To the Editor:
Introduction
Rivastigmine is a reversible acetyl- and butyryl-cholinesterase inhibitor used to ameliorate cognitive deficits associated with Alzheimer's disease Citation[1&2]. We report a case of intentional rivastigmine poisoning in which we obtained serial measurements of butyrylcholinesterase.
Case Report
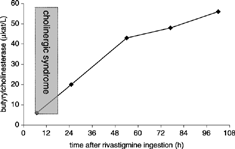
A 59-year-old man with a noncontributory medical history was found comatose and soiled after having urinated and defecated while unconscious. During transport to the Emergency Department (ED) he had two seizures that resolved spontaneously. On arrival, he was somnolent and he vomited. He had miosis, nystagmus, and excessive salivary and bronchial secretions. Breathing was shallow at a respiratory rate of 28 per minute between apneic pauses. Diffuse crackles were heard in his lungs. His tympanic temperature was 33.5°C, pulse 79 beats per minute, supine blood pressure 180/90 mmHg, and SpO2 76% on room air. The patient was intubated, suctioned, and ventilated. Atropine 3 mg intravenously cleared the bronchial secretions. Gastric lavage was performed and activated charcoal was given 1 hour after presentation. Gastric content had a normal smell. His initial laboratory test results were serum sodium 135 mmol/L, potassium 3.0 mmol/L, BUN 4.5 mmol/L, creatinine 65 mmol/L, glucose 12.9 mmol/L, and bicarbonate 26 mmol/L. Plasma butyrylcholinesterase was 6.0 µkat/L (normal 60–190 µkat/L). An electrocardiogram showed a sinus tachycardia and a brain CT scan revealed no pathological changes. Between 5 and 13 hours after presentation he had three periods of bradycardia (30 beats per minute), which were reversed with atropine 1 mg each time. Artificial ventilation was stopped 23 hours after presentation and he was extubated 3 hours later. After extubation his physical examination was unremarkable, lungs were clear, and cholinergic signs were absent. He reported that he had taken 48 tablets of Exelon® (288 mg of rivastigmine) and 28 tablets of Cipramil® (280 mg of citalopram) in a suicide attempt 5 hours before he was found. The drugs belonged to his mother who has Alzheimer's disease. A toxicological screening of gastric content by gas chromatography–mass spectrometry (incapable of detecting rivastigmine) was positive only for citalopram. During the rest of his hospitalization he felt dizziness and fatigue, but his physical examination was otherwise unremarkable. Plasma butyrylcholinesterase gradually returned to normal values in 5 days (). Red blood cell acetylcholinesterase was not measured. The patient was discharged after psychiatric evaluation on the fifth day.
Discussion
Rivastigmine inhibits cholinesterase enzymes at peripheral muscarinic and nicotinic receptors, and cholinergic receptors in a central nervous system Citation[1]. In our patient, we documented low activity levels of butyrylcholinesterase on presentation. Butyrylcholinesterase activity levels can be useful for confirmating cholinergic syndromes, but decreased activity levels are not specific for medical preparations such as rivastigmine.
The depressed level of consciousness and seizures in our patient could have been due to either the citalopram or the rivastigmine Citation[1], Citation[3]. Rivastigmine inhibition of cholinesterase at peripheral muscarinic receptors resulted in bradycardia and bronchosecretion. The last period of bradycardia appeared approximately 18 hours after ingestion. This is surprising since rivastigmine should be rapidly absorbed and rapidly metabolized with acetylcholinesterase activity returning to baseline levels about 9 hours after the maximum inhibitory effect was achieved Citation[1]. It is possible that rivastigmine activity and its plasma half-life are prolonged in overdose due to enzyme saturation, but there are no pharmacological studies to confirm this hypothesis. Serial serum rivastigmine concentrations could provide confirmatory data, but we were not able to measure rivastigmine in serum samples. Butyrylcholinesterase activity did not correlate with clinical improvement.
Treatment of rivastigmine poisoning includes atropine and respiratory support. Oxime therapy is not necessary unless one is unable to exclude organophosphate poisoning Citation[4&5].
Conclusion
Patients presenting with a cholinergic syndrome may have ingested a carbamate type of medical preparation such as rivastigmine. The cholinergic syndrome caused by rivastigmine is of short duration, yet it can be life threatening. Reduced butyrylcholinesterase activity levels confirm poisoning with a cholinesterase inhibitor but are not diagnostic of rivastigmine ingestion. Treatment of rivastigmine poisoning includes atropine and supportive therapy.
References
- Exelon. Summary of Product Characteristic. www.emea.eu.int Accessed October 25, 2004.
- Rogawski M A. What is the rationale for new treatment strategies in Alzheimer's disease?CNS Spectr 2004; 9(7):6–12, [PUBMED], [INFOTRIEVE], [CSA]
- Kelly C A, Dhaun N, Laing W J, Strachan F E, Good A M, Bateman D N. Comparative toxicity of citalopram and the newer antidepressants after overdose. J Toxicol, Clin Toxicol 2004; 42(1):67–71, [CROSSREF], [CSA]
- Ragoucy-Sengler C, Tracqui A, Chavonnet A, Daijardin J B, Simonetti M, Kintz P, Pileire B. Aldicarb poisoning. Hum Exp Toxicol 2000; 19(12):657–662, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lifshitz M, Rotenberg M, Sofer S, Tamir T, Shahak E, Almog S. Carbamate poisoning and oxime treatment in children: a clinical and laboratory study. Pediatrics 1994; 94:652–655, [CSA]