Abstract
The objective of this guideline is to assist poison center personnel in the out-of-hospital triage and initial management of patients with suspected exposure to long-acting anticoagulant rodenticides (LAAR). An evidence-based expert consensus process was used to create this guideline. It is based on an assessment of current scientific and clinical information. The panel recognizes that specific patient care decisions may be at variance with this guideline and are the prerogative of the patient and health professionals providing care. The grade of recommendation is in parentheses. 1) Patients with exposure due to suspected self-harm, abuse, misuse, or potentially malicious administration should be referred to an emergency department immediately regardless of the doses reported (Grade D). 2) Patients with symptoms of LAAR poisoning (e.g., bleeding, bruising) should be referred immediately to an emergency department for evaluation regardless of the doses reported (Grade C). 3) Patients with chronic ingestion of LAAR should be referred immediately to an emergency department for evaluation of intent and potential coagulopathy (Grade B). 4) Patients taking anticoagulants therapeutically and who ingest any dose of a LAAR should have a baseline prothrombin time measured and then again at 48–72 hours after ingestion (Grade D). 5) Patients with unintentional ingestion of less than 1 mg of LAAR active ingredient can be safely observed at home without laboratory monitoring. This includes practically all unintentional ingestions in children less than 6 years of age (Grade C). 6) Pregnant patients with unintentional exposure to less than 1 mg of LAAR active ingredient should be evaluated by their obstetrician or primary care provider as an outpatient. Immediate referral to an ED or clinic is not required (Grade D). 7) Patients with unintentional ingestion of 1 mg or more of active ingredient and are asymptomatic should be evaluated for coagulopathy at 48–72 hours after exposure (Grade B). 8) Physicians' offices or outpatient clinics must be able to obtain coagulation study results in a timely manner, preferably in less than 24 hours, for patients who require outpatient monitoring (Grade D). 9) Gastrointestinal decontamination with ipecac syrup or gastric lavage is not recommended (Grade D). 10) Transportation to an emergency department should not be delayed for administration of activated charcoal (Grade D). 11) Patients with dermal exposures should be decontaminated by washing the skin with mild soap and water (Grade D). 12) The administration of vitamin K is not recommended prior to evaluation for coagulopathy (Grade D).
Introduction
Rodenticides often contain an anticoagulant as an active ingredient. Warfarin was the initial ingredient in these products, but rodents quickly developed resistance to its effects. Longer-acting, more potent anticoagulants were developed in the 1970s and the term “superwarfarins” was coined to describe them. Currently, most mouse and rat baits available to the public contain these long-acting anticoagulant rodenticides (LAARs).
Scope of the problem and importance of the guideline
During the 4-year period of 2000 to 2003, poison centers in the US reported over 16,000 human exposures per year, for a total of 65,891 exposures to LAARs. Of these exposures, 63,146 (96%) were unintentional and 58,710 (89%) involved children under 6 years of age. The route of exposure was primarily by ingestion (95%). The most frequent sites of exposure were the home (98%) and workplace or public area (1.3%). The overwhelming majority of exposures (99%) were acute in nature (Citation1). In adults, LAAR poisoning may be due to self-harm attempts (Citation2CitationCitation–4) and, rarely, Munchausen syndrome (Citation3) or occupational exposure (Citation5,Citation6). Childhood poisoning resulting from Munchausen-by-proxy or child abuse has been reported (Citation7).
An analysis of the American Association of Poison Control Centers' Toxic Exposure Surveillance System (TESS) database for ingestion of LAARs by all ages and with a known outcome revealed that unintentional ingestions were 39 times more likely than intentional ingestions to have no effect or minor effects (). Of all unintentional exposures (n = 63,146), only 0.2% developed a moderate or major effect. However, it should be noted that over 60% of exposures were not followed to a known outcome in this database (Citation1).
Table 1. Known outcome by reason for ingestion of LAAR for all ages reported to TESS 2000–2003
In 2003, 4,811 patients (29% of all exposure) were evaluated in healthcare facilities, and seven died according to TESS. All deaths were intentional exposures in adults. A review of the TESS database from 1985 to 2004 did not reveal any LAAR-related deaths in children less than 6 years of age. Only one unintentional adult death was reported during this 20-year review, but there was not enough clinical information provided to attribute causation to brodifacoum (W.A. Watson, Pharm.D., Associate Director, AAPCC, personal communication, January 2005).
Substances and definitions
This guideline is intended to address exposures to LAARs only. The compounds included in this classification are brodifacoum, bromadiolone, chlorophacinone, difenacoum, and diphacinone (Citation2). Guidance for exposures to other anticoagulants, such as warfarin, or other non-anticoagulant rodenticides (e.g., bromethalin, cholecalciferol) is not included in this document. The term “out-of-hospital” is defined as the period before a patient reaches a healthcare facility. An acute ingestion is defined as any number of ingestions that occur within a period of up to 8 hours.
Sources and physical properties
Currently, the majority of anticoagulant compounds in commercially available rodenticides are LAARs (superwarfarins). These compounds include the 4-hydroxycoumarin derivatives brodifacoum, bromadiolone, and difenacoum and the indandione derivatives diphacinone and chlorophacione. Residential and industrial formulations are available with active ingredient concentrations ranging from 0.005% to 99% (). Brodifacoum is the most common active ingredient in commercially available rodenticides in the US and is usually found in a 0.005% concentration. It is used in agriculture, urban settings, and households for rodent control as ready-to-use solid, granular, and pellet baits. Most of the recommendations in this guideline are based on exposure to brodifacoum.
Table 2. Long-acting anticoagulant rodenticides
Pharmacology and pharmacokinetics
Long-acting anticoagulant rodenticides are absorbed from the gastrointestinal tract. The bioavailability is unknown and may vary depending on the formulation (e.g., solid vs. liquid). Dermal absorption of a liquid preparation has been reported (Citation8), and smoking solid formulations have resulted in systemic anticoagulation (Citation9,Citation10). From animal studies, brodifacoum is known to be highly lipid soluble and to concentrate in the liver. It is about 100 times more potent than warfarin (Citation11CitationCitation13).
Serum brodifacoum half-life ranged from 16 to 34 days after overdose in six adult patients (Citation7,Citation14CitationCitation16). Another adult patient demonstrated zero-order elimination kinetics (Citation17). The duration of action of brodifacoum ranged from 51 days to 9 months after overdose (Citation2,Citation4,Citation7,Citation18).
The terminal half-life of chlophacinone ranged from 3.3 to 23 days and can be decreased by phenobarbital administration (Citation19,Citation20). The half-life of difenacoum was 11.5 days in one case of covert poisoning (Citation21).
The anticoagulant effects of LAARs are secondary to inhibition of vitamin K 2,3-epoxide reductase and vitamin K quinone reductase. The inhibition of these enzymes prevents the activation of vitamin K and subsequent activation of clotting factors II, VII, IX, and X. A vitamin K-dependent clotting factor depletion below 25–30% of normal results in prolongation of prothrombin time (PT), which is often expressed as an international normalized ratio (INR). The partial thromboplastin time (PTT) can also be prolonged. The onset of anticoagulation effects after ingestion of an LAAR is usually delayed for several hours to days due to the presence of active clotting factors in circulation at the time of ingestion (Citation22CitationCitation24).
No dose equivalency studies were identified, so the expert consensus panel could not assess the relative potency of the various compounds. The lowest concentration for consumer use among various products is consistent at 0.005%, which suggests equipotency by manufacturers. For purposes of this guideline, all compounds are assumed to be equipotent in humans.
Clinical toxicology
Bleeding is the major risk following ingestion of LAAR compounds. The clinical manifestations after ingestion of LAARs range from being asymptomatic to active bleeding manifested as hematuria, epistaxis, menometrorrhagia, soft tissue bruising, hemarthrosis, anemia, hemoptysis, and retroperitoneal and intracranial hemorrhage (Citation16,Citation18,Citation25CitationCitationCitation28). Patients can require weeks to months of supplemental vitamin K therapy to correct the coagulation defect (Citation2,Citation7,Citation14,Citation19,Citation29,Citation30).
The PT or INR is the best screening test when performed 48–72 hours after exposure (Citation24). Serum brodifacoum and chlorophacinone concentrations have been reported as useful in documenting exposure and determining when vitamin K therapy can be discontinued (Citation14,Citation17,Citation19). Case reports suggest that coagulopathy is unlikely with serum brodifacoum concentrations less than 10 ng/mL (Citation14,Citation17). Most laboratories do not measure brodifacoum or other LAAR concentrations but can arrange for transportation of a sample to a reference laboratory for analysis for patients with LAAR-associated coagulopathy.
Intended users of this guideline
The intended users of this guideline are personnel in US poison centers. This guideline has been developed for the conditions prevalent in the US. While the toxicity of common LAARs is not expected to vary in a clinically significant manner in other nations, available formulations and active ingredients may differ for some LAAR products. In addition, out-of-hospital conditions could be much different. This guideline should not be extrapolated to other settings unless it has been determined that the conditions assumed in this guideline are present.
Objective of this guideline
The objective of this guideline is to assist poison center personnel in the appropriate out-of-hospital triage and initial management of patients with a suspected exposure to LAARs by 1) describing the process by which an exposure to LAAR might be evaluated, 2) identifying the key decision elements in managing cases of LAAR exposure, 3) providing clear and practical recommendations that reflect the current state of knowledge, and 4) identifying needs for research. This guideline applies to exposure to LAARs alone. Exposure to additional substances could require different referral and management recommendations, depending on the individual or combined toxicities of the substances.
This guideline is based on an assessment of current scientific and clinical information. The expert consensus panel recognizes that specific patient care decisions may be at variance with this guideline and are the prerogative of the patient and health professionals providing care, considering all of the circumstances involved. This guideline does not substitute for clinical judgment.
Methodology
The methodology used for the preparation of this guideline was developed after reviewing the key elements of practice guidelines (Citation31,Citation32). An expert consensus panel was established to develop the guideline (Appendix 1). The American Association of Poison Control Centers (AAPCC), the American Academy of Clinical Toxicology (AACT), and the American College of Medical Toxicology (ACMT) appointed members of their organizations to serve as panel members. To serve on the expert consensus panel, an individual had to have an exceptional track record in clinical care and scientific research in toxicology, board certification as a clinical or medical toxicologist, significant US poison center experience, and be an opinion leader with broad esteem. Two specialists in poison information were included as full panel members to provide the viewpoint of potential end-users of the guideline.
Literature search
The National Library of Medicine's PubMed database was searched (through 2004) using “brodifacoum or difenacoum or bromadiolone or chlorophacinone or diphacinone or pindone or valone or coumatetralyl or superwarfarin or rodenticide*” as textwords (title, abstract, MeSH term, CAS registry), limited to humans. The CAS registry numbers for these compounds were also used as search terms. This process was repeated in International Pharmaceutical Abstracts (1970–2004, excluding abstracts of meeting presentations), Science Citation Index (1977–2004), Database of Abstracts of Reviews of Effects (accessed December 2004), Cochrane Database of Systematic Reviews (accessed December 2004), and Cochrane Central Register of Controlled Trials (accessed December 2004). Reactions (1980–2004), the “Anticoagulants-long acting” poisoning management in Poisindex and the bibliographies of recovered articles were reviewed to identify previously undiscovered articles. Furthermore, North American Congress of Clinical Toxicology abstracts published in the Journal of Toxicology-Clinical Toxicology (1995–2004) were reviewed for original human data.
The chapter bibliographies in four major toxicology textbooks were reviewed for citations of additional articles with original human data. The Toxic Exposure Surveillance System (TESS) database maintained by the American Association of Poison Control Centers was searched for deaths resulting from unintentional LAAR poisoning or any deaths from LAAR poisoning in children.
Article selection
The recovered citations were entered into an EndNote library and duplicate entries were eliminated. The abstracts of these articles were reviewed, looking specifically for those that dealt with estimations of exposure doses with or without subsequent signs or symptoms, time of onset of symptoms, and management techniques that might be suitable for out-of-hospital use (e.g., gastrointestinal decontamination). Articles were excluded that did not meet the preceding criteria, did not add new data (e.g., some reviews, editorials), or that exclusively described inpatient-only procedures (e.g., dialysis).
Data extraction
All articles that were retrieved from the search were reviewed by a single abstractor. Each article was examined for original human data regarding the toxic effects of LAARs or original human data directly relevant to the out-of-hospital management of patients with LAAR toxicity or overdose. Relevant data (e.g., dose, resultant effects, time of onset of effects, therapeutic interventions or decontamination measures given, efficacy or results of any interventions, and overall patient outcome) were compiled into a table and a brief summary description of each article was written. This full evidence is available at http://www.aapcc.org/DiscGuidelines/LAAR%20evidence%20table.pdf. The completed table of all abstracted articles was then forwarded to the panel members for review and consideration in developing the guideline. Every attempt was made to locate significant foreign language articles and have their crucial information extracted, translated and tabulated. Copies of all of the articles were made available for reading by the panel members on a secure AAPCC website.
Criteria used to evaluate studies and assign levels of evidence
The articles were assigned level-of-evidence scores based on the Grades of Recommendation table developed by the Centre for Evidence-Based Medicine at Oxford University (Appendix 2). Single case reports and case series were classified as level 4.
Guideline writing and review
A guideline draft was prepared by the lead author (listed first). The draft was submitted to the expert consensus panel for comment. Using a modified Delphi process, comments from the expert consensus panel members were collected, copied into a table of comments, and submitted to the lead author for response. The lead author responded to each comment in the table and, when appropriate, the guideline draft was modified to incorporate changes suggested by the panel. The revised guideline draft was again reviewed by the panel and, if there was no strong objection by any panelist to any of the changes made by the lead author, the draft was prepared for the external review process. External review of the second draft was conducted by distributing it electronically to AAPCC, AACT, and ACMT members and the secondary review panel. The secondary review panel consisted of representatives from the federal government, public health, emergency services, pediatrics, pharmacy practice, and consumer organizations (Appendix 3). Comments were submitted via a discussion thread on the AAPCC web site or privately through email communication to AAPCC staff. All submitted comments were stripped of any information that would identify their sources, copied into a table of comments, and reviewed by the expert consensus panel and the lead author. The lead author responded to each comment in the table and his responses and subsequent changes in the guideline were reviewed and accepted by the panel. Following a meeting of the expert consensus panel, the final revision of the guideline was prepared.
Evaluation of evidence
Current poison center practice
The expert consensus panel solicited local referral and management guidelines for LAAR from all US poison centers in 2004 and received 14 documents (). One state poison center system (four poison centers) and 13 other individual poison centers submitted guidelines. Two centers specifically replied that they did not have guidelines for LAAR exposures. Review of the submitted guidelines revealed recommendations for home observation that ranged from “all unintentional” exposures to various dose thresholds (less than 0.5 mg to less than 1.25 mg of active ingredient). Likewise, referral for prothrombin time (PT) or INR determinations varied from “all exposures” to no referral if the patient was asymptomatic. The recommendations for the timing of outpatient PT or INR determination varied from 24 to 72 hours after exposure. Out-of-hospital decontamination was not addressed by many of the guidelines, but ipecac-induced emesis was recommended by one guideline in certain situations. Nine guidelines recommended the administration of activated charcoal if the patient presented soon after ingestion. Prophylactic vitamin K was not recommended by any center.
Table 3. Summary of individual US poison control center guidelines, 2004
This wide range of approaches, particularly for referral thresholds, suggested the need for a clear, evidence-based guideline. A guideline that determines the threshold dose for emergency department referral and the need for prehospital decontamination could potentially avoid unnecessary emergency department and clinic visits, reduce laboratory testing, and enhance the quality of care. In addition, a more consistent approach to this exposure might facilitate research that results in better care.
Review of textbooks
A review of anticoagulant poisoning in four toxicology textbooks revealed variation in their recommendations (Citation33CitationCitationCitation36). One textbook recommended that all children be followed with INR studies at 24 and 48 hours after a possible exposure (Citation35). Another textbook suggested that unintentional exposures in children might not need any evaluation (Citation33), while another limited at-home observation to patients with a known ingestion of a few pellets (Citation34). One textbook listed the toxic dose of LAAR active ingredient as greater than 0.1 mg/kg (Citation34), another as 0.0125 mg/kg (Citation33), and one suggested 10–14 g/kg of brodifacoum rodenticide bait is required for anticoagulant effects (Citation36).
Review of poisindex system
This toxicology information resource recommended obtaining a PT or INR in asymptomatic children with unintentional ingestions at 48 hours after the ingestion. Adults with intentional ingestions and children with evidence of bleeding are recommended to have an initial INR or PT and PTT measured and repeated at 24 and 48 hours after ingestion (Citation37).
Review of TESS mortality data
The American Association of Poison Control Centers' Toxic Exposure Surveillance System (TESS) database was analyzed for deaths from LAAR poisoning for all childhood exposures or for unintentional adult exposures over a 20-year period (1985–2004). There were no deaths reported in children less than 6 years of age. One elderly man with dementia unintentionally ingested an unspecified amount of brodifacoum rodenticide pellets (0.005% brodifacoum). During his hospitalization, he developed a prolonged PT (48 seconds) and multiple hypoglycemic episodes. He was treated with vitamin K, dextrose, and fresh frozen plasma. He became comatose and died 67 hours after admission. There was no brodifacoum dose information or evidence of clinical bleeding provided in the death abstract (W.A. Watson, Pharm.D., Associate Director, AAPCC, personal communication, January 2005).
Review of the literature
A summary of the evidence from all the reviewed articles on long-acting anticoagulant rodenticides (LAAR) appears below. In general, there were few articles specifically addressing out-of-hospital management of LAAR exposures. There were some limited in-hospital data that the expert consensus panel felt could contribute to the development of out-of-hospital guidelines. Therefore, both in-hospital and out-of-hospital data are included in this summary of the evidence.
Route of exposure
Skin was the route of exposure in only 3.3% (n = 554) of LAAR cases reported to US poison centers in 2003 (Citation1). No human cases of local or systemic toxicity from dermal exposure to solid LAARs were identified from the literature review. One case of toxicity associated with chronic skin exposure to a concentrated liquid formulation was identified (level 4). An 18-year-old man spilled 50 mL of 0.106% diphacinone liquid into his boot while working and wore it for 6–8 hours that day and for “several days” thereafter. He presented with hematuria and flank pain 7 days after the initial exposure. His PT and PTT 7 days after exposure were greater than 40 and 90 seconds, respectively (Citation8).
Only 1% (n = 176) of LAAR cases reported to US poison centers in 2003 had inhalation/nasal coded as the route of exposure (Citation1). The literature review found two cases (level 4) of poisoning via inhalation, both resulting from intentional abuse. A 37-year-old man smoked an unknown amount of brodifacoum mixed with cocaine in an attempt to potentiate the effect of the cocaine. He presented with epistaxis and a prothrombin time of 65.8 seconds (reference range, 9.7–12.5). His serum brodifacoum concentration was 680 ng/mL (Citation10). A 17-year-old man with a history of drug and alcohol abuse, smoked marijuana mixed with 3/4 of a box of 0.005% brodifacoum containing LAAR over an unknown period of time. He developed a coagulopathy that persisted for more than 1 year after he reportedly stopped smoking the mixture (Citation9).
Ingestion was the most common route of exposure (95%, n = 15,957) for LAAR cases reported to US poison centers (Citation1). All fatalities reported in the literature occurred by this route.
Toxic dose of LAAR by ingestion
There were very limited data concerning minimum toxic doses for LAARs in humans. There were two prospective studies and only a small number of retrospective studies that contained any toxic threshold or dose-effect information. Even when such information was present, there were often questions regarding the accuracy of the dose estimate due to uncertainties in the history. For example, in many cases the history was obtained days or weeks after the suspected exposure or there were differences in rodenticide content among available preparations. Retrospective data with dose-effect information were often confounded by the presence of co-exposures or differences in decontamination or treatment measures that could have altered the clinical presentation or outcome. It was difficult to account for inter-individual differences in age, weight, underlying health condition, or genetic factors that might affect LAAR toxicokinetics and toxicodynamics. It was also difficult to eliminate the possibility of recurrent ingestion or non-compliance with treatment in some cases of prolonged or recurrent coagulopathy. Among larger retrospective case series (level 4), many of the patients remained asymptomatic, and ingested doses and clinical effects were typically reported as ranges, percentages, or mean values for the populations so that individual doses resulting in specific effects could not be assigned. Very few cases had exposures confirmed with a serum LAAR concentrations. No data were identified concerning LAAR exposure in patients taking anticoagulants therapeutically.
Despite these limitations, some dose-response information was available for evaluation by the panel, most of which was represented by case reports and case series. The toxic dose evidence is summarized below and is divided into five general categories: 1) acute ingestions by patients 6 years of age and older, an age group in which non-therapeutic or suicidal exposures are difficult to exclude reliably; 2) acute ingestions by children less than 6 years old, an age group in which self-harm intent is extremely unusual; 3) chronic ingestions by patients 6 years of age and older; 4) chronic ingestions by patients less than 6 years of age; and 5) ingestions of unknown acuity. These data were further divided into subcategories based on the product involved because of potential differences in toxicokinetics and toxicodynamics. The articles cited in this summary are summarized in . The conversion of selected LAAR concentrations to dry weight for 1 mg of active ingredient (toxic dose) is illustrated in .
Table 4. Cases with dose information on acute toxicity in patients 6 years of age and older
Table 5. Cases with dose information on chronic toxicity
Table 6. Cases with dose information of unknown acuity
Table 7. Toxic dose conversion: LAAR concentration to dry weight
For the purposes of this guideline, minimal toxicity is defined as the presence of local effects only (e.g., irritation at the site of exposure); moderate toxicity as the presence of systemic effects, such as coagulopathy with or without non-life-threatening bleeding; and severe toxicity as the presence of coagulopathy with severe or life-threatening hemorrhage.
Acute ingestion by patients less than 6 years of age
There were two reports with dose-effect information on unknown or unspecified LAAR products. One was a prospective case series poison center study (level 4) of 110 children (7 months to 4 years of age) with acute ingestions of various LAARs over a 9-month period. Eight (7.3%) of these children developed mildly “elevated” PT-INRs (INR range 1.20–1.44) at 24–48 hours after ingestion. In three of these eight patients the dose was listed as a “taste,” in one patient it was listed as “1/2–1 pack,” and in four patients the dose was unknown. No correlation between doses and abnormal prothrombin times could be established. None of these children developed symptoms or clinical signs of bleeding. The child with the highest INR (1.44) ingested an unknown amount and reportedly had “prolonged bleeding” after minor cuts for 1 month after ingestion but did not seek medical care. None of the exposures were confirmed with serum brodifacoum measurements (Citation24).
The second article was a prospective case series (level 4) of 545 children with suspected acute, unintentional LAAR ingestions conducted over a 16-month period. Ingestions of unknown doses or greater than one pellet but less than one box were evaluated for coagulopathy. No decontamination was performed and prothrombin times were obtained between 48 and 96 hours after exposure. Mild, transient INR elevations, defined as INR of 1.5 or greater, were found in two asymptomatic patients (0.37%). Both patients ingested an unknown amount of LAAR. Their INRs were 1.5 and 1.8 and repeat prothrombin times on days 5 and 10, respectively, after ingestion were normal. One other child had an INR of 1.7 attributed to inadequate filling of the collection tube with blood (Citation22).
Another study evaluated clinical effects after acute LAAR ingestion by children, but no dose information was available. This was a retrospective analysis (level 4) of acute, unintentional ingestions of brodifacoum by children 6 years of age and younger with known clinical outcomes reported to the TESS database over a 4-year period (1993–1996). A total of 23,293 acute exposures occurred, but only 10,762 (46%) were followed to know outcomes. Of these, 308 (2.8%) were coded as having “minor” effects and 54 (0.5%) with “moderate” effects as defined by TESS. There were no “major” effects or deaths reported. There were 67 cases that had clinical or laboratory evidence of coagulopathy coded and the authors were able to review 38 poison center charts of these patients. Thirty-six of the 38 patients (95%) did not have evidence of bleeding or a prolongation of the prothrombin time greater than 50% of control (i.e., INR greater than 1.5). One patient had an INR of 2.6 at 48 hours after ingestion and remained asymptomatic for 6 days of follow-up. A 2-year-old boy presented with a prothrombin time of 15.1 seconds approximately 72 hours after the ingestion. He was treated with one dose of vitamin K and his PT was 12.1 seconds the following day. A third PT was 16 seconds at 3 weeks after ingestion. He was again treated with vitamin K and followed for 12 weeks without evidence of bleeding. They did not find any child with an elevated prothrombin time and evidence of bleeding (Citation38).
There were several other articles and abstracts (levels 4–6) that suggested that acute unintentional LAAR ingestions in children less than 6 years of age typically did not result in clinical effects or were associated with only transient unrelated effects (Citation3,Citation39CitationCitationCitationCitationCitationCitation45). Some of these articles did not specify children's ages. Mullins et al. (Citation43) reviewed 542 pediatric cases reported to a poison center over a 4-year period. Only one child in this case series (level 4) had an abnormal prothrombin time (18.8 seconds). No children had evidence of bleeding. Morrissey et al. (Citation42) evaluated exposures to warfarin-like compounds reported to a poison center over a 5-year period (level 4). A total of 1,789 cases were recorded (82% involved superwarfarins and 85% involved children of unspecified age) with no evidence of bleeding. One patient had a “minimally elevated” prothrombin time. An anecdotal report (level 5) from the National Poisons Unit for London states they have had “no serious adverse outcomes” related to possible superwarfarin ingestion by children over a five year period (approximately 1,000 cases) (Citation41). The abstract of a retrospective chart review of all pediatric (average age 22 months) superwarfarin cases reported to a poison center over an 11-month period reported no evidence of bleeding 48 hours after ingestion in 258 patients (level 6). In this study, there were 88 patients who reportedly ingested more than 5 pellets of rodenticide and had normal PT-INR measurements at 48 hours after ingestion (Citation44).
The preponderance of evidence suggests that acute, unintentional ingestion of ready-to-use LAARs by children will not result in a clinically significant coagulopathy or bleeding. Of more than 20,000 unintentional childhood exposures reviewed in the literature, only 14 cases of prolonged PT-INR (INR range: 1.22–2.6) were reported and none demonstrated bleeding. Only one patient had an INR greater than 2. It is unknown if any of these abnormal prothrombin times were false positives due to inadequate filling of blood tubes. Blood sample fill volumes of less than 90% for pediatric tubes can result in falsely elevated prothrombin times and INRs (Citation46). Another limitation of these studies is the lack of confirmation of ingestion with serum brodifacoum concentrations. Thus, it is difficult to conclude that the ingestions and reported doses actually occurred.
Acute ingestion by patients 6 years of age and older
Brodifacoum
There were several case reports, case series, and abstracts (levels 4 and 6) in which individual case information regarding a dose-effect relationship after acute ingestion was presented in detail. Specifically, there were 14 separate cases reported among 13 articles () (Citation2CitationCitation4,Citation17,Citation27,Citation47CitationCitationCitationCitationCitationCitation53). The lowest dose of brodifacoum associated with any toxicity was reported in an abstract (level 6) and was approximately 20 mL of a 0.005% solution (1 mg of brodifacoum), which resulted in moderate toxicity (coagulopathy, hematuria, tarry stools) (Citation48). A 44-year-old man admitted to ingesting a “handful” of Talon (0.005% brodifacoum), which resulted in gross hematuria, ecchymoses, a serum brodifacoum concentration of 78 ng/mL, and a PT greater than 90 seconds (level 4). He required treatment with vitamin K for 5 months (Citation3). A 62-year-old man ingested 2.15 mg of brodifacoum (43 g container of 0.005% brodifacoum) and presented with gross hematuria and a PT of 53.4 seconds 4 weeks later (level 4) (Citation51).
Bromadiolone
There was a single report (level 4) of two patients who ingested bromadiolone. A 41-year-old patient ingested 12.5 mg, and a 32-year-old patient ingested 62.5 mg of bromadiolone from rodenticides. Both patients developed coagulopathy and were treated with vitamin K (Citation53).
Chlorphacinone
There were five articles (level 4) with individual case data on dose-effect relationships for ingestion of chlorphacinone (Citation19,Citation20,Citation55CitationCitation57). Four of the articles were case reports and one was a case series involving nine patients. The doses ranged from 100 mg to 1825 mg; all patients developed coagulopathies and were treated with vitamin K. The lowest dose was ingested by an 18-year-old woman (approximately 100 mg or 1.6 mg/kg) who presented 3 days after ingestion with a prolonged prothrombin time (79 seconds) that persisted for 7 weeks (Citation57).
Difenacoum
There was a single case report (level 4) of a 17-year-old woman who ingested 500 g of a difenacoum-containing rat poison (difenacoum concentration not reported) and developed a coagulopathy (British corrected ratio 15) but no evidence of bleeding (Citation58).
Diphacinone
A single dose of 2 mg resulted in a slight reduction of prothrombin, but a 4-mg dose produced a clear reduction of prothrombin in a drug trial in adults (Citation59).
A prospective, uncontrolled trial (level 2c) with a single dose of a “new anticoagulant” was performed in 75 hospitalized patients without the need for anticoagulant therapy. They were given oral diphacinone as single doses, ranging from 0.5 to 20 mg, and serial coagulation studies were performed. The lowest single dose resulting in hypoprothrombinemia was 2 mg. The onset of effect was within 1–2 days of administration (Citation60).
Another prospective, uncontrolled trial with single or repeated doses of diphacinone was performed in 80 hospitalized patients. Thirty patients received various single oral doses ranging from 1 to 25 mg and coagulation tests were measured serially. The lowest dose associated with decreased prothrombin levels was 3 mg, usually occurring within 1–2 days of administration (Citation61).
Chronic ingestion by patients less than 6 years of age
There were no articles found with precise chronic dose-effect information for any LAAR exposure in children less than 6 years of age. In one report, a mother “sprinkled” an LAAR product containing 0.005% brodifacoum on her 24-month-old son's cereal over 4 days, which resulted in skin bruising and a prothrombin time greater than 125 seconds () (Citation7).
Chronic ingestion by patients 6 years of age and older
Brodifacoum
There were four case reports (levels 4 and 6) with quantifiable dose-effect information on chronic brodifacoum exposure in patients 6 years of age and older () (Citation18,Citation27,Citation62,Citation63). The lowest dose associated with toxicity was an ingestion of 168 g of a 0.005% preparation (8.4 mg brodifacoum) over approximately 12 hours by a 25-year-old man, which resulted in severe toxicity (Citation27).
Difenacoum
There were three case reports (level 4) with quantifiable dose-effect information on chronic difenacoum exposure in patients 6 years of age and older (Citation58,Citation64,Citation65). Among these cases, the lowest dose resulting in toxicity was in a 34-year-old man who ingested 4 mg daily for 1 month, which was associated with hematuria, dermal bleeding, and a prothrombin time greater than 90 seconds (Citation64).
Diphacinone
A prospective, uncontrolled trial (level 2b) of single and repeated doses of diphacinone was performed on 109 subjects with the need for anticoagulant therapy. Various dosing regimens were used in chronic treatment with individual daily doses ranging from 2 to 30 mg. Hypoprothrombinemia could be easily maintained in patients on chronic therapy with doses averaging 2–4 mg/day (Citation60).
Exposures of unknown acuity
Two case reports (level 4) were identified in which three children developed toxicity after LAAR exposures of unknown dose over an uncertain duration (Citation66,Citation67).
There were three case reports (level 4) with dose-effect information on brodifacoum in patients 6 years of age and older, but the duration of exposure was not clear () (Citation9,Citation16,Citation29). The lowest dose associated with toxicity was in a man who smoked about 3/4 of a box of D-Con Mouse Prufe II (0.005% brodifacoum) mixed with marijuana over an unclear time period and developed moderate toxicity (Citation9).
Onset of anticoagulant effects
The expert consensus panel was interested in determining the time of onset for toxicity (e.g., laboratory coagulopathy) after acute LAAR exposure in order to help make decisions about out-of-hospital triage, transportation, and management. Therefore, all articles documenting toxicity were searched for evidence of a time of onset of toxic effects. Unfortunately, the time of ingestion in relation to symptom or prolonged prothrombin time development was not reported or was not possible to assess in the vast majority of articles. The time of onset of coagulopathy is likely to be unreliable in retrospective cases because the initial development of a prolonged PT or INR is often asymptomatic. In only a few reports was a time of onset reported and felt to be reasonably accurate but, even in these cases, uncertainty remained.
There did not appear to be any difference in onset of symptoms after ingestion between different LAAR products, either because the data for each product were poor or because there are few toxicokinetic or toxicodynamic differences between products. For the purposes of this discussion on effect onset, all LAAR products and the data for both children and adults were considered together.
The onset of clinical systemic effects was delayed by hours to several days in the vast majority of cases (Citation2,Citation17,Citation20,Citation27,Citation30,Citation43,Citation47,Citation51CitationCitation53,Citation57,Citation62,Citation68). A few cases were delayed up to a week or longer. One woman presented with hematuria and back pain 7 days after ingesting 250 mg of chlorophaconone (level 4) (Citation19). A 61-year-old woman presented with a 3-day history of vaginal bleeding 2 weeks after ingesting two boxes of a brodifacoum-containing rodenticide (level 4) (Citation29).
The onset of laboratory evidence of coagulopathy was difficult to assess because most patients only presented after symptoms developed. However, in a few reports, laboratory evidence of toxicity was present as early as 8–48 hours after ingestion (Citation22CitationCitation24). In a prospective case series (level 4), prothrombin times obtained at 48 hours after ingestion were more likely to be abnormal that those at 24 hours (17% vs. 1.9%, p <0.005). A single PT measurement at 24 hours would have missed four of eight cases. It was not clear from the data whether larger doses resulted in more rapid onsets of effects (Citation24).
In long-acting anticoagulant single dosing trials (level 2b), the onset of laboratory confirmed coagulopathy was within 1–2 days of administration (Citation60,Citation61). Diphacinone was studied as an oral anticoagulant in humans in the 1950s. Adults receiving 4 mg had a clearly detectable reduction of prothrombin within 14 hours of ingestion (Citation59).
Duration of anticoagulant effect
The duration of action of brodifacoum ranged from 46 days to 9 months after intentional overdose (Citation2,Citation4,Citation7,Citation17CitationCitation19,Citation57). Adults receiving single 4-mg doses of diphacionone recovered from hypothrombinemia after 3 days. The effects of a 20-mg dose of diphacionone persisted for 6–10 days (Citation59).
Pregnancy and lactation
A 19-year-old pregnant woman ingested “a box of rat poison containing brodifacoum” at 21 weeks gestation (level 4). Eight days after exposure she presented with back pain and hematuria for several days. Her prothrombin time was greater than 60 seconds and her serum brodifacoum concentration was 220 ng/mL. Her coagulopathy was reversed with intravenous vitamin K and daily oral vitamin K1 (50 mg) until delivery. She delivered a healthy infant at 40 weeks. The mother and infant had normal coagulation studies at delivery (Citation53).
A 31-year-old woman ingested 30 50-g packages of Talon (total dose, 75 mg of brodifacoum) over a 2-day period (level 4). Five days after ingestion, her PT was 72 seconds (control 12 seconds). She was treated with vitamin K, fresh frozen plasma, and phenobarbital. Five weeks later, presumably during the first trimester, she had a spontaneous abortion (Citation18).
Therapeutic use of coumarin derivatives (i.e., dicumarol, warfarin) is associated with spontaneous abortion, hemorrhage, stillbirth, and embryopathy (Citation69).
There are no data on whether LAAR compounds cross the placenta or are excreted in breast milk.
Potential out-of-hospital management
The expert consensus panel identified potential methods for reducing LAAR toxicity in the out-of-hospital setting. These were reducing gastrointestinal absorption and administering supplemental vitamin K. Absorption could theoretically be decreased by early gastrointestinal decontamination such as emesis, gastric aspiration, or administration of activated charcoal soon after ingestion of large amounts. Supplemental vitamin K allows production of clotting factors that are depleted as a result of LAAR toxicity. No studies were found that specifically addressed out-of-hospital decontamination or use of antidotes or treatments.
Decontamination
There were many decontamination measures reported, including activated charcoal (single and multiple doses), ipecac syrup, and gastric lavage (Citation38,Citation39,Citation42,Citation45,Citation56,Citation70). With the exception of one study, all of the data were associated with case reports or case series (level 4), which limits any conclusions concerning efficacy. There were no prospective trials of decontamination methods.
The association of decontamination with clinical outcome for unintentional pediatric brodifacoum ingestions was evaluated by an examination (level 2b) of TESS data over a 4-year period (Citation38). There was no significant difference in outcome between patients who received any form of gastrointestinal decontamination compared to those who did not receive decontamination. Forty-two (0.75%) patients were reported to have minor complications related to decontamination procedures. Since these were unintentional pediatric ingestions, the likelihood of a toxic ingestion was small, and it is unlikely that activated charcoal would have had any effect on the outcome of these patients (Citation22,Citation43). Gastrointestinal decontamination in patients with known toxic ingestions has not been studied.
There were no human studies located that assessed the binding capacity of activated charcoal for LAARs. A 1-day course of multiple doses of activated charcoal (25 g every 4 hours) did not affect the duration of vitamin K therapy required in two cases (level 6) of brodifacoum poisoning (Citation70).
The administration of ipecac syrup resulted in severe vomiting and subsequent subarachnoid hemorrhage and death in an adult patient with brodifacoum poisoning (level 4) (Citation27).
There were no studies found that addressed skin decontamination.
Type of healthcare facility and mode of travel
There were no studies that addressed the type of healthcare facility to which patients should be referred or how they should be transported.
Out-of-hospital treatment measures
There were several treatments utilized for LAAR toxicity in published reports. These primarily included antidotal treatment such as vitamin K, fresh frozen plasma, and blood transfusion to correct coagulopathies. Recombinant activated factor VII was used in four patients (level 4) (Citation28). Treatments to control bleeding or replace lost blood such as compression, surgery, endoscopy, blood transfusion, or iron supplementation were also reported. Other treatments included general supportive measures for complications such as benzodiazepines for seizures caused by intracerebral hemorrhage, intravenous fluids, intubation, oxygen, and drugs (e.g., phenobarbital) designed to enhance the metabolism of the LAAR or its metabolites. There were no prospective or controlled retrospective data to support the efficacy for any of these treatment measures.
There are several case reports or case series or their abstracts (levels 4 and 6) in which temporal improvement in coagulopathy or bleeding clearly occurred after vitamin K or blood product administration. When described, treatment with vitamin K was often required for weeks to months after exposure (Citation5,Citation7,Citation17,Citation19,Citation29,Citation55).
Limitations of the published data
The strength of evidence for this guideline is limited to prospective case series, two uncontrolled prospective drug trials, retrospective case series, and case reports. Level 4 data do not provide a sound basis for toxic dose estimation or triage recommendations. The case reports and case series varied widely in the level of clinical detail presented, severity of clinical effects of the poisoning, timing of interventions, co-ingestants, estimated dose, and treatments administered.
The lack of precision in dose measurement is a major limitation of this literature analysis. The estimates are subject to many assumptions. Data for amount ingested are often inaccurate or incomplete. Parents might under- or overestimate the ingested dose because of denial or anxiety. Poison center staffs often record the dose taken as the worst case scenario in order to provide a wide margin of safety. Estimating the amount ingested from examining most packets or boxes of LAARs is unreliable. In most case reports and case series the estimates of exposure were not independently verified. Confirmation of exposure by measuring serum LAAR concentrations was rarely obtained.
Conclusions
Key decision elements
The expert consensus panel identified the patient's age, intent, route of exposure, estimated dose of LAARs, time since exposure, and symptoms as critical information needed in order to make a sound triage decision. The laboratory and treatment capabilities of the referral healthcare facility were also considered important in decision making. The expert consensus panel agreed that in each case, the judgment of the specialist in poison information or the poison center medical director might override any specific recommendation from this guideline.
Patient intent
All patients in whom suicidal, abuse, misuse, or malicious intent (e.g., child abuse, neglect, or homicide) is known or suspected should be referred to an emergency department for medical evaluation regardless of the dose ingested. Unintentional exposures in children less than 6 years of age have an extremely low rate of clinical toxicity. No cases of bleeding have been reported in this age group from unintentional exposure to LAARs.
Route of exposure
Exposure to LAARs can occur by ingestion, inhalation or dermal contact. Systemic toxicity has been documented via ingestion of liquid and solid formulations, chronic dermal exposure to a liquid formulation, and smoking pellets/granular formulation for abuse.
Toxic dose of LAAR
The minimum toxic dose has not been reliably established for children or adults. Case data for brodifacoum and diphacinone suggest that ingestion of 1 mg of active ingredient can be potentially toxic to an adult. The data do not allow determination of toxic doses for each compound or for classes of compounds, nor do the data allow for a reliable weight-based toxic dose estimate in children (i.e., mg/kg toxic dose). Unintentional ingestion of ready-to-use LAAR bait containing 0.005% active ingredient by children less than 6 years of age has not resulted in clinical bleeding in over 20,000 exposures reported in the literature and TESS database. The panel concluded that these children do not ingest toxic doses of LAAR active ingredients under usual circumstances. They can be observed at home without laboratory monitoring if asymptomatic. Under extenuating circumstances (e.g., pica), a child may ingest large amounts of LAAR bait (e.g., greater than 20 g or 2/3 oz net weight) that would warrant laboratory evaluation for coagulopathy, but this would be an extremely rare event.
Time of onset and duration of toxicity after overdose
The onset of anticoagulation effects after acute ingestion ranged from 8 to 48 hours. Laboratory evaluation for coagulopathy at less than 48 hours after exposure in asymptomatic patients can miss some patients who ingested a toxic dose. There are many case reports of delayed presentation to the hospital, but assessment of onset of symptoms or laboratory evidence of anticoagulation before presentation was not possible.
The duration of anticoagulation after overdose can be prolonged for several months. Therefore, evaluation for potential coagulopathy should occur in patients who ingest a toxic dose and present days to weeks after exposure, even if asymptomatic.
Presence of symptoms
The absence of signs or symptoms of bleeding does not exclude a potentially toxic ingestion. Referral for evaluation should not be withheld based on the absence of symptoms if a potential toxic dose was ingested.
Chronicity of exposure
There is insufficient evidence in the literature to recommend that the toxic dose of chronic LAAR ingestion is less than that of a single acute ingestion (i.e., 1 mg/day) (Field). However, the panel recommends that those rare patients with chronic ingestion of a LAAR be referred for medical evaluation of intent and coagulopathy, regardless of the dose. This is due to the uncertainty that likely surrounds the circumstances of such cases and the unknown kinetics of chronic administration of these chemicals in humans.
Type of healthcare facility and mode of travel
The expert consensus panel concluded that patients should be referred to facilities that have the ability to assess and manage coagulopathy in a timely manner. This requires referral to a hospital emergency department if the patient is actively bleeding or an outpatient clinic or physician's office that can obtain prothrombin time results within 24 hours if the patient is asymptomatic. For symptomatic patients, facilities that can obtain prothrombin times and have blood transfusion, fresh frozen plasma, and vitamin K therapy available are preferred. The patient's clinical condition, local protocols, and transportation resources should dictate the mode of transportation.
Pregnancy
The panel concluded that unintentional ingestion of less than 1 mg LAAR active ingredient by a pregnant woman, as with any unintended exposure to a drug or chemical, should be evaluated in follow-up by their primary care physician or obstetrician. Immediate referral to an ED is not necessary.
Potential out-of-hospital management
Decontamination
Patients with dermal exposures should be washed with mild soap and water. There was no evidence that out-of-hospital gastrointestinal decontamination offered benefit to the patient.
Ipecac syrup should not be administered (Citation27). The efficacy of oral activated charcoal in preventing the absorption of LAAR has not been adequately studied. Transportation to an emergency department should not be delayed in order to attempt activated charcoal administration.
Treatment
The expert consensus panel felt that vitamin K treatment should be based on laboratory evidence of coagulopathy and not administered prophylactically. Administration prior to laboratory evaluation can delay the onset of anticoagulation and makes the diagnosis and laboratory monitoring more difficult. The out-of-hospital use of vitamin K as an antidote cannot be advocated at this time.
Recommendations
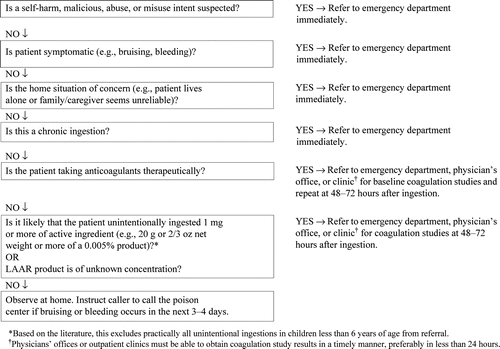
Patients with exposure due to suspected self-harm, abuse, misuse, or potentially malicious administration should be referred to an emergency department immediately regardless of the doses reported (Grade D).
Patients with symptoms of LAAR poisoning (e.g., bleeding, bruising) should be referred immediately to an emergency department for evaluation regardless of the doses reported (Grade C).
Patients with chronic ingestion of LAAR should be referred immediately to an emergency department for evaluation of intent and potential coagulopathy (Grade B).
Patients taking anticoagulants therapeutically and who ingest any dose of a LAAR should have a baseline prothrombin time measured and then again at 48–72 hours after ingestion (Grade D).
Patients with unintentional ingestion of less than 1 mg of LAAR active ingredient can be safely observed at home without laboratory monitoring. This includes practically all unintentional ingestions in children less than 6 years of age (Grade C).
Pregnant patients with unintentional exposure to less than 1 mg of LAAR active ingredient should be evaluated by their obstetrician or primary care provider as an outpatient. Immediate referral to an ED or clinic is not required.
Patients with unintentional ingestion of 1 mg or more of active ingredient and are asymptomatic should be evaluated for coagulopathy at 48–72 hours after exposure (Grade B).
Physicians' offices or outpatient clinics must be able to obtain coagulation study results in a timely manner, preferably in less than 24 hours, for patients who require outpatient monitoring (Grade D).
Gastrointestinal decontamination with ipecac syrup is not recommended (Grade D).
Transportation to an emergency department should not be delayed for administration of activated charcoal (Grade D).
Patients with dermal exposures should be decontaminated by washing the skin with mild soap and water Grade D)
The administration of vitamin K is not recommended prior to evaluation for coagulopathy (Grade D).
An algorithm that reflects many of these recommendations is in Appendix 4.
Implications for research
The expert consensus panel identified the following topics where additional research might be useful.
The correlation between reported LAAR ingestion, serum LAAR concentration, and abnormal prothrombin time.
Evaluation of patient adherence to poison center recommendations for medical evaluation and PT-INR measurement at 48–72 hours after LAAR exposure.
Disclosures
There are no potential conflicts of interest reported by the expert consensus panel or project staff regarding this guideline.
Guidelines for the Management of Poisoining. Supported in full by Cooperative Agreement 8 U4BHS00084 between the American Association of Poison Control Centers and the Healthcare Systems Bureau, Health Resources and Services Administration, Department of Health and Human Services.
References
- 1. Toxic Exposure Surveillance System (TESS). Washington (DC): American Association of Poison Control Centers, 2004. Database accessed June 2005
- Chong LL, Chau WK, Ho CH. A case of “superwarfarin” poisoning. Scand J Haematol 1986; 36: 314–315
- Chua JD, Friedenberg WR. Superwarfarin poisoning. Arch Intern Med 1998; 158: 1929–1932
- Jones EC, Growe GH, Naiman SC. Prolonged anticoagulation in rat poisoning. JAMA 1984; 252: 3005–3007
- Park BK, Choonara IA, Haynes BP, Breckenridge AM, Malia RG, Preston FE. Abnormal vitamin K metabolism in the presence of normal clotting factor activity in factory workers exposed to 4-hydroxycoumarins. Br J Clin Pharmacol 1986; 21: 289–293
- Svendsen SW, Kolstad HA, Steesby E. Bleeding problems associated with occupational exposure to anticoagulant rodenticides. Int Arch Occup Environ Health 2002; 75: 515–517
- Babcock J, Hartman K, Pedersen A, Murphy M, Alving B. Rodenticide-induced coagulopathy in a young child. A case of Munchausen syndrome by proxy. Am J Pediatr Hematol Oncol 1993; 15: 126–130
- Spiller HA, Gallenstein GL, Murphy MJ. Dermal absorption of a liquid diphacinone rodenticide causing coagulaopathy. Vet Hum Toxicol 2003; 45: 313–314
- La Rosa FG, Clarke SH, Lefkowitz JB. Brodifacoum intoxication with marijuana smoking. Arch Pathol Lab Med 1997; 121: 67–69
- Waien SA, Hayes D, Leonardo JM. Severe coagulopathy as a consequence of smoking crack cocaine laced with rodenticide. N Engl J Med 2001; 345: 700–701
- Bachmann KA, Sullivan TJ. Dispositional and pharmacodynamic characteristics of brodifacoum in warfarin-sensitive rats. Pharmacology 1983; 27: 281–288
- Leck JB, Park BK. A comparative study of the effects of warfarin and brodifacoum on the relationship between vitamin K1 metabolism and clotting factor activity in warfarin-susceptible and warfarin-resistant rats. Biochem Pharmacol 1981; 30: 123–128
- Park BK, Leck JB. A comparison of vitamin K antagonism by warfarin, difenacoum and brodifacoum in the rabbit. Biochem Pharmacol 1982; 31: 3635–3639
- Hollinger BR, Pastoor TP. Case management and plasma half-life in a case of brodifacoum poisoning. Arch Intern Med 1993; 153: 1925–1928
- Pavlu J, Harrington DJ, Voong K, Savidge GF, Jan-Mohamed R, Kaczmarski R. Superwarfarin poisoning. Lancet 2005; 365. 628
- Weitzel JN, Sadowski JA, Furie BC, Moroose R, Kim H, Mount ME, Murphy MJ, Furie B. Surreptitious ingestion of a long-acting vitamin K antagonist/rodenticide, brodifacoum: clinical and metabolic studies of three cases. Blood 1990; 76: 2555–2559
- Bruno GR, Howland MA, McMeeking A, Hoffman RS. Long-acting anticoagulant overdose: brodifacoum kinetics and optimal vitamin K dosing. Ann Emerg Med 2000; 36: 262–267
- Lipton RA, Klass EM. Human ingestion of a “superwarfarin” rodenticide resulting in a prolonged anticoagulant effect. JAMA 1984; 252: 3004–3005
- Burucoa C, Mura P, Robert R, Boinot C, Bouquet S, Piriou A. Chlorophacinone intoxication. A biological and toxicological study. J Toxicol Clin Toxicol 1989; 27: 79–89
- Lagrange F, Corniot AG, Titier K, Bedry R, Pehourcq F. Toxicological management of chlorophacinone poisoning. Acta Clin Belg Suppl 1999; 1: 13–16
- McCarthy PT, Cox AD, Harrington DJ, Evely RS, Hampton E, al-Sabah AI, Massey E, Jackson H, Ferguson T. Covert poisoning with difenacoum: clinical and toxicological observations. Hum Exp Toxicol 1997; 16: 166–170
- Ingels M, Lai C, Tai W, Manning BH, Rangan C, Williams SR, Manoguerra AS, Albertson T, Clark RF. A prospective study of acute, unintentional, pediatric superwarfarin ingestions managed without decontamination. Ann Emerg Med 2002; 40: 73–78
- Seidelmann S, Kubic V, Burton E, Schmitz L. Combined superwarfarin and ethylene glycol ingestion. A unique case report with misleading clinical history. Am J Clin Pathol 1995; 104: 663–666
- Smolinske SC, Scherger DL, Kearns PS, Wruk KM, Kulig KW, Rumack BH. Superwarfarin poisoning in children: a prospective study. Pediatrics 1989; 84: 490–494
- Barnett VT, Bergmann F, Humphrey H, Chediak J. Diffuse alveolar hemorrhage secondary to superwarfarin ingestion. Chest 1992; 102: 1301–1302
- Emre S, Kitabayashi K, Miller CM. Successful liver transplantation from a donor with brodifacoum intoxication. Liver Transpl Surg 1999; 5: 509–511
- Kruse JA, Carlson RW. Fatal rodenticide poisoning with brodifacoum. Ann Emerg Med 1992; 21: 331–336
- Zupancic-Salek S, Kovacevic-Metelko J, Radman I. Successful reversal of anticoagulant effect of superwarfarin poisoning with recombinant activated factor VII. Blood Coagul Fibrinolysis 2005; 16: 239–244
- Casner PR. Superwarfarin toxicity. Am J Ther 1998; 5: 117–120
- Hoffman RS, Smilkstein MJ, Goldfrank LR. Evaluation of coagulation factor abnormalities in long-acting anticoagulant overdose. J Toxicol Clin Toxicol 1988; 26: 233–248
- Shaneyfelt TM, Mayo-Smith MF, Rothwangl J. Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer-reviewed medical literature. JAMA 1999; 281: 1900–1905
- Shiffman RN, Shekelle P, Overhage JM, Slutsky J, Grimshaw J, Deshpande AM. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med 2003; 139: 493–498
- Burkhart KK. Anticoagulant rodenticides. Clinical toxicology, MD Ford, KA Delaney, J Ling, T Erickson. WB Saunders, Philadelphia 2001; 848–853
- Metts BC, Stewart NJ. Rodenticides. Clinical management of poisoning and drug overdose3rd, LM Haddad, MW Shannon, JF Winchester. WB Saunders, Philadelphia 1998; 864–875
- Su M, Hoffman RS. Anticoagulants. Goldfrank's toxicologic emergencies7th, LR Goldfrank, NE Flomenbaum, NA Lewin, MA Howland, RS Hoffman, LS Nelson. McGraw-Hill, New York 2002; 631–646
- Yip L. Anticoagulant rodenticides. Medical toxicology3rd, RC Dart. Williams & Wilkins, Baltimore 2004; 1497–1507
- Anticoagulants. long-acting management. Poisindex System, RK Klasco. Thomson Micromedex, Greenwood Village, CO, edition expires September 2004
- Shepherd G, Klein-Schwartz W, erson BD. Acute, unintentional pediatric brodifacoum ingestions. Pediatr Emerg Care 2002; 18: 174–178
- Bennett DL, Caravati EM, Veltri JC. Long-acting anticoagulant ingestion: a prospective study [abstract]. Vet Hum Toxicol 1987; 29: 472–473
- Brands CS, Bartkus EA, Daya MR. Accidental superwarfarin ingestion: compliance with current Poisindex® guidelines [abstract]. J Toxicol Clin Toxicol 1995; 33. 487
- Kanabar D, Volans G. Accidental superwarfarin poisoning in children—less treatment is better. Lancet 2002; 360. 963
- Morrissey B, Burgess JL, Robertson WO. Washington's experience and recommendations. Re: anticoagulant rodenticides. Vet Hum Toxicol 1995; 37: 362–363
- Mullins ME, Brands CL, Daya MR. Unintentional pediatric superwarfarin exposures: do we really need a prothrombin time?. Pediatrics 2000; 105: 402–404
- Polley K, Nichols MH, King WD. Superwarfarin ingestion: is observation alone the answer? [abstract]. J Investig Med 1996; 44. A28
- Sullivan MP, Dean BS, Krenzelok EP. Long-acting anticoagulant rodenticides: an evaluation of 88 cases [abstract]. Vet Hum Toxicol 1989; 31. 361
- Chuang J, Sadler MA, Witt DM. Impact of evacuated collection tube fill volume and mixing on routine coagulation testing using 2.5-mL (pediatric) tubes. Chest 2004; 126: 1262–1266
- Boyer LV, Griffith L, Fosdick CC, III, Fernandez M. Prolonged coagulopathy after brodifacoum ingestion and the challenges in provision of continuing care [abstract]. J Toxicol Clin Toxicol 1998; 36. 462
- Chen TW, Deng JF. A brodifacoum intoxication case of mouthful amount [abstract]. Vet Hum Toxicol 1986; 28. 488
- Lewis-Younger C, Horowitz Z. Elimination of brodifacoum [abstract]. J Toxicol Clin Toxicol 2001; 39: 474–475
- Morgan BW, Tomaszewski C, Rotker I. Spontaneous hemoperitoneum from brodifacoum overdose. Am J Emerg Med 1996; 14: 656–659
- Ross GS, Zacharski LR, Robert D, Rabin DL. An acquired hemorrhagic disorder from long-acting rodenticide ingestion. Arch Intern Med 1992; 152: 410–412
- Sheen SR, Spiller HA, Grossman D. Symptomatic brodifacoum ingestion requiring high-dose phytonadione therapy. Vet Hum Toxicol 1994; 36: 216–217
- Zurawski JM, Kelly EA. Pregnancy outcome after maternal poisoning with brodifacoum, a long-acting warfarin-like rodenticide. Obstet Gynecol 1997; 90: 672–674
- Haug B, Schjodt-Iversen L, Rygh J. Forgiftninger med langtidsvirkende antikoagulanter. Tidsskr Nor Laegeforen 1992; 112: 1958–1960
- Chataigner D, Garnier R, Elmalem J, Efthymiou ML. Hypocoagulabilit& eacute; prolong& eacute;e secondaire & agrave; l'ingestion de raticides anticoagulants. Ann Med Interne (Paris) 1989; 139: 537–541
- Murdoch DA. Prolonged anticoagulation in chlorphacinone poisoning. Lancet 1983; 1: 355–356
- Vogel JJ, de Moerloose P, Bouvier CA, Gaspoz J, Riant P. Anticoagulation prolong& eacute;e lors d'une intoxication & agrave; la chlorphacinone. Schweiz Med Wochenschr 1988; 118: 1915–1917
- Barlow AM, Gay AL, Park BK. Difenacoum (Neosorexa) poisoning. Br Med J (Clin Res Ed) 1982; 285. 541
- Hayes WJ. Pesticides studied in man. Williams & Wilkins, Baltimore 1982; 512–513
- Field JB, Goldfarb MS, Ware AG, Griffith GC. Dipaxin: 2-diphenylacetyl-1,3-indandione; clinical evaluation of a new anticoagulant. Circulation 1955; 11: 576–583
- Pascale LR, Olwin JH. Clinical experience with a new anticoagulant, dipaxin (2-diphenylacetyl-1,3-indandione). Circulation 1954; 9: 230–237
- Corke PJ. Superwarfarin (brodifacoum) poisoning. Anaesth Intensive Care 1997; 25: 707–709
- Tsutaoka BT, Miller M, Fung SM, Patel MM, Olson KR. Superwarfarin and glass ingestion with prolonged coagulopathy requiring high-dose vitamin K1 therapy. Pharmacotherapy 2003; 23: 1186–1189
- Butcher GP, Shearer MJ, MacNicoll AD, Kelly MJ, Ind PW. Difenacoum poisoning as a cause of haematuria. Hum Exp Toxicol 1992; 11: 553–554
- Terneu S, Verhelst D, Thys F, Ketelslegers E, Hantson P, Wittebole X. An unusual cause of abdominal pain. Acta Clin Belg 2003; 58: 241–244
- Greeff MC, Mashile O, MacDougall LG. “Superwarfarin” (bromodialone) poisoning in two children resulting in prolonged anticoagulation. Lancet 1987; 2. 1269
- Watts RG, Castleberry RP, Sadowski JA. Accidental poisoning with a superwarfarin compound (brodifacoum) in a child. Pediatrics 1990; 86: 883–887
- Dahl B, Caravati M, Dunson W. Surreptitious brodifacoum poisoning [abstract]. J Toxicol Clin Toxicol 1998; 36: 475–476
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactationSixth. Williams and Wilkins, Baltimore 2002; 327–334
- Donovan JW, Ballard JO, Murphy MJ. Brodifacoum therapy with activated charcoal: effect on elimination kinetics [abstract]. Vet Hum Toxicol 1990; 32. 350
- www.epa.gov/REDs/2100red.pdf, Environmental Protection Agency (US), Reregistration Eligibility Decision (RED): Rodenticide ClusterJulyEPA 738-R-98–007. 1998Document accessed June 6, 2005
Appendix 1
Expert Consensus Panel Members
Lisa L. Booze, PharmDCertified Specialist in Poison InformationMaryland Poison CenterUniversity of Maryland School of PharmacyBaltimore, Maryland
E. Martin Caravati, MD, MPH, FACMT, FACEPProfessor of Surgery (Emergency Medicine)University of UtahMedical DirectorUtah Poison Control CenterSalt Lake City, Utah
Gwenn Christianson, RN, MSNCertified Specialist in Poison InformationIndiana Poison Center Indianapolis, Indiana
Peter A. Chyka, PharmD. FAACT, DABATProfessor of Clinical PharmacyCollege of PharmacyUniversity of TennesseeKnoxville, Tennessee
Daniel J. Cobaugh, PharmD, FAACT, DABATDirector of ResearchAmerican Society of Health-System PharmacistsResearch and Education FoundationBethesda, MarylandFormer Associate Director, American Association of Poison Control Centers
Daniel C. Keyes, MD, MPHMedical DirectorPine Bluff Chemical Demilitarization FacilityAssociate Professor, Southwestern Toxicology Training ProgramDallas, Texas
Anthony S. Manoguerra, PharmD, DABAT, FAACTProfessor of Clinical Pharmacy and Associate DeanSchool of Pharmacy and Pharmaceutical SciencesUniversity of California San DiegoFormer Director, California Poison Control System, San Diego DivisionSan Diego, California
Lewis S. Nelson, MD, FACEP, FACMTAssistant Professor of Emergency MedicineNew York University School of MedicineAssociate Medical DirectorNew York City Poison Control CenterNew York, New York
Kent R. Olson, MD, FACEP, FAACT, FACMTMedical DirectorCalifornia Poison Control System, San Francisco DivisionClinical Professor of Medicine & PharmacyUniversity of California, San FranciscoSan Francisco, California
Elizabeth J. Scharman, PharmD, DABAT, BCPS, FAACTDirector, West Virginia Poison CenterProfessor, West Virginia University School of PharmacyDept. Clinical PharmacyCharleston, West Virginia
Paul M. Wax, MD, FACMTAttending ToxicologistUT Southwestern Medical CenterDallas, Texas
Alan D. Woolf, MD, MPH, FACMTDirector, Program in Environmental MedicineChildren's Hospital, BostonAssociate Professor of PediatricsHarvard Medical SchoolBoston, Massachusetts
Appendix 2
Grades of Recommendation and Levels of Evidence
Appendix 3
Secondary Review Panel Organizations
Ambulatory Pediatric Association
American Academy of Breastfeeding Medicine
American Academy of Emergency Medicine
American Academy of Pediatrics
American Association for Health Education
American College of Clinical Pharmacy
American College of Emergency Physicians
American College of Occupational and Environmental Medicine
American Pharmacists Association
American Public Health Association
American Society of Health-System Pharmacists
Association of Maternal and Child Health Programs
Association of Occupational and Environmental Clinics
Association of State and Territorial Health Officials
Canadian Association of Poison Control Centres
Centers for Disease Control and Prevention – National Center for Injury Prevention and Control
Consumer Federation of America
Consumer Product Safety Commission
Department of Transportation
Emergency Medical Services for Children
Emergency Nurses Association
Environmental Protection Agency
Food and Drug Administration
National Association of Children's Hospitals and Related Institutions
National Association of Emergency Medical Services Physicians
National Association of Emergency Medical Technicians
National Association of School Nurses
National Association of State Emergency Medical Services Directors
National Safe Kids Campaign
Teratology Society
World Health Organization International Programme on Chemical Safety
Appendix 4