Abstract
Introduction
Common major co-formulants in glyphosate-based herbicides, polyethoxylated tallow amine surfactants, are suspected of being more toxic than glyphosate, contributing to the toxicity in humans. However, limited information exists on using polyethoxylated tallow amine concentrations to predict clinical outcomes. We investigated if plasma concentrations of glyphosate, its metabolite and polyethoxylated tallow amines can predict acute kidney injury and case fatality in glyphosate poisoning.
Methods
We enrolled 151 patients with acute glyphosate poisoning between 2010 and 2013. Plasma concentrations of glyphosate, its metabolite, aminomethylphosphonic acid, and polyethoxylated tallow amines were determined in 2020 using liquid chromatography-tandem mass spectrometry. Associations between exposure and poisoning severity were assessed.
Results
Plasma concentrations of glyphosate and aminomethylphosphonic acid demonstrated good and moderate performances in predicting acute kidney injury (≥2), with an area under the receiver operating characteristic curve of 0.83 (95% CI 0.69–0.97) and 0.76 (95% CI 0.59–0.94), respectively. Polyethoxylated tallow amines were detected in one-fifth of symptomatic patients, including one of four fatalities and those with unsaturated tallow moieties being good indicators of acute kidney injury (area under the receiver operating characteristic curve ≥0.7). As the number of repeating ethoxylate units in tallow moieties decreased, the odds of acute kidney injury increased. Glyphosate and aminomethylphosphonic acid concentrations were excellent predictors of case fatality (area under the receiver operating characteristic curve >0.9).
Discussion
The 2.7% case fatality rate with 49% acute, albeit mild, acute kidney injury following glyphosate poisoning is consistent with previously published data. A population approach using model-based metrics might better explore the relationship of exposure to severity of poisoning.
Conclusions
Plasma concentrations of glyphosate and its metabolite predicted the severity of clinical toxicity in glyphosate poisoning. The co-formulated polyethoxylated tallow amine surfactants were even more strongly predictive of acute kidney injury but were only detected in a minority of patients.
Introduction
Glyphosate is a common agent used in pesticide self-poisoning, predominantly in low- and middle-income Asia-Pacific countries [Citation1, Citation2]. Fatality rates following glyphosate poisoning vary significantly, ranging from 1 to 30% [Citation3, Citation4].
Although the exact composition of the co-formulants in glyphosate-based herbicides remains undisclosed to the public, polyethoxylated tallow amine (POEA) surfactants have been widely recognized as the most common surfactants in glyphosate formulations over the last 40 years [Citation5, Citation6]. The past decade has seen a growing concern regarding the potential toxicity of POEA, which in animals displayed higher toxicity across all investigated endpoints compared to glyphosate [Citation7]. Despite extensive research on glyphosate poisoning, there is a paucity of information on POEA-related glyphosate toxicity following acute intoxication, primarily due to the analytical challenges associated with measuring these compounds in biological samples.
We have recently developed bioanalytical methods that enable the evaluation of exposure to glyphosate, its metabolite, aminomethylphosphonic acid, and POEA surfactants after acute poisoning [Citation8, Citation9]. This study further investigates the correlation between exposure to these compounds and the severity of poisoning, as well as their prognostic values in predicting clinical outcomes.
Glyphosate poisoning is known to be associated with various toxic effects, including gastrointestinal symptoms, renal and hepatic impairment, respiratory distress, hypotension, impaired consciousness, metabolic acidosis, dysrhythmias, pulmonary toxicity, shock, and even death [Citation10–12]. Among these effects, acute kidney injury is recognized as a key clinical feature associated with moderate to severe poisoning and a significant predictor of poor outcome [Citation13]. However, there has been no research on the relationship between glyphosate and POEA surfactant concentrations and acute kidney injury.
Here, we describe the clinical outcomes of acute glyphosate self-poisoning in a large, multi-centre cohort study in Sri Lanka. The relationships of the plasma concentrations of glyphosate, aminomethylphosphonic acid, and POEA surfactants to acute kidney injury and case fatality are investigated.
Materials and methods
Study design, clinical data and specimen collection
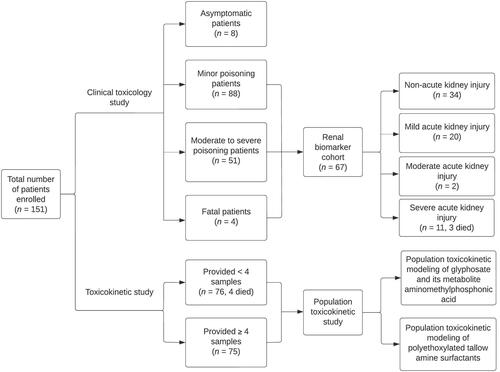
This is part of a multi-centre prospective cohort study of patients with acute self-poisoning in Sri Lanka. Patients presenting to study hospitals with a history of glyphosate poisoning between October 2010 and January 2013 were approached for consent and eligible for enrolment in this study (glyphosate cohort). All patients received standard care according to local hospital management guidelines. Patients with co-ingestion of other substances (except for alcohol) were excluded. Informed written consent was obtained from all participating patients or their relatives. Demographic and clinical details were obtained from consenting patients upon presentation. Regular clinical examinations were performed at least twice daily, and observations were recorded by on-site study doctors during hospitalization. All patients provided a blood sample upon admission. Additional serial samples were collected from a subset of patients at 4, 8, 16 and 24 h when possible, then daily until discharge or death and at follow-up, up to 3 months. A sub-group of patients (glyphosate renal biomarker cohort) provided multiple samples and were also enrolled in a study exploring novel biomarkers of glyphosate-induced nephrotoxicity [Citation13], and blood and urine samples were collected following the same sampling time point where possible. All specimens were promptly processed and stored at −20 °C for up to 3 months and then transferred to a −80 °C freezer until analysis. The study was approved by the human research ethics committees of the University of Sydney (HREC 10023) and the University of Peradeniya (2007/EC/46).
Renal biomarker assays
We have used serum creatinine concentrations previously measured on the same patients as part of a renal biomarker cohort [Citation13–15]. The serum creatinine concentration was measured as described elsewhere [Citation13–15] using the Jaffe method (kinetic method, rate blank and compensated) on a Hitachi 912 automatic analyzer (Roche, Japan).
Glyphosate, aminomethylphosphonic acid and polyethoxylated tallow amine surfactants assays
Plasma concentrations of glyphosate, aminomethylphosphonic acid, and POEA surfactants were determined using liquid chromatography-tandem mass spectrometry methods, which we have recently established [Citation8, Citation9]. The validated methods showed good specificity, accuracy and precision with a lower limit of quantification of 50 µg/L for both glyphosate and aminomethylphosphonic acid and ranging from 0.35–10.8 µg/L for all selected POEA homologues.
The following naming convention was used to represent POEA homologues in this paper: Cz(s/u)(EO)x, where z refers to the number of carbon atoms in the alkyl chain, s/u is either saturated or unsaturated tallow moiety, and x is the total number of ethoxylate units from the two ethoxylated chains [Citation8]. The first generation of POEA surfactants found in Monsanto’s Roundup® formulation contained the POEA surfactants with an average of 15 ethoxylate units, commonly known as "POE (15) tallow amines". Although the formulations of glyphosate-containing herbicides in Sri Lanka are not publicly available, some products are known to be based on the original Monsanto formulation, which contained POE (15) tallow amines as the surfactant [Citation5, Citation12]. Therefore, the clinical analysis in this study included the five POE (15) tallow amines to represent the POEA surfactants to which poisoning patients in our cohort were exposed. Additionally, the concentrations of these five tallow amines were divided by their corresponding proportions and the average value was used to estimate the concentration of total POE (15) tallow amines (total POEA).
Clinical staging and acute kidney injury staging of severity of poisoning
The severity of poisoning in all patients enrolled in the study was graded into four categories (asymptomatic, minor, moderate to severe, and fatal) (Supplementary Table S1) based on predetermined clinical criteria using clinical features of glyphosate poisoning [Citation12].
For those patients enrolled in the renal biomarker cohort, we performed a subgroup analysis by grading the severity of acute kidney injury based on an increase in serum creatinine concentration into three categories: no acute kidney injury (non-acute kidney injury, acute kidney injury 0), mild acute kidney injury (acute kidney injury 1) and moderate to severe acute kidney injury (acute kidney injury ≥2) using the 2012 Kidney Disease: Improving Global Outcomes Clinical Practice Guideline for acute kidney injury criteria [Citation16]. For sensitivity analyses, acute kidney injury cases were also grouped as two binary variables, with the first being no-acute kidney injury (0) versus acute kidney injury (1–3), and the second no- or mild acute kidney injury (0–1) versus moderate to severe acute kidney injury (2–3).
Glyphosate overall exposure metrics analysis
The overall exposure metrics for glyphosate, specifically the total extent of exposure, quantified by area under the concentration versus time curve (AUC0-∞), and the average duration of exposure, as calculated by mean residence time, were computed to investigate their correlation with the severity of poisoning. The AUC, area under the first moment curve (AUMC) and mean residence time were analyzed for glyphosate using the "noncompartmental analysis, extravascular" module within the PK Solver 2.0 program [Citation17]. The AUC and AUMC were derived using the linear trapezoidal method. The terminal elimination slope was automatically calculated using regression analysis based on sequential data points, starting with the last three, then four, and so on. The regression with the highest adjusted R2 value was then chosen. Patients with unreported ingested volume or fewer than three data points defining the terminal slope were excluded from the noncompartmental pharmacokinetic analysis.
Statistical analysis
Continuous variables were reported as medians with interquartile ranges, while categorical variables were expressed as counts and percentages. The Mann-Whitney U test was used to compare ranks between two groups for continuous nonparametric variables. The Kruskal-Wallis test, with post hoc Dunn’s multiple comparison test, was used to compare between three or more groups. Chi-square (and Fisher’s exact) test was used to compare categorical variables between groups.
Demographic and clinical variables, along with the measured concentrations, were tested in a univariable logistic regression to predict no acute kidney injury versus acute kidney injury or no/mild acute kidney injury versus moderate/severe acute kidney injury. All concentration data were transformed to log2 to represent the risk estimates associated with a doubling in corresponding concentrations. The results are presented as odds ratios (ORs) with 95% confidence intervals (CIs). Significant determinants of case fatality were estimated using exact logistic regression because of the low number of deaths. Concentration data were log2-transformed to reduce the skewness and kurtosis. Only variables demonstrating a significant relationship (P < 0.05) with the dependent variable were then added to the multivariable model. We used PROC GENMOD in SAS to output the Hessian weights of a model comparing all the continuous predictors with death as the dependent variable. Next, we examined the collinearity amongst the weighted variables. This revealed that both the Glasgow Coma Scale (GCS) (variation proportion: 0.99) and time post-ingestion (variation proportion: 0.85) were collinear with the intercept. The individual tallow amine values were also very strongly correlated (all >0.90), and thus could not be used in regression concurrently. Thus, we introduced total POEA into the model as a replacement for the five POE (15) tallow amines. Additionally, the discriminative performance of the variables in predicting moderate to severe acute kidney injury (≥2) and death was evaluated at the univariate level using the area under the receiver operating characteristic curves (AUC-ROC). The optimal cut-point for each variable was determined based on the highest Youden’s index. Sensitivity, specificity and the likelihood ratio at this cut-off value for each parameter are also presented.
For the measured concentrations, values below the limit of detection were assigned one-quarter of the lower limit of quantification, while values above the limit of detection but lower than the lower limit of quantification were assigned half of the lower limit of quantification for both univariate and multivariate statistical tests. Statistics analyses were conducted using GraphPad Prism version 9.3.1 (GraphPad software, San Diego, CA, USA) and SAS/STAT of the SAS system for Windows, version 9.04. Two-tailed P < 0.05 was considered statistically significant.
Results
A total of 151 patients with a history of acute glyphosate poisoning were eligible for inclusion in the glyphosate cohort between October 2010 and January 2013 (). More than 600 plasma samples were analyzed for glyphosate, aminomethylphosphonic acid, and POEA surfactants in 2020, allowing clinical toxicology and population toxicokinetic studies (to be addressed in a further paper) to be performed. Sixty-seven patients were enrolled in the glyphosate renal biomarker cohort.
Baseline demographic and clinical characteristics
Out of the 151 patients, 5% remained asymptomatic during hospital stay (). More than half of the patients (88/151; 58%) experienced minor symptoms, and one-third of the patients (51/151; 34%) exhibited signs of moderate to severe poisoning following ingestion. There were four fatal cases, all male patients, giving a case fatality of 2.7% (Clopper-Pearson 95% CI: 0.7%–6.6%). Additional information regarding the clinical characteristics and the correlation of the estimated volume ingested with the measured concentrations of glyphosate, aminomethylphosphonic acid and POEA surfactants is provided in the Supplementary Material, including Supplementary Figure S1.
Table 1. Baseline demographics and clinical features for 151 patients with glyphosate poisoning grouped by clinical stage.
Association of plasma concentrations of glyphosate, aminomethylphosphonic acid, and polyethoxylated tallow amine surfactants with severity of poisoning
A significant association was observed between the clinical severity and the admission plasma concentrations of glyphosate and peak concentrations of aminomethylphosphonic acid. Specifically, median (interquartile range [IQR]) concentrations of glyphosate were 0.5 mg/L (IQR 0.1–1.1 mg/L), 3.3 mg/L (IQR 1.0–11.0 mg/L), 21.1 mg/L (IQR 3.1–88.4 mg/L) and 734.0 mg/L (IQR 448.2–1,083 mg/L) for asymptomatic, minor, moderate to severe poisoning and fatal cases, respectively (). Meanwhile, median aminomethylphosphonic acid concentrations were less than the lower limit of quantification, 0.1 mg/L (IQR 0.1–0.5 mg/L), 0.3 mg/L (IQR 0.2–1.1 mg/L) and 2.4 mg/L (IQR 2.0–4.5 mg/L) for these respective patient groups. Glyphosate and aminomethylphosphonic acid concentrations correlated with increasing clinical severity (). Polyethoxylated tallow amine surfactants were not detectable in asymptomatic patients and were only detected in approximately one-fifth of symptomatic patients (30/143, 21%), including only one of the four fatal cases (). Although the one fatal case had the highest concentrations of all the five POE (15) tallow amines, the concentrations of POEA did not exhibit significant differences across the groups ().
Figure 2. Association of glyphosate and aminomethylphosphonic acid concentration with severity of poisoning. The scatter plots on top illustrate admission glyphosate concentrations (A) and peak aminomethylphosphonic acid concentrations (B) in glyphosate poisoned patients grouped by clinical stage (bars represent median and interquartile ranges). The scatter plots below show admission glyphosate concentrations (C) and peak aminomethylphosphonic acid concentrations (D) relative to the time post ingestion and severity of poisoning. Symbols representing different severity of poisoning groups are as follows: black – asymptomatic patients, yellow – minor poisoning patients, blue – moderate to severe poisoning patients, red – fatal patients.
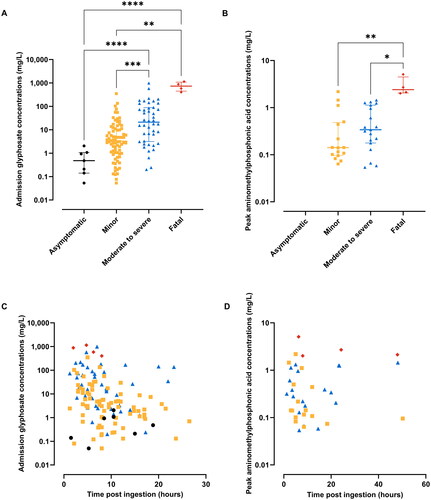
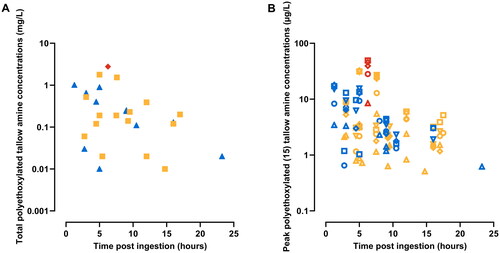
Figure 3. Association of concentrations of polyethoxylated tallow amine (POEA) surfactants with severity of poisoning. Scatter plots showing the estimated total POEA concentrations (A) and peak plasma concentrations of five POE (15) tallow amines (B) relative to the time post ingestion and severity of poisoning in glyphosate poisoned patients. Symbols representing different severity of poisoning groups and different POE (15) tallow amines are as follows: yellow – minor poisoning patients, blue – moderate to severe poisoning patients, red – fatal patients; open circle – C18u(EO)10, open triangle – C18s(EO)10, open square – C18u(EO)11, open reversed triangle – C18u(EO)12, open diamond – C18u(EO)14.
Baseline demographic and clinical characteristics for patients grouped by Kidney Disease: Improving Global Outcomes acute kidney injury criteria
Among the patients enrolled in the renal biomarker cohort (n = 67 of 151), approximately half (33/67, 49%) developed acute kidney injury, mostly mild acute kidney injury (20/33; 61%), while around 40% developed moderate acute kidney injury (2/33; 6%) to severe acute kidney injury (11/33; 33%) and three patients died (). The development of moderate to severe acute kidney injury (≥2) was significantly associated with higher concentrations of glyphosate and aminomethylphosphonic acid (). Polyethoxylated (15) tallow amines displayed higher median concentrations in patients with acute kidney injury ≥2, although the differences were not statistically significant. One exception was the significantly elevated median concentration of C18u(EO)11 in the acute kidney injury ≥2 groups compared to the mild acute kidney injury group.
Figure 4. Scatter plots showing admission glyphosate concentrations (a), peak aminomethylphosphonic acid concentrations (B) and total polyethoxylated tallow amine concentrations (C) in glyphosate poisoned patients grouped by Kidney Disease: Improving Global Outcomes acute kidney injury criteria. Bars represent median and interquartile ranges. Symbols representing different severity of poisoning groups are as follows: yellow – patients with no acute kidney injury (non-acute kidney injury), blue – patients with mild acute kidney injury (acute kidney injury 1), red – patients with moderate to severe acute kidney injury (acute kidney injury ≥2).
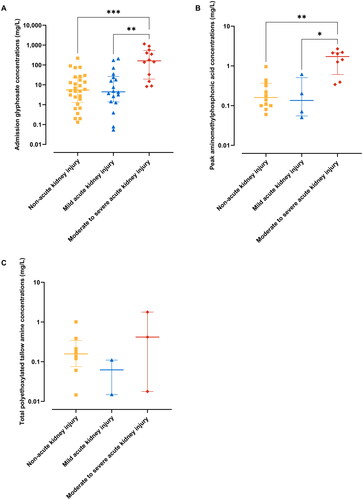
Table 2. Baseline demographics and clinical features for 67 glyphosate poisoned patients grouped by kidney disease: Improving global outcomes acute kidney injury criteria.
Prognostic factors predicting moderate to severe acute kidney injury (≥2) and death in glyphosate poisoning
As predictors of acute kidney injury ≥2, plasma concentrations of glyphosate displayed good performance with an AUC-ROC of 0.83 (95% CI 0.69–0.97), whereas plasma concentrations of aminomethylphosphonic acid displayed moderate performance with an AUC-ROC of 0.76 (95% CI 0.59–0.94) (, ). Both glyphosate and aminomethylphosphonic acid concentrations were excellent predictors (AUC-ROC >0.9) of case fatality with cut-off values of 376 mg/L and 1.7 mg/L, respectively. Polyethoxylated tallow amine surfactants with unsaturated (u) tallow moieties were good indicators for predicting acute kidney injury ≥2 (AUC-ROC ≥0.7), with C18u(EO)11 being the best predictor (AUC-ROC = 0.94; 95% CI 0.77–1.00).
Figure 5. Receiver operator characteristic curves demonstrating the diagnostic performances of demographic and clinical variables in predicting moderate to severe acute kidney injury (acute kidney injury ≥2) (A, C) and death (B). Polyethoxylated tallow amine surfactants were only detected in one fatal case and hence area under the receiver operating characteristic curves was not constructed.
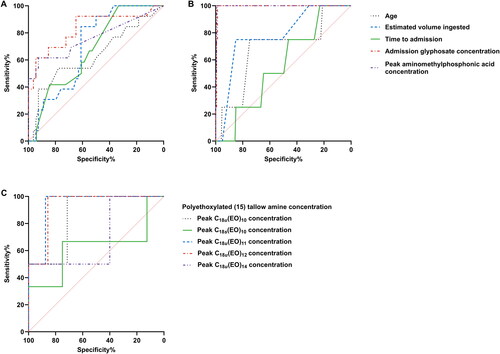
Table 3. Diagnostic profiles of demographic and clinical variables in predicting moderate to severe acute kidney injury (≥2) and death following glyphosate poisoning.
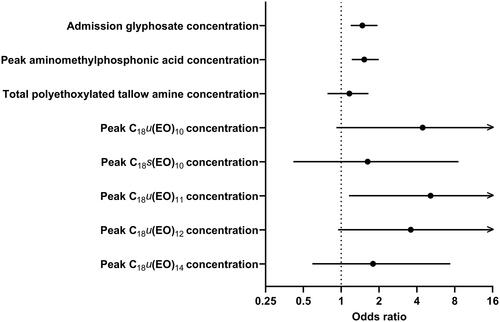
We observed that a doubling in aminomethylphosphonic acid concentrations was significantly associated with an approximately 53% increase in the risk of developing acute kidney injury ≥2 (OR 1.526; 95% CI 1.214–1.993); the corresponding risk estimates for glyphosate and total POEA were 1.472 (95% CI 1.189–1.942) and 1.160 (95% CI 0.7774–1.646), respectively (). Individual concentrations of POE (15) tallow amines showed stronger associations with the risk of developing acute kidney injury ≥2 compared to glyphosate, aminomethylphosphonic acid and total POEA concentrations. Additionally, a general trend was observed: as the number of repeating ethoxylate units in unsaturated tallow moieties decreased, the odds of acute kidney injury ≥2 increased. However, only C18u(EO)11 showed a statistically significant correlation, with a risk estimate of 5.147 for a doubling in its concentration (95% CI 1.153–176.4). The association of demographic and clinical variables with acute kidney injury ≥2 is provided in Supplementary Figure S2.
Figure 6. Univariate binary logistic regression analysis demonstrating the prognostic utility of glyphosate, aminomethylphosphonic acid, total polyethoxylated tallow amine (POEA) and five POE (15) tallow amines concentrations in predicting acute kidney injury ≥2 in glyphosate poisoning. All concentration data were transformed to log2 so that the risk estimates would represent a doubling of corresponding concentrations.
Because the low number of deaths caused a quasi-complete separation of the data when examining the effect of various categorical predictors on death, we used exact logistic regression to estimate the effect of categorical predictors and some continuous predictors on death, first each in a univariate analysis (Supplementary Table S2). Only GCS, admission glyphosate concentration, and co-ingestion of alcohol were significant univariate predictors of death. However, a multivariable analysis using these predictors would not converge due to the low numbers of deaths, and an analysis including only GCS and admission glyphosate concentration resulted in only the latter being a significant predictor of death (GCS was strongly correlated to admission concentration, R = −0.56).
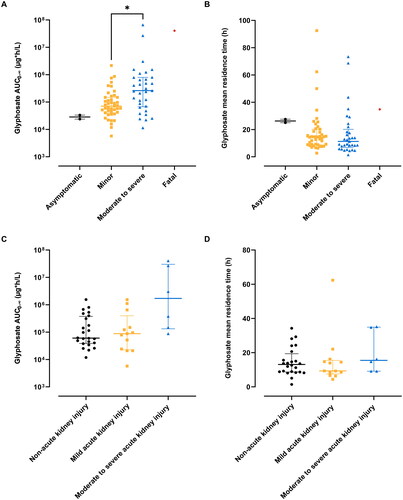
In addition to investigating the relationship between acute exposure and severity of poisoning, the association between glyphosate overall exposure metrics and the severity of poisoning is presented in . The total extent of glyphosate exposure was significantly higher in the moderate to severe poisoning group compared to the minor poisoning group. While the overall extent of glyphosate exposure was higher in the acute kidney injury ≥2 groups compared to the non-acute kidney injury and acute kidney injury 1 group, this variance did not reach statistical significance. No significant differences were observed in the average duration of exposure between the groups.
Figure 7. Association of glyphosate area under the curve (AUC) and mean residence time with severity of poisoning. The scatter plots on top illustrate glyphosate AUC0-∞ (A) and mean residence time (B) in glyphosate poisoning patients grouped by clinical stage. The scatter plots below show glyphosate AUC0-∞ (C) and mean residence time (D) in glyphosate poisoning patients grouped by Kidney Disease: Improving Global Outcomes acute kidney injury criteria. Bars represent median and interquartile ranges.
Discussion
A comprehensive investigation was undertaken in this study to assess the utility of glyphosate, aminomethylphosphonic acid, and POEA exposure in predicting acute kidney injury and mortality following acute glyphosate product self-poisoning. Glyphosate and aminomethylphosphonic acid concentrations exhibited moderate to excellent predictive performance for acute kidney injury ≥ 2 and mortality. The co-formulated POEA surfactants displayed even stronger predictive power for acute kidney injury ≥ 2, though detected in only a minority of patients. Further research involving larger sample sizes is required to fully elucidate the significance of POEA concentrations in predicting death.
In the current study, the case fatality was 2.7%, which is comparable to previous studies in Sri Lanka [Citation2, Citation12, Citation13, Citation19–21]. Acute kidney injury in the renal biomarker cohort was common (49%) but mostly mild, which is consistent with the findings of a previous study [Citation13]. Higher fatality rates are often reported in East Asia, including Taiwan, South Korea and Japan [Citation4, Citation10, Citation22–32]. Glyphosate formulations can vary across different brands, countries and over time, particularly in terms of glyphosate salts, concentrations and surfactant systems [Citation5, Citation33]. A recent retrospective study demonstrated that increased severity, including severe respiratory symptoms, was associated with POEA rather than other co-formulants [Citation34]. Ingestion of products containing glyphosate isopropylamine or ammonium salts and POEA surfactants was shown to cause severe acute lung injury and liver injury, while ingestion of products containing glyphosate potassium salt and non-POEA surfactants resulted in hyperkalaemia and potentially fatal ventricular arrhythmia or cardiac arrest [Citation24]. Therefore, variations in commercial formulations may contribute to the marked regional differences in case fatality observed in glyphosate poisoning.
Our analysis of plasma concentrations of glyphosate and aminomethylphosphonic acid is consistent with previous studies that have shown an association between high glyphosate concentrations and death [Citation12, Citation35]. However, the reported blood concentrations in previous studies were relatively higher in fatalities. For example, in a large prospective case series in Sri Lanka, non-survivors at the time of hospital presentation had a median glyphosate concentration of 1,373 mg/L (IQR 821–1,929 mg/L) [Citation12]. Furthermore, a previous study in France involving 13 cases of acute glyphosate intoxication reported median glyphosate and aminomethylphosphonic acid concentrations of 4,260 mg/L (IQR 1,335–6,850 mg/L) and 9.2 mg/L (IQR 1.7–18.1 mg/L), respectively, in fatal cases [Citation35]. Correspondingly, the optimal cut-off for plasma glyphosate concentration in predicting death was 734 mg/L in the previous study, which was about twice the concentration we reported. The utility of plasma concentrations of aminomethylphosphonic acid as predictors of severity of poisoning was only reported in one study where clinical severity was assessed using the Poisoning Severity Score (PSS) [Citation36]. The cut-off plasma concentrations for predicting high severity of poisoning (PSS ≥3) were 600 mg/L and 0.88 mg/L for glyphosate and aminomethylphosphonic acid, respectively [Citation36]. Besides investigating the relationship between acute exposure and the severity of poisoning, we attempted to relate the overall extent of exposure with the severity of poisoning. However, the relatively sparse clinical data prevented us from demonstrating statistically significant differences between groups, with the only significant difference being glyphosate AUC0-∞ between minor poisoning and moderate to severe poisoning groups. A population approach using model-based exposure metrics might be a better option for investigating this relationship.
Furthermore, we investigated the association between POEA exposure and the severity of poisoning. Plasma concentrations of POEA surfactants, differentiating between various homologues, were presented in relation to clinical staging and acute kidney injury staging in glyphosate-poisoned patients. We were able to identify one study that has described the blood concentrations of POEAs, which involved seven glyphosate-poisoned patients, three of whom ingested glyphosate isopropylamine and glufosinate ammonium as a mixed agent [Citation37]. However, that study only reported total concentrations of POEAs without distinguishing between various homologues. They found that patients with an initial blood concentration of POEAs higher than 8 mg/L exhibited severe symptoms, including decreased mean arterial pressure and respiratory failure; conversely, concentrations lower than 3.5 mg/L did not significantly affect the clinical presentations [Citation37]. In contrast, in our data, death occurred at a concentration of total POEA as low as 2.8 mg/L. The reasons for this discrepancy are likely multifaceted. Firstly, regional differences in ingested formulations may contribute to the variations in observed POEA concentrations in relation to clinical severity. Secondly, the co-ingestion of glufosinate is highly likely to affect the absorption of other compounds as well as the severity of poisoning in their cohort. Glufosinate co-ingestion could worsen poisoning, potentially due to its inhibition of glutamine synthetase and the associated toxicity from the anionic surfactant, sodium polyoxyethylene alkyl ether sulfate [Citation38–40]. Acute glufosinate poisoning commonly presents with gastrointestinal symptoms, followed by central nervous system toxicities, cardiovascular and respiratory symptoms [Citation38, Citation39]. Gastrointestinal absorption kinetics may be altered by the toxic effects of glufosinate, such as vomiting and diarrhea [Citation39], potentially affecting the absorption of other co-ingested compounds. Thirdly, there was uncertainty regarding the accuracy of their results due to the absence of both the blood sample preparation method and validation data. However, glyphosate and aminomethylphosphonic acid concentrations were not measured in their study, making it difficult to compare with our results and further investigate the reasons underlying the differences.
Based on our observation, all individual POEA surfactants demonstrated stronger associations with acute kidney injury ≥2 than glyphosate and aminomethylphosphonic acid. Furthermore, there was a trend indicating that individual POEA surfactants with unsaturated tallow moieties and fewer ethoxylate units served as good indicators for predicting acute kidney injury ≥2. This finding aligns with the inherent physicochemical properties of POEA surfactants, where the hydrophobicity of the molecule decreases with longer water-soluble ethoxylate chains, resulting in decreased toxicity due to less efficient interaction with cell membranes [Citation5]. However, the limited number of measurable POEA surfactants in our cohort prevented the use of more sophisticated statistical analyses, potentially affecting the level of significance [Citation41]. It is challenging to determine whether the undetected POEA surfactants were absent in the ingested products or present in low concentrations in the plasma samples due to the commercial confidentiality regarding the exact formulation, including the types and quantities of surfactants in different brands of glyphosate products. Nonetheless, a larger sample size is required to further examine the contribution of POEAs to the toxicity.
Our study has several limitations. Firstly, the plasma samples were 7–10 years old at the time of analysis. While there was no evidence to prove that the degradation did not occur, glyphosate appears to be stable according to previous research [Citation42], and the plasma samples were stored at −80 °C to minimize potential degradation. Secondly, the lack of standardized treatment guidelines for glyphosate-poisoned patients across different study hospitals in our cohort might impact the interpretation and comparability of outcomes within our study. Nevertheless, this variability is likely to be indicative of the real-world challenges faced in managing glyphosate poisoning in certain developing countries, giving an accurate reflection of clinical outcomes following such poisonings. Thirdly, acute kidney injury staging was only conducted for 67 patients in the renal biomarker cohort, while the remaining patients lacked serum creatinine concentrations, thus preventing acute kidney injury staging for them. This underscores the inherent limitations and complexities associated with conducting studies in uncontrolled settings, particularly in poisoning studies. The patients in our cohort were suicidal, sometimes unconscious, mentally ill, or otherwise unable to provide informed consent. Consequently, their eligibility for participation in the sub-studies was largely influenced by the acuteness and variability of their clinical conditions. Moreover, out of the 67 patients, only 13 (19%) developed acute kidney injury ≥2. Although this finding aligns with previous studies [Citation43], the relatively small sample size of patients who experienced moderate to severe acute kidney injury may limit the generalizability of our study findings to other cohorts with more pronounced renal dysfunction due to glyphosate poisoning. Additionally, our POEA assay does not cover all POEA homologues; instead, we measured the five most abundant ones, which represents about 17.6% of the total POEA. This approach allowed us to provide a reasonable estimate of the total POEA content, which was supported by liquid chromatography–mass spectrometry characterization [Citation8, Citation44, Citation45]. Assays for each type of surfactant used in various products were not available, and therefore, comparisons between different categories of surfactants, such as newer generation of ethoxylated etheramines and non-POEA surfactants and the glyphosate salt (potassium, isopropylamine and ammonium salt) in terms of their contributions to toxicity following glyphosate poisoning were also not feasible in our study.
Despite these limitations, our study supports concerns about an important role for POEA ingestion in the toxicity from glyphosate herbicide products. It is worth noting that ongoing concerns regarding the potential toxicity of POEA have prompted its phase-out in glyphosate products in Europe since 2017, while its use remains unrestricted in some other countries, such as the United States [Citation5, Citation46]. As POEA surfactants are still commonly employed in glyphosate-based herbicides globally, they remain a public health concern. Therefore, further studies are necessary to evaluate the POEA-related glyphosate toxicity to guide risk assessment.
The classification of glyphosate composition as confidential commercial information, and the regulatory position of considering pesticide co-formulants as inert result in the lack of official monitoring of these substances [Citation5]. Consequently, there has been limited exploration into assessing their exposure for predicting clinical outcomes in glyphosate-poisoned patients. Our investigation into the relationship between glyphosate and POEA exposure and toxicity in a large cohort of poisoned patients represents a promising start. Future studies with larger sample sizes are essential to thoroughly explore the contribution of POEA surfactants to toxicity, expanding upon our initial investigation. More research to understand the health implications of ingesting these substances will provide more scientific data, revealing their specific mechanisms and effects in humans. This understanding can guide improved management approaches, including raising awareness of potential POEA-related toxicity, implementing legislation requiring disclosure of pesticide product compositions, and introducing new generations of surfactants with more favorable toxicity profiles into pesticide formulations.
Conclusion
The present study offers a comprehensive investigation into the clinical utility of concentrations of glyphosate, its metabolite aminomethylphosphonic acid, and POEA surfactants in predicting moderate to severe acute kidney injury (≥2) and case fatality in acute glyphosate self-poisoning. There remains a need for future research with larger sample sizes to better understand POEA-related glyphosate toxicity and whether concentrations may be useful to guide the treatment of acute glyphosate poisoning.
Supplementary Figure S2.tiff
Download TIFF Image (1.6 MB)Glyphosate clinical paper_Supplementary_4Oct_24Mar.docx
Download MS Word (1.9 MB)Supplementary Figure S1.tiff
Download TIFF Image (2 MB)Supplementary Figure S3.tiff
Download TIFF Image (1.8 MB)Glyphosate clinical paper_Supplementary_27May.docx
Download MS Word (4.1 MB)Disclosure statement
The authors declare that no potential conflicts of interest are relevant to the contents of this manuscript.
Additional information
Funding
References
- World Health Organization and Food and Agriculture Organization of the United Nations. Preventing suicide: a resource for pesticide registrars and regulators. Geneva: WHO Press; 2019.
- Buckley NA, Fahim M, Raubenheimer J, et al. Case fatality of agricultural pesticides after self-poisoning in Sri Lanka: a prospective cohort study. Lancet Glob Health. 2021; Jun9(6):e854–e862. doi: 10.1016/S2214-109X(21)00086-3.
- Gummin DD, Mowry JB, Beuhler MC, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59(12):1282–1501. doi: 10.1080/15563650.2021.1989785.
- Lee CH, Shih CP, Hsu KH, et al. The early prognostic factors of glyphosate-surfactant intoxication. Am J Emerg Med. 2008;26(3):275–281. doi: 10.1016/j.ajem.2007.05.011.
- Mesnage R, Benbrook C, Antoniou MN. Insight into the confusion over surfactant co-formulants in glyphosate-based herbicides. Food Chem Toxicol. 2019;128:137–145. doi: 10.1016/j.fct.2019.03.053.
- Novotny E. Glyphosate, roundup and the failures of regulatory assessment. Toxics. 2022;10(6):321. doi: 10.3390/toxics10060321.
- European Food Safety Authority. Request for the evaluation of the toxicological assessment of the co‐formulant POE‐tallowamine. Efsa J. 2015;13(11):4303.
- Qiang S, Mohamed F, Mackenzie L, et al. Rapid determination of polyethoxylated tallow amine surfactants in human plasma by LC-MSMS. Talanta. 2023;254:124115. doi: 10.1016/j.talanta.2022.124115.
- Qiang S, Abdalla A, Mohamed F, et al. Acute human exposure to ingested glyphosate herbicide products: rapid analysis of glyphosate, its major metabolite and surfactant excipients in human plasma using HILIC- and RPLC-MSMS. [Manuscript in preparation for submission]. J Anal Toxicol. 2024.
- Chen YJ, Wu ML, Deng JF, et al. The epidemiology of glyphosate-surfactant herbicide poisoning in Taiwan, 1986-2007: a poison center study. Clin Toxicol (Phila). 2009;47(7):670–677. doi: 10.1080/15563650903140399.
- Bradberry SM, Proudfoot AT, Vale JA. Glyphosate poisoning. Toxicol Rev. 2004;23(3):159–167. doi: 10.2165/00139709-200423030-00003.
- Roberts DM, Buckley NA, Mohamed F, et al. A prospective observational study of the clinical toxicology of glyphosate-containing herbicides in adults with acute self-poisoning. Clin Toxicol (Phila). 2010;48(2):129–136. doi: 10.3109/15563650903476491.
- Mohamed F, Endre ZH, Pickering JW, et al. Mechanism-specific injury biomarkers predict nephrotoxicity early following glyphosate surfactant herbicide (GPSH) poisoning. Toxicol Lett. 2016;258:1–10. doi: 10.1016/j.toxlet.2016.06.001.
- Mohamed F, Buckley NA, Jayamanne S, et al. Kidney damage biomarkers detect acute kidney injury but only functional markers predict mortality after paraquat ingestion. Toxicol Lett. 2015;237(2):140–150. doi: 10.1016/j.toxlet.2015.06.008.
- Mohamed F, Endre Z, Jayamanne S, et al. Mechanisms underlying early rapid increases in creatinine in paraquat poisoning. PLoS One. 2015;10(3):e0122357. doi: 10.1371/journal.pone.0122357.
- Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138.
- Zhang Y, Huo M, Zhou J, et al. Solver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99(3):306–314. doi: 10.1016/j.cmpb.2010.01.007.
- Nahm FS. Receiver operating characteristic curve: overview and practical use for clinicians. Korean J Anesthesiol. 2022;75(1):25–36. doi: 10.4097/kja.21209.
- Carroll R, Metcalfe C, Gunnell D, et al. Diurnal variation in probability of death following self-poisoning in Sri Lanka–evidence for chronotoxicity in humans. Int J Epidemiol. 2012;41(6):1821–1828. doi: 10.1093/ije/dys191.
- Dawson AH, Eddleston M, Senarathna L, et al. Acute human lethal toxicity of agricultural pesticides: a prospective cohort study. PLoS Med. 2010;7(10):e1000357. doi: 10.1371/journal.pmed.1000357.
- van der Hoek W, Konradsen F. Analysis of 8000 hospital admissions for acute poisoning in a rural area of Sri Lanka. Clin Toxicol (Phila). 2006;44(3):225–231. doi: 10.1080/15563650600584246.
- Moon JM, Chun BJ, Cho YS, et al. Cardiovascular Effects and Fatality May Differ According to the Formulation of Glyphosate Salt Herbicide. Cardiovasc Toxicol. 2018;18(1):99–107. doi: 10.1007/s12012-017-9418-y.
- Cho YS, Moon JM, Chun BJ, et al. Use of qSOFA score in predicting the outcomes of patients with glyphosate surfactant herbicide poisoning immediately upon arrival at the emergency department. Shock. 2019;51(4):447–452. doi: 10.1097/SHK.0000000000001201.
- Kamijo Y, Takai M, Sakamoto T. A multicenter retrospective survey of poisoning after ingestion of herbicides containing glyphosate potassium salt or other glyphosate salts in Japan. Clin Toxicol (Phila). 2016;54(2):147–151. doi: 10.3109/15563650.2015.1121271.
- Kim YH, Lee JH, Hong CK, et al. Heart rate-corrected QT interval predicts mortality in glyphosate-surfactant herbicide-poisoned patients. Am J Emerg Med. 2014;32(3):203–207. doi: 10.1016/j.ajem.2013.09.025.
- Kim YH, Lee JH, Cho KW, et al. Prognostic Factors in Emergency Department Patients with Glyphosate Surfactant Intoxication: point-of-Care Lactate Testing. Basic Clin Pharmacol Toxicol. 2016;119(6):604–610. doi: 10.1111/bcpt.12624.
- Chen HH, Lin JL, Huang WH, et al. Spectrum of corrosive esophageal injury after intentional paraquat or glyphosate-surfactant herbicide ingestion. Int J Gen Med. 2013;6:677–683. doi: 10.2147/IJGM.S48273.
- Lee HL, Chen KW, Chi CH, et al. Clinical presentations and prognostic factors of a glyphosate-surfactant herbicide intoxication: a review of 131 cases. Acad Emerg Med. 2000;7(8):906–910. doi: 10.1111/j.1553-2712.2000.tb02069.x.
- Tominack RL, Yang GY, Tsai WJ, et al. Taiwan National Poison Center survey of glyphosate–surfactant herbicide ingestions. J Toxicol Clin Toxicol. 1991;29(1):91–109. doi: 10.3109/15563659109038601.
- Yang CC, Wu JF, Ong HC, et al. Taiwan National Poison Center: epidemiologic data 1985-1993. J Toxicol Clin Toxicol. 1996;34(6):651–663. doi: 10.3109/15563659609013825.
- Sawada Y, Nagai Y, Ueyama M, et al. Probable toxicity of surface-active agent in commercial herbicide containing glyphosate. Lancet. 1988;1(8580):299. doi: 10.1016/s0140-6736(88)90379-0.
- Talbot AR, Shiaw MH, Huang JS, et al. Acute poisoning with a glyphosate-surfactant herbicide ('Roundup’): a review of 93 cases. Hum Exp Toxicol. 1991;10(1):1–8. doi: 10.1177/096032719101000101.
- Mesnage R, Defarge N, Spiroux de Vendomois J, et al. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem Toxicol. 2015;84:133–153. doi: 10.1016/j.fct.2015.08.012.
- Langrand J, Blanc-Brisset I, Boucaud-Maitre D, et al. Increased severity associated with tallowamine in acute glyphosate poisoning. Clin Toxicol (Phila). 2020;58(3):201–203. doi: 10.1080/15563650.2019.1623406.
- Zouaoui K, Dulaurent S, Gaulier JM, et al. Determination of glyphosate and AMPA in blood and urine from humans: about 13 cases of acute intoxication. Forensic Sci Int. 2013;226(1-3):e20-5–e25. doi: 10.1016/j.forsciint.2012.12.010.
- Cellier M, Anthony N, Bruneau C, et al. Determination of Glyphosate and AMPA in Blood Can Predict the Severity of Acute Glyphosate Herbicide Poisoning. Lab Med. 2022;53(4):394–398. doi: 10.1093/labmed/lmac002.
- Park J, Kim SC, Jeon Y, et al. Serial blood concentration of polyethoxylated tallow amine and clinical presentations in acute herbicide poisoning. World J Emerg Med. 2022;13(4):305–308. doi: 10.5847/wjem.j.1920-8642.2022.061.
- Hsiao JT, Pan HY, Kung CT, et al. Assessment of glufosinate-containing herbicide exposure: a multi-center retrospective study. Am J Emerg Med. 2021;50:232–236. doi: 10.1016/j.ajem.2021.08.017.
- Mao YC, Hung DZ, Wu ML, et al. Acute human glufosinate-containing herbicide poisoning. Clin Toxicol (Phila). 2012;50(5):396–402. doi: 10.3109/15563650.2012.676646.
- Inoue Y, Onodera M, Fujita Y, et al. Factors associated with severe effects following acute glufosinate poisoning. Clin Toxicol (Phila). 2013;51(9):846–849. doi: 10.3109/15563650.2013.841180.
- Thiese MS, Ronna B, Ott U. P value interpretations and considerations. J Thorac Dis. 2016;8(9):E928–e931. doi: 10.21037/jtd.2016.08.16.
- Motojyuku M, Saito T, Akieda K, et al. Determination of glyphosate, glyphosate metabolites, and glufosinate in human serum by gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875(2):509–514. doi: 10.1016/j.jchromb.2008.10.003.
- Cho AY, Oh JH, Oh SS, et al. Clinical characteristics of acute kidney injury in patients with glyphosate surfactant herbicide poisoning. Kidney Res Clin Pract. 2023;42(3):349–357. doi: 10.23876/j.krcp.22.051.
- Corbera M, Simonet BM, Salvadó V, et al. Characterization of alkylamine ethoxylates (ANEOs) in commercial herbicide formulations using liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2010;24(20):2931–2937. doi: 10.1002/rcm.4698.
- Tush D, Loftin KA, Meyer MT. Characterization of polyoxyethylene tallow amine surfactants in technical mixtures and glyphosate formulations using ultra-high performance liquid chromatography and triple quadrupole mass spectrometry. J Chromatogr A. 2013;1319:80–87. doi: 10.1016/j.chroma.2013.10.032.
- Kudsk P, Mathiassen SK. Pesticide regulation in the European Union and the glyphosate controversy. Weed Sci. 2020;68(3):214–222. doi: 10.1017/wsc.2019.59.