusted for birth year, sex, insurance type, income level, location of residence, body mass index, smoking status, pulmonary tuberculosis history, and charlson comorbidity index score at the index date.
Discussion
In this nationwide population-based cohort, we found that previous carbon monoxide poisoning was associated with an increased risk of lung diseases, namely lung cancer (1.8-fold), chronic obstructive pulmonary disease (1.6-fold), pulmonary tuberculosis (1.5-fold), and non-tuberculous mycobacterial infection (1.5-fold) when compared to matched controls. Therefore, we suggest that the long-term management of survivors of acute carbon monoxide poisoning should include monitoring for these lung diseases.
Our previous experimental study [Citation16] demonstrated that emphysematous changes, such as the enlargement of the alveolar airspaces and destruction of the septal walls, and alterations in biomarkers of inflammatory activity that mediate the pathogenesis of emphysema occurred in the alveoli of rat lungs at 6 weeks after a single high-concentration exposure to carbon monoxide. Exposure to high concentrations of carbon monoxide can lead to the dysregulation of the signalling pathways, which may become pathological and eventually fatal [Citation19]. Finck and colleagues [Citation20] reported that the gross pathological alterations in the lungs of 351 patients with fatal carbon monoxide poisoning included congestion and/or oedema (66%) and haemorrhage (7%). Çolakoğlu and colleagues [Citation21] evaluated rat lung cells using the argyrophilic nucleolar-organizing region staining method and found that carbon monoxide poisoning could damage the lung cells. Two key processes that contribute to the development of chronic obstructive pulmonary disease are oxidative stress and increased proteinase synthesis [Citation22]. Cigarette smokers are at a higher risk of developing chronic obstructive pulmonary disease due to oxidative stress and an imbalance between antioxidants and oxidants [Citation23]. This is similar to carbon monoxide poisoning in that free radicals and oxidative stress are the main damaging mechanisms [Citation24].
In this study, the carbon monoxide poisoning group exhibited a higher incidence of lung cancer as well as chronic obstructive pulmonary disease. As cigarette smoking-induced epigenetic alterations play important roles in the development of lung cancer by regulating the specificity and duration of gene transcription [Citation25], carbon monoxide poisoning might also play a role in epigenetic alteration. Although cigarettes and carbon monoxide have common inflammatory pathways in terms of organ damage [Citation24,Citation26], the association and mechanism between acute carbon monoxide poisoning and lung cancer development are not clear. Further studies are required.
The relationship between some respiratory infections, including tuberculosis and non-tuberculous mycobacterium pulmonary diseases and carbon monoxide poisoning are not clear in terms of causation and mechanism. However, respiratory pathogens usually easily cause an infection in the damaged lung architecture. In a population-based cohort study, chronic obstructive pulmonary disease patients had an increased risk of developing tuberculosis [Citation27]. Patients with emphysema showed delayed sputum culture conversion after initiation of treatment for pulmonary tuberculosis [Citation28]. Chronic respiratory disease, including chronic obstructive pulmonary disease, was a strong risk factor for non-tuberculous mycobacterium pulmonary disease [Citation29]. Initiation of lung architectural damage after carbon monoxide poisoning might predispose the patients to respiratory infections such as tuberculosis and non-tuberculous mycobacterium.
The long-term mortality of patients with carbon monoxide poisoning compared to the general population is not much investigated. In one single-centre cohort study, survivors after carbon monoxide poisoning requiring hyperbaric treatment showed a higher mortality [Citation30]. The investigators considered the increased risk might come from psychiatric illnesses and behaviour because the mortality is higher in the group of intentional carbon monoxide poisoning. Another study based on nationwide cohort data also showed higher long-term mortality, and the deaths occurred particularly during the first year, which is similar to our result [Citation31]. However, besides acute stage mortality, increased comorbid disorders after events and the diseases-associated long-term mortality were revealed in previous studies [Citation15,Citation31,Citation32]. If lung diseases occurred commonly after carbon monoxide poisoning, the disease progression might contribute to their higher long-term mortalities.
Our study has several strengths compared to previous studies on carbon monoxide poisoning. First, the association between previous carbon monoxide poisoning and overall risk of lung diseases has rarely been studied. Second, the Korean National Health Insurance Service database, which includes almost all medical information for 50 million Korean individuals, was used in this study; we consider this population sufficient to explore the risk of lung diseases while avoiding selection bias [Citation33]. Third, the controls were matched for the basis of demographic and socioeconomic factors, and multivariate analysis was performed after adjustment for a range of variables to eliminate the effect of potential confounders [Citation34].
This study had a few limitations. First, although we validated our criteria for identifying patients with carbon monoxide poisoning, misclassification may still occur when collecting patient data from a nationwide database. Second, our retrospective cohort study design did not establish the causality. Third, our results may have been influenced by the fact that charcoal burning is one of the source of carbon monoxide in Korea, followed by toxic materials generated by the incomplete combustion of co-contaminants. Unfortunately, the T58 code in the Korean National Health Insurance Service database (claim database) is not sub-classified according to the origin of carbon monoxide and the intention of poisoning. Therefore, we cannot determine the proportion of unintentional and intentional cases. In addition, the operational definition of intentional carbon monoxide poisoning is not accurate because there are quite a few cases where suicide codes or codes for intentionality were not included due to the possibility of medical disputes. Third, the study has limitations related to time-to-outcome analysis. For example, it was not possible to perform Cox proportional hazard analysis for specific outcomes where a significant violation of the proportional hazard assumption was observed. Additionally, caution is advised when interpreting because there might be residual time-varying effects in the other conditions. Fourth, the observation period was significantly shorter in cases than that in controls. The observed differences in survival times are thought to be due to higher short-term mortality in the patient group, as well as variations in long-term mortality rates due to various conditions. Despite our efforts to compensate for variable observational periods between the two groups through survival analysis, it is possible that we might have overestimated or underestimated the effect size for certain outcomes, especially those requiring long-term observation. Fifth, except for idiopathic pulmonary fibrosis detected by the International Classification of Diseases, 10th Revision codes, subtle fibrotic changes could not be confirmed by the administrative database. Finally, the generalizability of our findings may be limited by the homogeneity of our predominantly Korean study population; therefore, further studies with different ethnic and regional groups are needed.
Conclusions
Previous carbon monoxide poisoning was associated with an increased risk of lung diseases, but the relative importance of the causes and sources of exposure is not known. The long-term management of survivors of acute carbon monoxide poisoning should include monitoring for lung cancer, chronic obstructive pulmonary disease, pulmonary tuberculosis, and non-tuberculous mycobacterial infection.
Author contributions
YSC conceived and designed the study and drafted and approved the final manuscript. SJL drafted the manuscript. SL analyzed and interpreted the data and drafted the manuscript. SL and YHK critically revised the manuscript. All authors have read the manuscript and approved its submission.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data supporting the findings of this study were available from the National Health Insurance Service of Korea. Access restrictions, however, apply as these data were used under license for the current study and are not publicly available. However, the authors will make data available upon reasonable request and with permission from the National Health Insurance Service of Korea.
Additional information
Funding
References
- Ryter SW, Choi AM. Therapeutic applications of carbon monoxide in lung disease. Curr Opin Pharmacol. 2006;6(3):257–262. doi: 10.1016/j.coph.2006.03.002.
- Morse D, Pischke SE, Zhou Z, et al. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J Biol Chem. 2003;278(39):36993–36998. doi: 10.1074/jbc.M302942200.
- Wang X, Wang Y, Kim HP, et al. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem. 2007;282(3):1718–1726. doi: 10.1074/jbc.M607610200.
- Foresti R, Hammad J, Clark JE, et al. Vasoactive properties of CORM-3, a novel water-soluble carbon monoxide-releasing molecule. Br J Pharmacol. 2004;142(3):453–460. doi: 10.1038/sj.bjp.0705825.
- Weaver LK. Carbon monoxide poisoning. In: Moon RE, editor. Hyperbaric oxygen therapy indications. 14th ed. North Palm Beach (FL): Best Publishing Company; 2019. p. 81–104.
- Hampson NB. U.S. mortality due to carbon monoxide poisoning, 1999–2014. Ann Am Thorac Soc. 2016;13(10):1768–1774. doi: 10.1513/AnnalsATS.201604-318OC.
- Hampson NB, Weaver LK. Carbon monoxide poisoning: a new incidence for an old disease. Undersea Hyperb Med. 2007;34(3):163–168.
- Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002;347(14):1057–1067. doi: 10.1056/NEJMoa013121.
- Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol. 1983;40(7):433–435. doi: 10.1001/archneur.1983.04050070063016.
- Satran D, Henry CR, Adkinson C, et al. Cardiovascular manifestations of moderate to severe carbon monoxide poisoning. J Am Coll Cardiol. 2005;45(9):1513–1516. doi: 10.1016/j.jacc.2005.01.044.
- Cha YS, Kim H, Hwang SO, et al. Incidence and patterns of cardiomyopathy in carbon monoxide-poisoned patients with myocardial injury. Clin Toxicol (Phila). 2016;54(6):481–487. doi: 10.3109/15563650.2016.1162310.
- Cho DH, Ko SM, Son JW, et al. Myocardial injury and fibrosis from acute carbon monoxide poisoning: a prospective observational study. JACC Cardiovasc Imaging. 2021;14(9):1758–1770. doi: 10.1016/j.jcmg.2021.02.020.
- Kim YJ, Sohn CH, Seo DW, et al. Analysis of the development and progression of carbon monoxide poisoning-related acute kidney injury according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria. Clin Toxicol (Phila). 2018;56(8):759–764. doi: 10.1080/15563650.2018.1424890.
- Huang TL, Tung MC, Lin CL, et al. Risk of acute kidney injury among patients with carbon monoxide poisoning. Medicine (Baltimore). 2021;100(38):e27239. doi: 10.1097/MD.0000000000027239.
- Henry CR, Satran D, Lindgren B, et al. Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning. JAMA. 2006;295(4):398–402. doi: 10.1001/jama.295.4.398.
- Lee SJ, Kim T, Cha YS, et al. Alveolar damage and development of emphysema in rats with carbon monoxide poisoning. Mol Cell Toxicol. 2023. doi: 10.1007/s13273-023-00405-7.
- Seong SC, Kim YY, Khang YH, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800.
- National Health Insurance Service (KR). National Health Insurance bigdata sharing service [Internet]. Wonju, Korea: National Health Insurance Service; 2022; [cited 2022 1 October]. Available from: https://nhiss.nhis.or.kr/bd/ab/bdaba011eng.do.
- Tien Vo TT, Vo QC, Tuan VP, et al. The potentials of carbon monoxide-releasing molecules in cancer treatment: an outlook from ROS biology and medicine. Redox Biol. 2021;46:102124. doi: 10.1016/j.redox.2021.102124.
- Finck PA. Exposure to carbon monoxide: review of the literature and 567 autopsies. Mil Med. 1966;131(12):1513–1539. doi: 10.1093/milmed/131.12.1513.
- Çolakoğlu S, Saritas A, Eroz R, et al. Is one-time carbon monoxide intoxication harmless? Evaluation by argyrophilic nucleolar-organizing regions staining method. Hum Exp Toxicol. 2015;34(1):24–31. doi: 10.1177/0960327114531994.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2022 report): Global Initiative for Chronic Obstructive Lung Disease; 2021.
- Domej W, Oettl K, Renner W. Oxidative stress and free radicals in COPD–implications and relevance for treatment. Int J Chron Obstruct Pulmon Dis. 2014;9:1207–1224. doi: 10.2147/COPD.S51226.
- Rose JJ, Wang L, Xu Q, et al. Carbon monoxide poisoning: pathogenesis, management, and future directions of therapy. Am J Respir Crit Care Med. 2017;195(5):596–606. doi: 10.1164/rccm.201606-1275CI.
- Zong D, Liu X, Li J, et al. The role of cigarette smoke-induced epigenetic alterations in inflammation. Epigenetics Chromatin. 2019;12(1):65. doi: 10.1186/s13072-019-0311-8.
- Zhou Z, Chen P, Peng H. Are healthy smokers really healthy? Tob Induc Dis. 2016;14:35. doi: 10.1186/s12971-016-0101-z.
- Inghammar M, Ekbom A, Engström G, et al. COPD and the risk of tuberculosis-a population-based cohort study. PLoS One. 2010;5(4):e10138. doi: 10.1371/journal.pone.0010138.
- Takasaka N, Seki Y, Fujisaki I, et al. Impact of emphysema on sputum culture conversion in male patients with pulmonary tuberculosis: a retrospective analysis. BMC Pulm Med. 2020;20(1):287. doi: 10.1186/s12890-020-01325-1.
- Andréjak C, Nielsen R, Thomsen V, et al. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68(3):256–262. doi: 10.1136/thoraxjnl-2012-201772.
- Hampson NB, Rudd RA, Hauff NM. Increased long-term mortality among survivors of acute carbon monoxide poisoning. Crit Care Med. 2009;37(6):1941–1947. doi: 10.1097/CCM.0b013e3181a0064f.
- Wong CS, Lin YC, Sung LC, et al. Increased long-term risk of major adverse cardiovascular events in patients with carbon monoxide poisoning: a population-based study in Taiwan. PLoS One. 2017;12(4):e0176465. doi: 10.1371/journal.pone.0176465.
- Wong CS, Lin YC, Hong LY, et al. Increased long-term risk of dementia in patients with carbon monoxide poisoning: a population-based study. Medicine (Baltimore). 2016;95(3):e2549. doi: 10.1097/MD.0000000000002549.
- Kim M, Choi KH, Hwang SW, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76(1):40–48. doi: 10.1016/j.jaad.2016.08.022.
- Bae JM, Chung KY, Yun SJ, et al. Markedly reduced risk of internal malignancies in patients with vitiligo: a nationwide population-based cohort study. J Clin Oncol. 2019;37(11):903–911. doi: 10.1200/JCO.18.01223.
