Abstract
A review of national poison center data from 1990 through 2003 showed approximately 10,000 annual ingestion exposures to camphor-containing products. A guideline that determines the threshold dose for emergency department referral and need for pre-hospital decontamination could potentially avoid unnecessary emergency department visits, reduce health care costs, optimize patient outcome, and reduce life disruption for patients and caregivers. An evidence-based expert consensus process was used to create the guideline. Relevant articles were abstracted by a trained physician researcher. The first draft of the guideline was created by the primary author. The entire panel discussed and refined the guideline before distribution to secondary reviewers for comment. The panel then made changes based on the secondary review comments. The objective of this guideline is to assist poison center personnel in the appropriate out-of-hospital triage and initial management of patients with suspected exposures to camphor-containing products by 1) describing the manner in which an exposure to camphor might be managed, 2) identifying the key decision elements in managing cases of camphor exposure, 3) providing clear and practical recommendations that reflect the current state of knowledge, and 4) identifying needs for research. This guideline applies to camphor exposure alone. Co-ingestion of additional substances, such as in commercial products of camphor combined with other ingredients, could require different referral and management recommendations depending on the combined toxicities of the substances. This guideline is based on an assessment of current scientific and clinical information. The expert consensus panel recognizes that specific patient care decisions may be at variance with this guideline, and are the prerogative of the patient and the health professionals providing care, considering all of the circumstances involved. This guideline does not substitute for clinical judgment. Recommendations are in chronological order of likely clinical use. The grade of recommendation is in parentheses. 1) Patients with stated or suspected self-harm or who are the recipients of malicious administration of a camphor-containing product should be referred to an emergency department immediately, regardless of the amount ingested (Grade D). 2) Patients who have ingested more than 30 mg/kg of a camphor-containing product or who are exhibiting symptoms of moderate to severe toxicity (e.g., convulsions, lethargy, ataxia, severe nausea and vomiting) by any route of exposure should be referred to an emergency department for observation and treatment (Grade D). 3) Patients exhibiting convulsions following a camphor exposure should be transported to an emergency department by pre-hospital emergency medical care providers (Grade D). A benzodiazepine should be used to control convulsions (Grade C). 4) Patients who have been exposed to a camphor product and who remain asymptomatic after 4 hours can be safely observed at home (Grade C). 5) Induction of emesis with ipecac syrup should not be performed in patients who have ingested camphor products (Grade C). 6) Activated charcoal administration should not be used for the ingestion of camphor products. However, it could be considered if there are other ingredients in the product that are effectively adsorbed by activated charcoal or if other substances have been co-ingested. (Grade C). 7) For asymptomatic patients with topical exposures to camphor products, the skin should be thoroughly washed with soap and water and the patient can be observed at home for development of symptoms (Grade C). 8) For patients with topical splash exposures of camphor to the eye(s), the eye(s) should be irrigated in accordance with usual poison center procedures and that referral take place based on the presence and severity of symptoms (Grade D). 9) Patients with camphor inhalation exposures should be moved to a fresh air environment and referred for medical care based on the presence and severity of symptoms. It is unlikely that symptoms will progress once the patient is removed from the exposure environment (Grade D).
INTRODUCTION
Camphor has been used historically as an aphrodisiac, contraceptive, abortifacient, analeptic, lactation suppressant, cardiac and central nervous system stimulant, cold remedy, muscle and joint liniment, substance of abuse, and rodent repellant (Citation1). It was first produced by the distillation of the bark of the Cinnamomum camphora tree, but today is produced synthetically from turpentine. It has a characteristic, penetrating odor and a pungent, aromatic taste (Citation2). The most common products involved in case reports of toxicity have been camphorated oil (20% camphor in cottonseed oil) and camphor spirits (10% camphor in alcohol or isopropyl alcohol). Following a large number of accidental ingestions by people mistaking camphorated oil for castor oil and through the dedicated efforts of pharmacist Carmine Varano (Citation3–5), the Food and Drug Administration ruled in 1983 that camphorated oil could no longer be sold over-the-counter (OTC). As a result of this ruling and improvements in the medical management of patients poisoned with camphor, fatal poisoning cases are now rare. Currently, OTC camphor-containing products cannot exceed 11% camphor. However, cases of severe toxicity and convulsions are still reported to occur even with the lower concentration products that remain on the market. In addition to pharmaceutical products containing camphor, there are imported, non-FDA approved, ethnic remedies sold in the US that contain camphor in amounts greater than the 11% limit set by the FDA.
Camphor Poisoning
The mechanism by which camphor produces toxicity is unknown. Absorption from the gastrointestinal tract occurs rapidly with detectable serum concentrations found within minutes after ingestion (Citation6, Citation7). Within 5–15 minutes, patients commonly complain of mucus membrane irritation, nausea, vomiting, and abdominal pain. Generalized tonic-clonic convulsions are often the first sign of significant toxicity and can occur soon after ingestion (Citation8–11). Central nervous system depression is commonly seen, as are headache, dizziness, confusion, agitation, anxiety, hallucinations, myoclonus, and hyperreflexia (Citation6, Citation7Citation12–18). Death is usually the result of respiratory failure or convulsions (Citation6, Citation12, Citation19, Citation20).
Even when applied to the skin in large quantities, camphor has only rarely been reported to cause systemic poisoning resembling the effects seen with acute ingestion exposures (Citation11, Citation21, Citation22).
Chronic oral administration of camphor has been reported to cause death (Citation23), while chronic, low-dose, dermal exposure over many years was reported to cause granulomatous hepatitis in one case (Citation24).
Camphor appears to readily cross the placenta. Fetal deaths, as well as uneventful deliveries, have been reported following camphor poisoning in the mother during the latter stages of pregnancy (Citation7, Citation25, Citation26).
Camphor-containing Products
Camphor is found in a variety of non-prescription products, either alone or in combination with other ingredients. It can also be purchased, particularly in shops providing alternative medications, as solid blocks of pure camphor. provides a partial list of common camphor-containing products sold in the US. Pharmaceutical products currently on the market in the US have camphor concentrations ranging from 0.5% to 10.8%.
TABLE 1 Selected camphor-containing products (Citation75, Citation76)
Definition of Terms
Acute exposure is defined as a single exposure or multiple exposures occurring within a period of 8 hours. Chronic exposure is defined as multiple exposures occurring over a period of greater than 8 hours (Citation27). The term out-of-hospital is defined as the period before a patient reaches a healthcare facility. A child is defined as a person less than 6 years of age.
When the mg/kg dose or a child's weight was not included in an article, the mg/kg dose was estimated by the use of pediatric growth charts (Citation28). The 95th percentile weight was used for a particular age and sex. When the sex of the child was not stated, the weight for boys was used. This approach errs on the side of estimating a lower mg/kg dose. Estimated mg/kg doses are italicized whenever they are presented.
Intended Users of the Guideline
The intended users of this guideline are personnel in US poison centers. This guideline has been developed for the conditions prevalent in the US. While the toxicity of camphor is not expected to vary in a clinically significant manner in other nations, the available forms of camphor and the out-of-hospital conditions could be much different. This guideline should not be extrapolated to other settings unless it has been determined that the conditions assumed in this guideline are present.
Objective of the Guideline
The objective of this guideline is to assist poison center personnel in the appropriate out-of-hospital triage and initial management of patients with suspected exposures to camphor-containing products by 1) describing the manner in which an exposure to camphor might be managed, 2) identifying the key decision elements in managing cases of camphor exposure, 3) providing clear and practical recommendations that reflect the current state of knowledge, and 4) identifying needs for research. This guideline applies to exposure to camphor alone. Co-ingestion of additional substances, such as in commercial products of camphor combined with other ingredients, could require different referral and management recommendations depending on the combined toxicities of the substances.
This guideline is based on an assessment of current scientific and clinical information. The expert consensus panel recognizes that specific patient care decisions may be at variance with this guideline and are the prerogative of the patient and the health professionals providing care, considering all of the circumstances involved. This guideline does not substitute for clinical judgment.
METHODOLOGY
The methodology used for the preparation of this guideline was developed after reviewing the key elements of practice guidelines (Citation29, Citation30). An expert consensus panel was established to develop the guideline (Appendix 1). The American Association of Poison Control Centers (AAPCC), the American Academy of Clinical Toxicology (AACT), and the American College of Medical Toxicology (ACMT) appointed members of their organizations to serve as panel members. To serve on the expert consensus panel, an individual had to have an exceptional record in clinical care and scientific research in toxicology, board certification as a clinical or medical toxicologist, significant US poison center experience, and be an opinion leader with broad esteem. Two specialists in poison information were included as full panel members to provide the viewpoint of the end-users of the guideline.
Literature Search
The National Library of Medicine's PubMed database was searched (to November 2004) using camphor (poisoning) or camphor (toxicity) or camphor (adverse effects) as MeSH terms, all limited to humans. PubMed was also searched using camphor as a textword (title, abstract, MeSH term, CAS number), plus either poison* or overdos* or tox* or intox*, limited to humans. The latter process was repeated in International Pharmaceutical Abstracts (1970–November 2004, excluding abstracts of meeting presentations), Science Citation Index (1977–November 2004), Database of Abstracts of Reviews of Effects (accessed November 2004), Cochrane Database of Systematic Reviews (accessed November 2004), and Cochrane Central Register of Controlled Trials (accessed November 2004). Reactions (1980–November 2004), the camphor poisoning management in Poisindex (Citation31), and the bibliographies of recovered articles were reviewed to identify previously undiscovered articles. Furthermore, North American Congress of Clinical Toxicology abstracts published in the Journal of Toxicology-Clinical Toxicology (1995–2004) were reviewed for original human data. The camphor chapter bibliographies in five major toxicology textbooks were reviewed for citations of additional articles with original human data (Citation32–36). US poison control centers were invited to submit their current guidelines for the management of camphor exposures. Finally, The Toxic Exposure Surveillance System (TESS) maintained by the American Association of Poison Control Centers was searched for deaths resulting from camphor poisoning (Citation37). These cases were abstracted for use by the panel.
Article Selection
The recovered citations were entered into an EndNote library and duplicate entries were eliminated. The abstracts of the remaining articles were reviewed, looking specifically for those that dealt with estimations of mg/kg, or ingested doses with or without subsequent signs or symptoms, time to onset of symptoms, and management techniques that might be suitable for out-of-hospital use (e.g., gastrointestinal decontamination). Articles excluded were those that did not meet any of the preceding criteria, did not add new data (e.g., some reviews, editorials), or that exclusively described inpatient-only procedures (e.g., whole bowel irrigation).
Data Extraction
All articles that were retrieved from the search were reviewed by a single abstractor. Each article was assigned a level of evidence score from 1 to 6 using a rating scheme based on that of the Centre for Evidence-based Medicine at Oxford University (Appendix 2). Single case reports were classified along with case series as level 4. The complete paper was then reviewed for original human data regarding the toxic effects of camphor or original human data directly relevant to the out-of-hospital management of patients with camphor poisoning. Relevant data (e.g., dose of camphor, resultant effects, time to onset of effects, therapeutic interventions or decontamination measures given, efficacy or results of any interventions, and overall patient outcome) were compiled into a table and a brief summary description of each article was written. This full evidence table is available at http://www.aapcc.org/DiscGuidelines/Guidelines%20Tables/Camphor%20evidence%20table.pdf. The completed table of all abstracted articles was then forwarded to the panel members for review and consideration in developing the guideline. Every attempt was made to locate significant foreign language articles and have their crucial information extracted, translated, and tabulated. Copies of all of the articles were made available for reading by the panel members on a secure AAPCC website.
Guideline Writing and Review
A guideline draft was prepared by the primary author (listed first). The draft was submitted to the expert consensus panel for comment. Using a modified Delphi process, comments from the expert consensus panel members were collected, copied into a table of comments, and submitted to the primary author for response. The primary author responded to each comment in the table and, when appropriate, the guideline draft was modified to incorporate changes suggested by the panel. The revised guideline draft was again reviewed by the panel and, if there was no strong objection by any panelist to any of the changes made by the primary author, the draft was prepared for the external review process. External review of the second draft was conducted by distributing it electronically to AAPCC, AACT, ACMT members, and the secondary review panel. The secondary review panel consisted of representatives from the federal government, public health, emergency services, pediatrics, pharmacy practice, and consumer organizations (Appendix 3). Comments were submitted via a discussion thread on the AAPCC web site or privately through e-mail communication to AAPCC staff. All submitted comments were stripped of any information that would identify their sources, copied into a table of comments, and reviewed by the expert consensus panel and the primary author. The primary author responded to each comment in the table, and his responses and subsequent changes in the guideline were reviewed and accepted by the panel. Following a meeting of the expert consensus panel, the final revision of the guideline was prepared.
EVALUATION OF EVIDENCE ON THE TOXIC DOSE OF CAMPHOR
Limitations
While there were some published data suggesting a general toxic threshold dose range for camphor, the data suffered from a number of limitations, including the following:
Understandably, there were no prospective studies that specifically investigated the toxic dose threshold for camphor.
The published data existing for camphor are almost entirely case reports and suffer from all of the usual problems attributed to case report data, including incomplete patient information and unconfirmed exposure to the toxin.
The bulk of the literature on camphor was published prior to 1970. Many of the case reports are from the early part of the 20th century and do not provide the same level of information that is considered standard for case reports published in the recent literature.
Only a small number of articles contained any toxic threshold or dose-effect information.
Even when such information was presented in an article, there were often questions regarding the accuracy of the dose estimate due to inherent uncertainties in the history, differences in camphor content between products, and sometimes uncertainty with the report itself (e.g., confusion between the total product ingested or total camphor ingested, use of ambiguous or outdated language).
Dose-effect information was often confounded by the presence of co-exposures, differences in decontamination or treatment measures, and concurrent medical conditions that could have altered the clinical presentation or outcome.
It was difficult, if not impossible, to account for inter-individual differences in age, weight, underlying health condition, or other factors that might affect camphor's toxicokinetics and toxicodynamics.
Among larger case series, many of the patients remained asymptomatic; doses and/or effects were typically reported as ranges, percentages, or means for the cases so that individual doses resulting in specific effects could not be determined.
In the few available prospective trials of therapeutic use of camphor, camphor was generally administered in small doses or by routes that are not expected to occur in the setting of a poisoning.
The basic premise upon which the recommendations are based is that all camphor products have similar bioavailability characteristics irrespective of the formulation and that the amount of camphor in a product is the primary determinant in the potential for toxicity. This is an assumption for which there are no data, for or against, in the literature. This might not be a valid assumption and formulation could be a factor in observed differences in toxicity.
Current Poison Control Center Practices
Following a request to all AAPCC member poison centers in early 2005, seven poison centers provided copies of guidelines on camphor. These are summarized in . There was no consensus among the poison centers that responded with referral thresholds ranging from 15 to 30 mg/kg. Seven other poison centers indicated that they did not have guidelines for camphor.
TABLE 2 Summary of poison center guidelines, March 2005
Review of TESS Mortality Data
A review of TESS data from 1990 through 2003 showed approximately 10,000 annual ingestion exposures to camphor-containing products. Annually, approximately 70–90 exposures progress to moderate outcomes and 10–15 exposures develop into severe outcomes (Citation38).
From 1990 to 2003 (Citation37), there were three fatal cases involving combination camphor-containing products. One was a 51-year-old who intentionally abused a camphor product that also contained methyl salicylate; the second was a 27-year-old patient who committed suicide; and the third was a 5-year-old, morbidly obese boy who was given a camphor and menthol cream mixed in tea for treatment of a viral syndrome. The contribution of camphor to the outcome in each case cannot be evaluated. There were no fatal reports from acute or chronic unintentional exposures to camphor products (Citation38).
Review of Textbooks
A review of camphor poisoning chapters in commonly used toxicology textbooks revealed variation in their recommendations. None of them made recommendations for doses that require referral to a healthcare facility. Two chapters stated that significant toxicity has not been reported at doses less than 30 mg/kg (Citation33, Citation36), two stated that the toxic dose in children is greater than 1 g (Citation34, Citation35), and one chapter provided no estimate of a toxic or referral dose (Citation32).
Poisindex, a computerized toxicology reference used by poison centers, suggested that all patients who have ingested more than 30 mg/kg of camphor be referred to a medical facility (Citation31).
Acute Camphor Ingestions in Patients Less Than 6 Years of Age
Camphor (solid)
In a case report from 1887, the ingestion of a bite of solid camphor (estimated by the author at 30 grains or 1950 mg of camphor–116 mg/kg) resulted in the death of a 34-month-old child. The child developed coma and convulsions at an unreported time after ingestion and died approximately 18 hours after ingestion (Citation19). In another case, a 4-year-old girl reportedly ingested an unknown number of camphor “rocks” that had been placed under her pillow. She developed vomiting, convulsions, coma, bradycardia, and mydriasis (Citation39).
Camphor Tincture (alcoholic solution of camphor)
In a case report from 1887 (level 4), a 2-year-old child died after ingesting a compounded solution of 15 grains (975 mg) of camphor dissolved in each dram (3.7 mL) of 60% alcohol. The author estimated that the child ingested approximately 15 grains (975 mg or 64 mg/kg) of camphor. Immediately after ingesting the solution, the child complained of a burning sensation in the throat. At 30 minutes after ingestion, the child lost consciousness and then developed convulsions. Death occurred at approximately 4 hours after ingestion (Citation40).
Campho-Phenique
Campho-Phenique is a 10.8% camphor product that also contains phenol 4.7%. There were two level 4 case reports of toxicity in patients less than 6 year of age after acute Campho-Phenique ingestions. In one case, the ingestion of 1–2 teaspoonfuls (equivalent to 0.5–1 g of camphor or 34–68 mg/kg) by a 2-year-old girl resulted in mild toxicity manifested as spontaneous emesis (Citation6). In the other case, ingestion of approximately 9.5 mL by a 2-year-old boy (estimated at 1050 mg or 68 mg/kg) resulted in severe toxicity with coma and convulsions. However, the product had also been previously applied to the child's canker sores (Citation41). As this is a combination product composed of camphor and phenol, the relative contribution of each ingredient to the toxic effects reported cannot be determined.
Camphorated Oil
There were a number of articles related to camphorated oil (20% solution of camphor in cottonseed oil) ingestion in patients less than 6 years of age. Among those with dose-effect information, were three level 4 case series (Citation8, Citation15, Citation42) and 14 individual cases reported in nine level 4 articles (Citation12, Citation20,Citation43–49). In several cases, ingestion of 5 mL of camphorated oil was associated with severe toxicity, including death, in children ranging from 3 weeks to 4 years of age (Citation15, Citation43,Citation45–47,Citation49). A 5-mL dose represents approximately 1000 mg camphor (50–225 mg/kg).
Vicks VapoRub Ointment
There were two level 4 articles reporting three cases with data on dose-toxicity relationships for Vicks VapoRub Ointment, a product containing 4.8% camphor (Citation6, Citation50). Between them, the lowest dose associated with severe toxicity was an ingestion of approximately 1 tablespoonful (equivalent to 0.7 g camphor–41 mg/kg) by a 3-year-old girl who developed vomiting, confusion, and convulsions (Citation6).
Unspecified Camphor Products
An abstract (level 6) briefly described a prospective study of 82 patients (ages not specified) who were reported to a poison center after ingesting Campho-Phenique, Vicks VapoRub, or Vicks VapoSteam during a 1-year period. The lowest dose resulting in convulsions was an ingestion of 124 mg/kg of camphor, but the exact product was not specified. Eight other patients in this study developed symptoms but the doses ingested were not reported (Citation51). There was a level 4 retrospective review describing 182 cases of camphor product exposure reported to two poison centers over a 3-year period. The ages of the patients and the exact products involved were not specified. In this review, all patients ingesting less than 2 mg/kg remained asymptomatic. Ingestion of as little as 5 mg/kg resulted in minor symptoms and ingestion of as little as 59 mg/kg resulted in major symptoms (Citation52).
Chronic Camphor Ingestions in Patients Less Than 6 Years of Age
There was a level 4 case report with information on a dose-toxicity relationship for chronic camphor exposures in patients less than 6 years of age. In this case, a 6-month-old boy died after being given dropperful quantities of camphor in a home remedy containing whiskey daily over the course of 5 months for a total dose of 24.5 g (3000 mg/kg). Postmortem examination revealed diffuse edema in the brain with neuronal degeneration and necrosis, as well as hepatomegaly with fatty infiltration of the liver and hepatocellular necrosis (Citation52).
Dermal or Inhalation Exposures in Patients Less Than 6 Years of Age
Camphor Spirits Dermal Exposure and Camphorated Vaporizer Solution Inhalation
A level 4 case report described a 15-month-old boy child who crawled through a puddle of spilled camphor spirits. He developed ataxia followed by generalized convulsions that persisted for 2 days despite phenobarbital administration. He had no further convulsions until 1 year later when he was exposed to a 4.8% camphorated vaporizer solution to relieve the symptoms of an acute upper respiratory illness. He developed a single, generalized major motor convulsion. He received phenobarbital, which was discontinued after 5 years with no further convulsions (Citation11).
Vicks VapoRub Ointment
There was a level 4 case report in which a malnourished 2-month-old girl developed serum transaminase elevations after application of the 4.8% camphor product to her chest and neck three times a day for 5 days (Citation53).
Camphorated Oil
A level 4 case report described a 25-month-old boy with an upper respiratory illness who developed delirium, visual hallucinations, and urinary incontinence after his chest was “soaked in” more than 1 ounce of camphorated oil for 80 hours. The product contained 6.4 g camphor per ounce (Citation21).
Unspecified Camphor Product
Joly et al. (Citation22) described a 9-month-old girl with a 20% body surface area scald burn who was treated with dermal application of a camphorated dressing (9.6 g camphor/100 g dressing) for 24 hours and developed severe toxicity, including convulsions (level 4). They estimated that she had been exposed to 15 g camphor.
Acute Camphor Ingestions in Patients 6 Years of Age or Older
Camphor (solid)
There were four cases of solid camphor ingestion reported in three articles (Citation17, Citation54, Citation55). Toxicity, characterized by hallucinations, agitation, anxiety and tachycardia occurred in two young adult men following the ingestion of 6–10 g (Citation17). A 16-year-old pregnant girl developed nausea, dizziness, cramping, and shortness of breath following the ingestion of 30 g of camphor dissolved in red wine as an abortifacient (Citation55). The lowest dose associated with severe toxicity with convulsions was 3 drams of camphor. The article did not specify the dram from which system of measurement (Citation54). If the dram was the avoirdupois dram (1.77185 g/dram) the dose was 5.2 g; if the dram was the apothecary dram (3.88793 g/dram), the dose was 12 g.
Campho-Phenique
Ingestion of 44 mL Campho-Phenique (4.75 g or 68 mg/kg of camphor) by a 20-year-old man resulted in severe toxicity with grand mal seizures (level 4) (Citation56).
Camphor Liniment
Camphor liniment was a 20% w/w camphor formulation in either cottonseed oil or arachis oil. Two level 4 articles had individual case data (Citation13, Citation57) that provided limited information on dose-toxicity relationships. In one case report, the author estimated that a 35-year-old man took 18–20 grains (1170–1300 mg) of camphor following the ingestion of “a good big teaspoonful” of camphor liniment, which resulted in delirium and convulsions (Citation57). In the other case, a patient was administered 45 mL of camphor liniment orally (approximately 9 g camphor). He developed an oral and esophageal burning sensation, followed by vomiting, convulsions, and coma. He made a full recovery (Citation13).
Camphorated Oil
Several articles described ingestions of camphorated oil by patients 6 years of age and older. Among those with dose-effect information was a level 4 retrospective review (Citation41), a brief level 4 composite case series (Citation8), and 23 individual cases reported in 16 level 4 articles (Citation7, Citation9, Citation10, Citation12, Citation14, Citation25, Citation26, Citation48,Citation58–65). The smallest dose of camphorated oil associated with toxicity was 0.5 teaspoonful (500 mg or 18.5 mg/kg), which caused mild toxicity in a 6-year-old boy with mumps (Citation48). The lowest dose associated with severe toxicity was 1–1.5 teaspoonful (1000–1500 mg), a dose that caused convulsions in a number of children aged 4–10 years (Citation9). The lowest dose causing toxicity in an adult was 12 mL, which resulted in convulsions in a 22-year-old man (Citation42).
Camphor Spirits
There was a level 4 case report of a 54-year-old woman who ingested an unknown amount, but no more than 200 mL, of camphor spirits and developed coma, respiratory depression, and convulsions (Citation18).
Unspecified Camphor Products and Patient Ages
A level 6 abstract briefly described 82 patients (ages not specified) who were reported to one poison center over a 1-year period after ingesting Campho-Phenique, Vicks VapoRub, or Vicks VapoSteam. The lowest dose resulting in convulsions was an ingestion of 124 mg/kg, but the product was not specified. Eight other patients in this study developed symptoms but their ingestion doses were also not reported (Citation51).
There was a single level 4 review of 182 cases of camphor exposure reported to two poison centers over a 3-year period. Patient ages and specific camphor products were not mentioned. In this review, all patients ingesting less than 2 mg/kg remained asymptomatic. Ingestion of as little as 5 mg/kg resulted in minor symptoms, and ingestion of as little as 59 mg/kg resulted in major symptoms (Citation52).
Chronic Camphor Exposures in Patients 6 Years of Age or Older
Camphor in Oil
A level 4 retrospective case series provided information on the dose-toxicity relationship with chronic administration of camphor in oil. In this study, 80 postpartum women were treated for breast engorgement with intramuscular injections of camphor dissolved in oil at a dosage of 3.0 grains (195 mg) the first day, followed by 1.5 grains (97 mg) each day for 3 days (total dose administered 486 mg). With this regimen, one patient developed nausea and vomiting (Citation66).
Dermal or Inhalation Exposures in Patients 6 Years of Age or Older
Camphor Fumes
Gronka et al. (Citation67) published a prospective survey (level 4) of workers at a camphor packaging facility who were exposed to air concentrations of camphor greater than 2 ppm (2 mg/m3). These concentrations produced mild to moderate symptoms described as eye, nose, and throat irritation. For reference, the National Institute for Occupational Safety and Health Recommended Exposure Limit Time Weighted Average (NIOSH REL TWA) is 2 mg/m3 (Citation68), the Occupational Safety and Health Administration Permissible Exposure Limit Time Weighted Average (OSHA PEL TWA) is 2 mg/m3, and the Immediately Dangerous to Life and Health (IDLH) level is 200 mg/m3 (Citation69).
Vicks Inhaler
A 48-year-old woman developed severe toxicity with convulsions after using a Vicks Inhaler (containing l-desoxyephedrine, menthol, camphor, and pine oil in unspecified amounts) every 30 minutes for approximately 8 hours (level 4). She had also ingested by way of nasal instillation approximately 3.75 mL of Vicks Va-Tro-Nol nasal drops (containing menthol, eucalyptol, camphor, ephedrine, and methyl salicylate in unspecified amounts) in the hour before developing symptoms (Citation16).
Vicks VapoRub Ointment
There was a level 6 abstract of a case report in which a 72-year-old woman developed granulomatous hepatitis following the repeated dermal application of five containers of Vicks VapoRub Ointment over a 5-year period. Following discontinuation of the product, she improved and the problem appeared to be resolving at the time of the report (Citation24).
Camphor/Menthol/Methyl Salicylate Patch
In a level 1b prospective volunteer study, 24 healthy adult male and female volunteers were randomly assigned to one of three dosage groups: 2, 4, or 8 Satogesic Medicated Adhesive Patches. Each patch contained 46.8 mg camphor, 37.44 mg menthol, and 74.88 mg methyl salicylate. Blood samples were drawn at regular intervals. The study found that at these doses and with this dosage form, dermal absorption of camphor was low (Citation70).
EVALUATION OF EVIDENCE FOR ONSET OF EFFECT
All articles reporting toxicity were searched for evidence documenting or estimating a time of onset of effect. The vast majority of articles reported times of presentation to healthcare facilities but not times of symptom onset, which might have occurred much earlier. Thus, in most cases, it was only possible to establish an upper limit for time to symptom onset. In the few reports with exact times of onset reported, mild or more localized and nonspecific symptoms might have been missed in the history or omitted by the authors. Onset after chronic exposures is not discussed since most chronic poisonings are likely to come to the attention of the poison center long after symptom onset.
Onset of Effects after Acute Ingestions
There did not appear to be any difference in onset of symptoms after ingestion between different camphor products. Therefore, all camphor products are considered together. The onset of symptoms was rapid in the vast majority of cases. In many cases, the first symptoms reflected direct gastrointestinal or mucous membrane irritation and occurred within 20 minutes (Citation6, Citation10, Citation13, Citation47, Citation64). There were numerous examples of severe symptoms, such as convulsions or coma, developing 30–60 minutes after ingestion, often without warning or prior effects (Citation12, Citation24, Citation41, Citation56, Citation58, Citation63, Citation71).
Occasionally, toxic effects did not arise until hours after ingestion. In one case report, an adult developed convulsions and coma 6 hours after ingesting camphorated oil. The authors did not state if any other symptoms were present prior to that time (Citation62). In another case report, a 4-year-old girl developed convulsions 9 hours after ingesting Vicks VapoRub Ointment. However, she had been found in bed lying in her vomitus at approximately 5 hours after the estimated time of ingestion; the time of onset of the first symptoms is unknown (Citation50). In a case series of 19 patients (level 4), the onset of effects occurred up to 125 minutes after ingestion (Citation15). A 3-week-old infant died suddenly and unpredictably of a cyanotic attack 43 hours after ingesting 1 teaspoonful of camphorated oil. However, this infant also had a concurrent respiratory illness and had appeared cyanotic and very ill within 1 hour after being given the camphorated oil (Citation45). These delayed cases are rare and in one 3-year, level 3b retrospective review of 182 camphor exposures, no patient developed symptoms more than 6 hours after ingestion (Citation52).
It should also be noted that many patients with toxicity continued to deteriorate after symptom onset, hospital presentation, and/or decontamination, usually over the course of several hours (Citation7, Citation12, Citation20, Citation40, Citation47, Citation49, Citation50). Most patients without complications recovered within 24 hours.
Onset of Systemic Effects after Dermal Exposures
There were no cases reviewed in which toxicity was documented to occur after the product had been adequately removed from the skin. In two case reports, dermal exposures led to symptom development after 24–48 hours of apparently continuous exposure (Citation11, Citation22).
EVALUATION OF EVIDENCE FOR OUT-OF-HOSPITAL TREATMENT
Gastrointestinal Decontamination
There were a number of different gastrointestinal decontamination measures used in case reports, including activated charcoal, ipecac syrup, gastric lavage, salt water, mustard water, milk, magnesium sulfate, castor oil, and various other emetics, cathartics, adsorptives, or evacuants. All of these measures had too little evidence to allow comment on their efficacy.
Dean et al. (Citation72) demonstrated in a rat model that activated charcoal in a ratio of 2:1 did not reduce the gastrointestinal absorption of camphor.
Anticonvulsants
There were a number of anecdotal reports describing temporal improvement in convulsions, agitation, or excessive neuromuscular activity in patients with camphor toxicity after the administration of benzodiazepines or barbiturates (Citation6, Citation7, Citation9, Citation14,Citation16–18,Citation22, Citation25, Citation26,Citation48–50,Citation60, Citation62, Citation63, Citation73, Citation74). There was a single report of some improvement after phenytoin administration (Citation62). In many of these reports, other treatment measures were given concurrently with the anticonvulsants and could have also had a beneficial effect. Improvement might also have coincided with the natural course of the toxicity. It is, therefore, difficult to derive a meaningful conclusion from these reports. In addition, there were reports of respiratory depression following sedative-hypnotic administration to patients with camphor toxicity (Citation49, Citation73).
CONCLUSIONS
The expert consensus panel chose to emphasize the importance of information that would be needed in order to make a triage decision for a patient exposed to a camphor-containing product. These variables include the patient's intent, the time of the exposure, the patient's symptoms or underlying medical condition(s), the dose and formulation of the specific product involved, and other co-exposures. As many camphor-containing products are combinations of multiple ingredients, the specialist must take each ingredient into consideration when evaluating a patient's exposure. The expert consensus panel agreed that in each case, the judgment of the specialist in poison information or the poison center medical director might override any specific recommendation from this guideline.
Patient Intent
The expert consensus panel concluded that all patients with suicidal intent or in whom a malicious exposure is suspected (e.g., child abuse or neglect) should be expeditiously transported to an emergency department. Patients without these characteristics are candidates for consideration of out-of-hospital management of their exposure.
Dose and Formulation Taken
The expert consensus panel concluded that the camphor content of the product appears to be a major factor in the toxicity of a specific formulation. Therefore, one of the first steps in assessing an exposure to a camphor-containing product should be to calculate the approximate quantity of camphor to which the victim was exposed. Severe toxicity with convulsions in adults was estimated to occur at approximately 34 mg/kg (Citation42). An estimated dose of 41 mg/kg is the lowest reported dose causing severe toxicity with convulsions in a child less than 6 years of age (Citation6). The evidence, however, provides little information upon which to establish the minimum toxic dose of camphor, as most reported cases were exposed to large quantities of camphor and only a few case reports included dosage information. In the only retrospective review, which reported 182 cases of camphor exposure reported to two poison centers over a 3-year period (Citation52), all patients ingesting less than 2 mg/kg remained asymptomatic while ingestion of as little as 5 mg/kg resulted in minor symptoms and ingestion of as little as 59 mg/kg resulted in major effects. From all of this evidence, the panel decided to set the dose for which referral to an emergency department should occur at 30 mg/kg. The following are examples of referral doses of common products for a 10-kg child using the 30 mg/kg dose:
Campho-Phenique (108 mg camphor/mL) 2.8 mL
Vicks VapoRub Ointment (48 mg camphor/cm3) 6.4 cm3
BenGay Ultra Strength Cream (40 mg camphor/cm3) 7.6 cm3
Time to Onset of Effects
The expert consensus panel concluded that the onset of symptoms is rapid following camphor ingestion, with most patients exhibiting symptoms within minutes of exposure. Convulsions could occur as early as 30 minutes following ingestion. The panel also concluded that patients who are asymptomatic at 4 hours are unlikely to develop toxicity.
Topical Dermal Exposures
As there were no cases of topical exposure to camphor that progressed following removal of the product from the skin, the expert consensus panel concluded that in asymptomatic cases of topical exposure, the skin should be thoroughly washed with soap and water and the patient observed at home for signs and symptoms of toxicity. Symptomatic patients should be referred to healthcare facilities as determined by the severity of their symptoms. Once the skin has been thoroughly washed it is unlikely that the symptoms will progress.
Topical Eye Exposures
There is no information published on the effects of topical eye exposures to camphor. The expert consensus panel concluded that patients with these exposures should have their eye(s) irrigated in accordance with usual poison center procedures and referred as symptoms require.
Inhalation Exposures
The expert consensus panel concluded that there is little information upon which to base a recommendation relative to inhalation exposures to camphor. The panel concluded that patients with inhalation exposures should be moved to a fresh air environment and referral to a healthcare facility should occur based on the presence and severity of symptoms.
Potential Out-of-Hospital Management
The expert consensus panel concluded that, in addition to standard supportive care normally provided by prehospital personnel, convulsions induced by a toxic dose of camphor should be managed with adequate doses of a benzodiazepine.
Gastrointestinal decontamination
As the risk of rapidly developing convulsions and coma is great following a camphor ingestion, the expert consensus panel concluded that emesis with ipecac syrup should not be performed. The panel concluded that there is no human evidence in support of or against the use of activated charcoal in camphor ingestions and an animal study showed no benefit (Citation72). Therefore, activated charcoal should not be used for camphor ingestions and should be considered only if other substances for which activated charcoal is indicated have also been ingested, either included with camphor in a product or co-ingested.
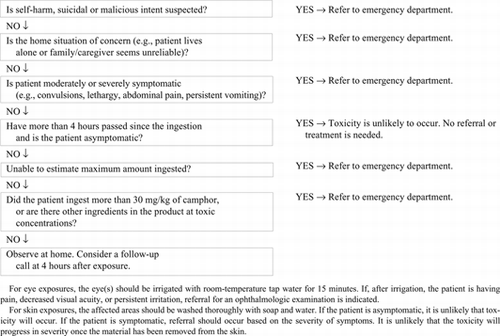
RECOMMENDATIONS
Patients with stated or suspected self-harm or who are the recipients of malicious administration of a camphor-containing product, should be referred to an emergency department immediately. This activity should be guided by local poison center procedures. In general, this should occur regardless of the amount ingested (Grade D).
Patients who have ingested more than 30 mg/kg of a camphor-containing product or who are exhibiting symptoms of moderate to severe toxicity (e.g., convulsions, lethargy, ataxia, severe nausea and vomiting) by any route of exposure should be referred to an emergency department for observation and treatment (Grade D).
Patients exhibiting convulsions following a camphor exposure should be transported to an emergency department by pre-hospital emergency medical care providers (Grade D). A benzodiazepine should be used to control convulsions (Grade C).
Patients who have been exposed to a camphor product and who remain asymptomatic after 4 hours can be safely observed at home (Grade C).
The induction of emesis with ipecac syrup should not be performed in patients who have ingested camphor products (Grade C).
Activated charcoal administration should not be used for the ingestion of camphor products. However, it could be considered if there are other ingredients in the product that are effectively adsorbed by activated charcoal or if other substances have been co-ingested. (Grade C).
For asymptomatic patients with topical exposures to camphor products, the skin should be thoroughly washed with soap and water and the patient can be observed at home for development of symptoms (Grade C).
For patients with topical splash exposures of camphor to the eye(s), the eye(s) should be irrigated in accordance with usual poison center procedures and that referral take place based on the presence and severity of symptoms (Grade D).
Patients with camphor inhalation exposures should be moved to a fresh air environment and referred for medical care based on the presence and severity of symptoms. It is unlikely that symptoms will progress once the patient is removed from the exposure environment (Grade D).
These recommendations are summarized in Appendix 4.
IMPLICATIONS FOR RESEARCH
The panel identified the following areas as needing additional research.
A prospective study of ingestions of camphor-containing products should be performed to attempt to identify the minimum dose at which toxicity may occur and to more definitively establish the appropriate referral dose.
Studies should be conducted to determine the effectiveness of prevention measures, such as public education, product labeling or product reformulation in reducing the toxicity of camphor-containing products.
Experience with the effects of camphor splash exposures to the eye should be published.
DISCLOSURES
There are no potential conflicts of interest reported by the expert consensus panel or project staff regarding this guideline.
REFERENCES
- Goldfrank LR, Flomenbaum NE, Lewin N, Howland MA, Hoffman RS, Nelson LS. Goldfrank's toxicologic emergencies 7th. McGraw-Hill, New York 2002
- Gennaro AR. Remington: the science and practice of pharmacy 21st. Lippincott Williams & Wilkins, Philadelphia 2005
- Varano C. Camphor poisoning. Drug Intell Clin Pharm 1977; 11: 110
- Varano C. Camphorated oil. JAMA 1979; 242: 240, [INFOTRIEVE], [CROSSREF]
- Varano C. Mistaken ingestion of camphorated oil for castor oil. Am J Hosp Pharm 1980; 37: 176, [INFOTRIEVE]
- Phelan WJ, 3rd. Camphor poisoning: over-the-counter dangers. Pediatrics 1976; 57: 428–431, [INFOTRIEVE]
- Riggs J, Hamilton R, Homel S, McCabe J. Camphorated oil intoxication in pregnancy; report of a case. Obstet Gynecol 1965; 25: 255–258, [INFOTRIEVE]
- Benz RW. Camphorated oil poisoning with no mortality. Report of twenty cases. J Am Med Assoc 1919; 72: 1217–1218
- Aronow R, Spigiel RW. Implications of camphor poisoning: therapeutic and administrative. Drug Intell Clin Pharm 1976; 10: 631–634, [INFOTRIEVE]
- Kopelman R, Miller S, Kelly R, Sunshine I. Camphor intoxication treated by resin hemoperfusion. JAMA 1979; 241: 727–728, [INFOTRIEVE], [CROSSREF]
- Skoglund RR, Ware LL, Jr., Schanberger JE. Prolonged seizures due to contact and inhalation exposure to camphor. A case report. Clin Pediatr (Phila) 1977; 16: 901–902
- Clark TL. Fatal case of camphor poisoning. Br Med J 1924; 1: 467
- Haft HH. Camphor liniment poisoning [letter]. J Am Med Assoc 1925; 84: 1571
- Klingensmith WR. Poisoning by camphor. J Am Med Assoc 1934; 102: 2182–2183
- Craig JO. Poisoning by the volatile oils in childhood. Arch Dis Child 1953; 28: 475–483, [INFOTRIEVE]
- Seife M, Leon JL. Camphor poisoning following ingestion of nose drops. J Am Med Assoc 1954; 155: 1059–1060, [INFOTRIEVE]
- Köppel C, Tenczer J, Schirop T, Ibe K. Camphor poisoning – abuse of camphor as a stimulant. Arch Toxicol 1982; 51: 101–106, [CROSSREF]
- Köppel C, Martens F, Schirop T, Ibe K. Hemoperfusion in acute camphor poisoning. Intensive Care Med 1988; 14: 431–433, [CROSSREF]
- Davies R. A fatal case of camphor poisoning [letter]. Br Med J 1887; 1: 726
- Barker F. A case of poisoning by camphorated oil [letter]. Br Med J 1910; 1: 921
- Summers GD. Case of camphor poisoning. Br Med J 1947; 2: 1009–1010
- Joly C, Bouillie C, Hummel M. Intoxication aiguë par le camphre administré par voie externe chez un nourrisson. Ann Pediatr (Paris) 1980; 27: 395–396
- Jimenez JF, Brown AL, Arnold WC, Byrne WJ. Chronic camphor ingestion mimicking Reye's syndrome. Gastroenterology 1983; 84: 394–398, [INFOTRIEVE]
- McCollam A, Block R, Lipscomb JW, Pompei P. Chronic camphor ingestion: a case report of granulomatous hepatitis [abstract]. Vet Hum Toxicol 1989; 31: 337
- Blackmon WP, Curry HB. Camphor poisoning: report of a case occurring during pregnancy. J Fla Med Assoc 1957; 43: 999–1000, [INFOTRIEVE]
- Weiss J, Catalano P. Camphorated oil intoxication during pregnancy. Pediatrics 1973; 52: 713–714, [INFOTRIEVE]
- AAPCC TESS manual 2002. http://www.aapcc.org/MEMBERS/TESS/tess1.htm
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Advance data from vital and health statistics; no. 314. Hyattsville(MD): National Center for Health Statistics, 2000
- Shaneyfelt TM, Mayo-Smith MF, Rothwangl J. Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer-reviewed medical literature. JAMA 1999; 281: 1900–1905, [PUBMED], [INFOTRIEVE], [CROSSREF]
- 30. Shiffman RN, Shekelle P, Overhage JM, Slutsky, J, Grimshaw J, Deshpande AM. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization”. Ann Intern Med 2003; 139:493–498
- Poisindex system, RK Klasco. Thomson Micromedex (Edition expires, Greenwood Village, (CO) 2005
- Sue Y-J, Pinkert H. Baby powder, borates, and camphor. Clinical management of poisoning and drug overdose 3rd, LM Haddad, MW Shannon, JF Winchester. Philadelphia, WB Saunders 1998; 1160–1168
- Anderson DL, Topliff AR. Camphor and mothballs. Clinical toxicology, MD Ford, KA Delaney, LJ Ling, T Erickson. Philadelphia, WB Saunders 2001; 339–342
- Kuffner EK. Camphor and moth repellants. Goldfrank's toxicologic emergencies 7th, LR Goldfrank, NE Flomenbaum, NA Lewin, MA Howland, RS Hoffman, LS Nelson. McGraw-Hill, New York 2002; 1295–1302
- Anderson IB. Camphor and other essential oils. Poisoning & drug overdose 4th, KR Olson. McGraw-Hill, New York 2004; 147–148
- Carracio TR, McGuigan MA. Over-the-counter products. Medical toxicology 3rd, RC Dart. Lippincott Williams & Wilkins, Philadelphia 2004; 1051–1062
- AAPCC TESS fatality abstracts 1985–2002
- AAPCC TESS reports. http://www.aapcc.org/annual.htm.
- Cristallo G, Tarallo MR, Vollono C, Tarallo D, Grandi E, Tarallo S. Un caso di avvelenamento da canfora. Pediatr Med Chir 1995; 17: 287–288, [INFOTRIEVE]
- Finley MJ. A fatal case of poisoning from camphor. Med Record January 29, 1887; 125–126
- Gibson DE, Moore GP, Pfaff JA. Camphor ingestion. Am J Emerg Med 1989; 7: 41–43, [INFOTRIEVE], [CROSSREF]
- Verhulst HC, Page LA, Crotty JJ. Communications from the National Clearinghouse for Poison Control Centers: Camphor. Amer J Dis Child 1961; 101: 536–537, [INFOTRIEVE]
- Tidcombe FS. Severe symptoms following the administration of a small teaspoonful of camphorated oil [letter]. Lancet 1897; 2: 660, [CROSSREF]
- Miller DGM. The toxicity of camphor (camphorated oil) [letter]. J Am Med Assoc 1914; 63: 579
- Haas SV. Death following ingestion of 1 dram of camphorated oil. Amer J Obstet 1916; 73: 1153
- Blair J. Camphorated oil poisoning. Ohio State Med J 1929; 25: 808–809
- Smith AG, Margolis G. Camphor poisoning; anatomical and pharmacologic study; report of a fatal case; experimental investigation of protective action of barbiturate. Am J Pathol 1954; 30: 857–869, [INFOTRIEVE]
- Jacobziner H, Raybin HW. Camphor poisoning. Arch Pediatr 1962; 79: 28–30, [INFOTRIEVE]
- Theis JG, Koren G. Camphorated oil: still endangering the lives of Canadian children. CMAJ 1995; 152: 1821–1824, [PUBMED], [INFOTRIEVE]
- Ruha AM, Graeme KA, Field A. Late seizure following ingestion of Vicks VapoRub. Acad Emerg Med 2003; 10: 691, [INFOTRIEVE], [CROSSREF]
- Alsop JA. Camphor toxicity from accidental ingestions [abstract]. Vet Hum Toxicol 1993; 35: 346
- Geller RJ, Spyker DA, Garrettson LK, Rogol AD. Camphor toxicity: development of a triage strategy. Vet Hum Toxicol 1984; 26(Suppl 2)8–10, [INFOTRIEVE]
- Aliye UC, Bishop WP, Sanders KD. Camphor hepatotoxicity. South Med J 2000; 93: 596–598
- Craig M. Case of camphor poisoning. Br Med J 1895; 2: 660–661
- Rabl W, Katzgraber F, Steinlechner M. Camphor ingestion for abortion (case report). Forensic Sci Int 1997; 89: 137–140, [INFOTRIEVE], [CROSSREF]
- Lahoud CA, March JA, Proctor DD. Campho-Phenique ingestion: an intentional overdose. South Med J 1997; 90: 647–648, [INFOTRIEVE]
- Hurd JL. Camphor poisoning. Medical Council Philadelphia 1914; 14: 76
- Cottrell J. Poisoning by camphorated oil. Br Med J 1931; 1: 96–97
- Ginn HE, Anderson KE, Mercier RK, Stevens TW, Matter BJ. Camphor intoxication treated by lipid dialysis. JAMA 1968; 203: 230–231, [INFOTRIEVE], [CROSSREF]
- Vasey RH, Karayannopoulos SJ. Camphorated oil. Br Med J 1972; 1: 112, [INFOTRIEVE]
- Trestrail JH, 3rd, Spartz ME. Camphorated and castor oil confusion and its toxic results. Clin Toxicol 1977; 11: 151–158, [INFOTRIEVE]
- Antman E, Jacob G, Volpe B, Finkel S, Savona M. Camphor overdosage. Therapeutic considerations. N Y State J Med 1978; 78: 896–897, [INFOTRIEVE]
- Reid FM. Accidental camphor ingestion [letter]. JACEP 1979; 8: 339–340, [INFOTRIEVE]
- Mascie-Taylor BH, Widdop B, Davison AM. Camphor intoxication treated by charcoal hæmoperfusion. Postgrad Med J 1981; 57: 725–726, [INFOTRIEVE]
- Bridge CK. Hazards of camphorated oil [letter]. CMAJ 1995; 153: 881, [PUBMED], [INFOTRIEVE]
- Philpott NW. Intramuscular injections of camphor in the treatment of engorgement of the breasts. Can Med Assoc J. 1929; 20: 494–495
- Gronka PA, Bobkoskie RL, Tomchick GJ, Rakow AB. Camphor exposures in a packaging plant. Am Ind Hyg Assoc J 1969; 30: 276–279, [INFOTRIEVE]
- National Institute for Occupational Safety and Health. NIOSH Pocket Guide to Chemical Hazards, 2005: http://www.cdc.gov/niosh/npg/npgd0096.html
- Occupational Safety and Health Administration Safety and Health Topics: Camphor: http://www.osha.gov/dts/chemicalsampling/data/CH_224600.html#exposure.
- Martin D, Valdez J, Boren J, Mayersohn M. Dermal absorption of camphor, menthol, and methyl salicylate in humans. J Clin Pharmacol 2004; 44: 1151–1157, [INFOTRIEVE], [CROSSREF]
- Moore S. Poisoning by linimentum camphorae. Br Med J 1898; 2: 717
- Dean BS, Burdick JD, Geotz CM, Bricker JD, Krenzelok EP. In vivo evaluation of the adsorptive capacity of activated charcoal for camphor. Vet Hum Toxicol 1992; 34: 297–300, [INFOTRIEVE]
- Gouin S, Patel H. Unusual cause of seizure. Pediatr Emerg Care 1996; 12: 298–300, [INFOTRIEVE]
- Emery DP, Corban JG. Camphor toxicity. J Paediatr Child Health 1999; 35: 105–106, [INFOTRIEVE], [CROSSREF]
- 75. Clinical pharmacology™, electronic drug Information medication management resource. Tampa (FL): Gold Standard Multimedia, 2004
- eFacts. Drug facts and comparisons. Wolters Kluwer Health, Chicago 2004; 10
APPENDIX 1 Expert Consensus Panel Members
Lisa L. Booze, Pharm.D.
Certified Specialist in Poison Information
Maryland Poison Center
University of Maryland School of Pharmacy
Baltimore, Maryland
E. Martin Caravati, M.D., M.P.H., F.A.C.M.T., F.A.C.E.P.
Professor of Surgery (Emergency Medicine)
University of Utah
Medical Director
Utah Poison Center
Salt Lake City, Utah
Gwenn Christianson, R.N., M.S.N.
Certified Specialist in Poison Information
Indiana Poison Center
Indianapolis, Indiana
Peter A. Chyka, Pharm.D., F.A.A.C.T., D.A.B.A.T.
Professor, Department of Pharmacy
University of Tennessee Health Science Center
Memphis, Tennessee
Daniel J. Cobaugh, Pharm.D., F.A.A.C.T., D.A.B.A.T.
Director of Research
ASHP Research and Education Foundation
Bethesda, Maryland
Former Associate Director, American Association of Poison Control Centers
Daniel C. Keyes, M.D., M.P.H., F.A.C.E.P., F.A.C.M.T.
Medical Director
Pine Bluff Chemical Demilitarization Facility
Associate Professor, Southwestern Toxicology Training Program
Dallas, Texas
Anthony S. Manoguerra, Pharm.D., D.A.B.A.T., F.A.A.C.T.
Professor of Clinical Pharmacy and Associate Dean
School of Pharmacy and Pharmaceutical Sciences
University of California San Diego
Former Director, California Poison Control System, San Diego Division
San Diego, California
Lewis S. Nelson, M.D., F.A.C.E.P., F.A.C.M.T.
Assistant Professor of Emergency Medicine
New York University School of Medicine
Associate Medical Director
New York City Poison Control Center
New York, New York
Kent R. Olson, M.D., F.A.C.E.P., F.A.A.C.T., F.A.C.M.T.
Medical Director
California Poison Control System, San Francisco Division
Clinical Professor of Medicine & Pharmacy
University of California, San Francisco
San Francisco, California
Elizabeth J. Scharman, Pharm.D., D.A.B.A.T., B.C.P.S., F.A.A.C.T.
Director, West Virginia Poison Center
Professor, West Virginia University School of Pharmacy
Department of Clinical Pharmacy
Charleston, West Virginia
Paul M. Wax, M.D., F.A.C.M.T.
Managing Director
Banner Poison Center
Professor of Clinical Emergency Medicine
University of Arizona School of Medicine
Phoenix, Arizona
Alan D. Woolf, M.D., M.P.H., F.A.C.M.T.
Director, Program in Environmental Medicine
Children's Hospital, Boston
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
APPENDIX 2 Grades of Recommendation and Levels of Evidence
APPENDIX 3 Secondary Review Panel Organizations
Ambulatory Pediatric Association
American Academy of Breastfeeding Medicine
American Academy of Emergency Medicine
American Academy of Pediatrics
American Association for Health Education
American College of Clinical Pharmacy
American College of Emergency Physicians
American College of Occupational and Environmental Medicine
American Pharmacists Association
American Public Health Association
American Society of Health-System Pharmacists
Association of Maternal and Child Health Programs
Association of Occupational and Environmental Clinics
Association of State and Territorial Health Officials
Canadian Association of Poison Control Centres
Centers for Disease Control and Prevention – National Center for Injury Prevention and Control
Consumer Federation of America
Consumer Product Safety Commission
Department of Transportation
Emergency Medical Services for Children
Emergency Nurses Association
Environmental Protection Agency
European Association of Poisons Control Centers and Clinical Toxicologists
Food and Drug Administration
National Association of Children's Hospitals and Related Institutions
National Association of Emergency Medical Services Physicians
National Association of Emergency Medical Technicians
National Association of School Nurses
National Association of State Emergency Medical Services Directors
National Safe Kids Campaign
Teratology Society
World Health Organization International Programme on Chemical Safety